Published online Jul 27, 2020. doi: 10.4254/wjh.v12.i7.332
Peer-review started: December 31, 2019
First decision: February 19, 2020
Revised: May 6, 2020
Accepted: May 15, 2020
Article in press: May 15, 2020
Published online: July 27, 2020
Processing time: 197 Days and 13.4 Hours
Alcohol consumption is one of the leading causes of the global burden of disease and results in high healthcare and economic costs. Heavy alcohol misuse leads to alcohol-related liver disease, which is responsible for a significant proportion of alcohol-attributable deaths globally. Other than reducing alcohol consumption, there are currently no effective treatments for alcohol-related liver disease. Oxidative stress refers to an imbalance in the production and elimination of reactive oxygen species and antioxidants. It plays important roles in several aspects of alcohol-related liver disease pathogenesis. Here, we review how chronic alcohol use results in oxidative stress through increased metabolism via the cytochrome P450 2E1 system producing reactive oxygen species, acetaldehyde and protein and DNA adducts. These trigger inflammatory signaling pathways within the liver leading to expression of pro-inflammatory mediators causing hepatocyte apoptosis and necrosis. Reactive oxygen species exposure also results in mitochondrial stress within hepatocytes causing structural and functional dysregulation of mitochondria and upregulating apoptotic signaling. There is also evidence that oxidative stress as well as the direct effect of alcohol influences epigenetic regulation. Increased global histone methylation and acetylation and specific histone acetylation inhibits antioxidant responses and promotes expression of key pro-inflammatory genes. This review highlights aspects of the role of oxidative stress in disease pathogenesis that warrant further study including mitochondrial stress and epigenetic regulation. Improved understanding of these processes may identify novel targets for therapy.
Core tip: Alcohol is a global health problem with alcohol-related liver disease forming a significant proportion of alcohol-attributable deaths. However, there are no effective treatments for alcohol-related liver disease. Oxidative stress plays multiple roles in disease pathogenesis, which if better understood may yield new therapeutic targets. Here, we review the current literature on how alcohol consumption leads to oxidative stress and how this results in hepatocyte apoptosis and necrosis through its contribution to mitochondrial stress, dysregulation of cell signalling pathways and epigenetic regulation.
- Citation: Tan HK, Yates E, Lilly K, Dhanda AD. Oxidative stress in alcohol-related liver disease. World J Hepatol 2020; 12(7): 332-349
- URL: https://www.wjgnet.com/1948-5182/full/v12/i7/332.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i7.332
Europe has the highest per capita alcohol consumption and alcohol-related loss of disability adjusted life years globally[1]. The European Union is the heaviest drinking region of the world, with 11 liters of pure alcohol drunk per adult each year[1]. In the last decade, this trend continues to rise in central and northern Europe. Alcohol is a dose-related risk factor for more than 200 diseases[1]. It causes 5.9% of all deaths globally and more than 25% of deaths in the age group 20-39 years[1].
Heavy alcohol use is the cause of alcohol-related liver disease (ALD). Therefore, it is not surprising that the incidence of ALD is on a rising trajectory[2]. The scale of ALD is estimated to burden the United Kingdom national health system with health-related costs of over £3.5 billion per year[2]. In Europe, the cost of treating ALD is estimated to be €17 billion, together with €5bn spent on treatment and prevention of harmful alcohol use and alcohol dependence[3]. Worldwide, alcohol-related liver cirrhosis deaths account for approximately 10% of all alcohol-attributable deaths resulting in the loss of 22.2 million disability-adjusted life years annually[4].
The ALD spectrum ranges from simple steatosis to steatohepatitis, fibrosis, and cirrhosis. While alcohol-related cirrhosis is no longer considered a completely irreversible condition, no effective anti-fibrotic therapies are currently available. Cirrhosis can be divided into compensated and decompensated stages, with differentiating clinical features and prognosis. The median survival of patients with compensated liver disease is approximately 6.5 years but only 2.5 years in those with decompensated cirrhosis[4]. Once a complication of cirrhosis develops, the 5-year survival rate decreases to less than 20%[4].
Alcoholic hepatitis (AH) is an acute inflammatory condition that occurs on the background of ALD. Severe AH has a mortality rate of 30% within 3 mo[5] but even non-severe AH has a significant 7% mortality within 3 mo[6]. The established treatment for AH is corticosteroids, which improve short-term survival but do not affect long-term survival[5].
The molecular mechanisms underlying ALD pathogenesis are complex and have not been fully elucidated. However, there is emerging evidence that oxidative stress plays a role in mediating the inflammatory response and in directly causing liver damage. Oxidative stress represents the body’s imbalance in the production and the elimination of reactive species (including reactive oxygen and nitrogen species) as well as decreased production of antioxidants[7]. Here, we review the role of oxidative stress in ALD focusing on its effect on mitochondrial stress, cell signaling and epigenetic regulation.
Comprehensive searches of MEDLINE, EMBASE, PubMed and TRIPS from their commencement to June 2019 were conducted. The search strategy included subject headings and keywords related to “alcohol” and “oxidative stress” and “liver”. The reference list of all included studies was screened for eligibility. This review included all study types in humans and animals. Studies published in all languages were considered. One author independently screened titles and abstracts and subsequently reviewed full-texts of retrieved studies for eligibility.
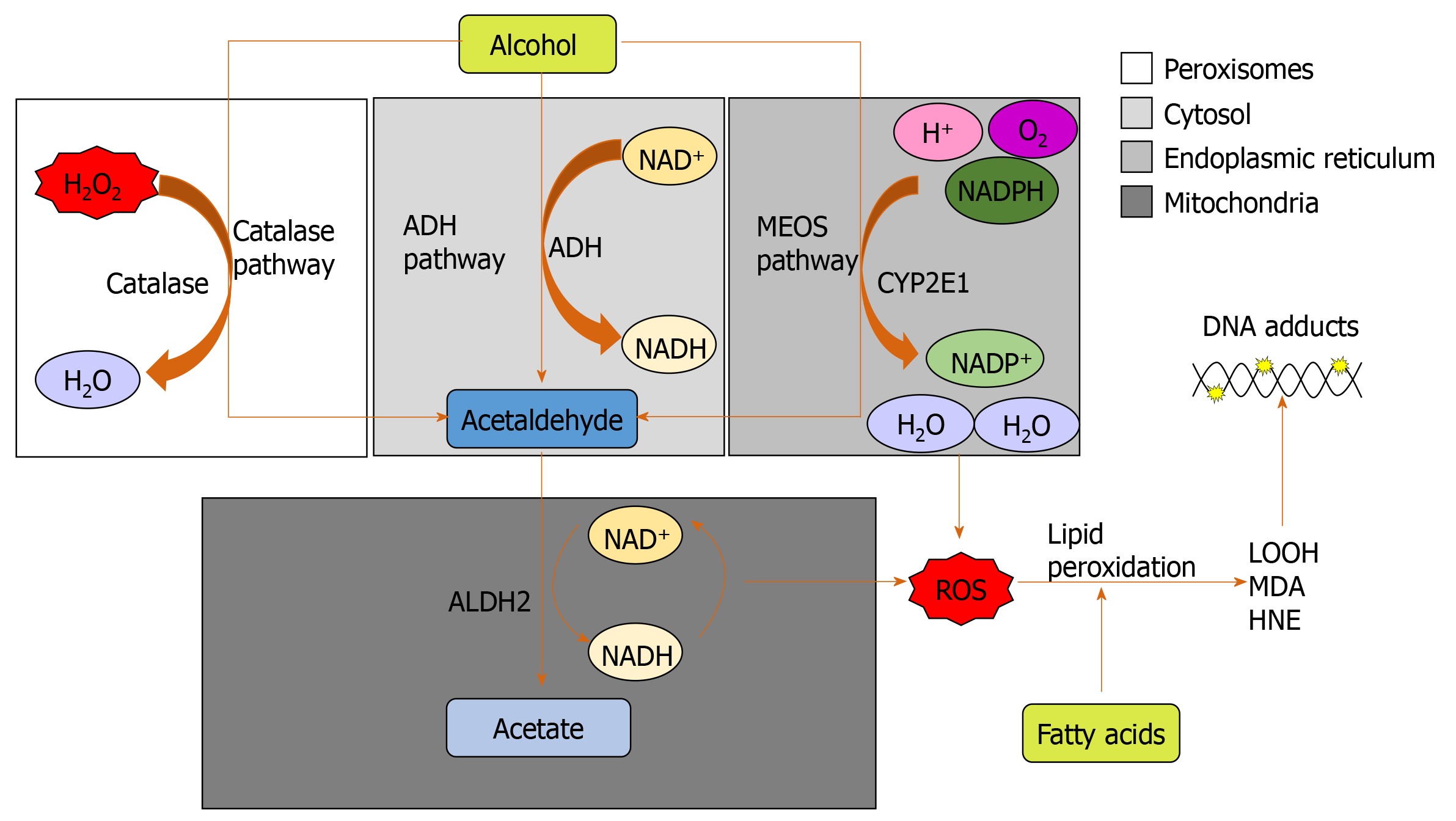
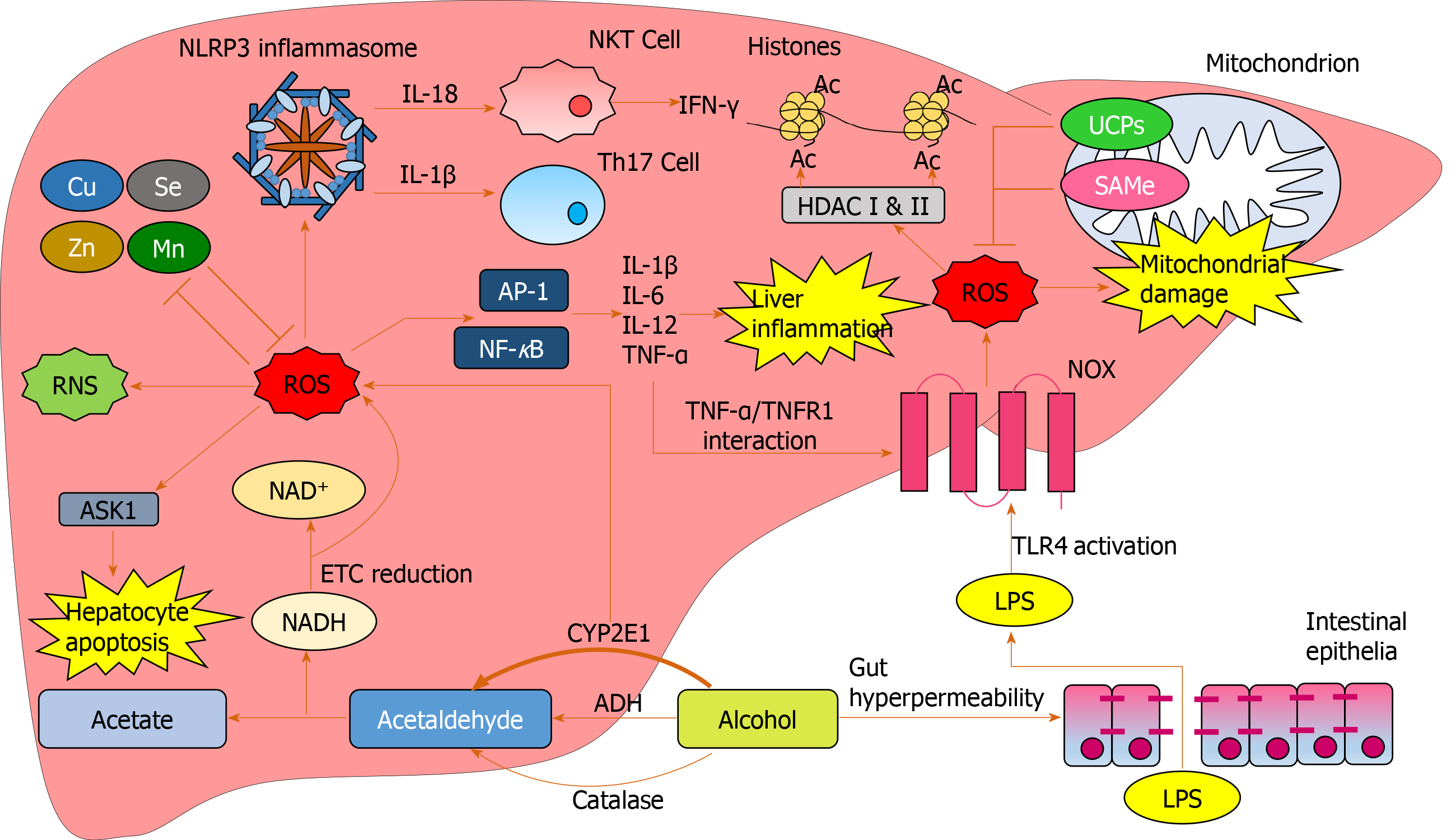
Alcohol (ethanol) is metabolized by three major pathways (Figure 1)[7]. The primary pathway is initiated by alcohol dehydrogenase (ADH), a NAD+ requiring enzyme expressed at high levels in hepatocytes, which oxidizes ethanol to acetaldehyde[7]. In a normal liver, acetaldehyde enters the mitochondria and is quickly metabolized to acetate by aldehyde dehydrogenase (ALDH). Acetate is then broken down to carbon dioxide and water for elimination[8]. In chronic alcohol users, the ADH/ALDH pathway becomes saturated and reactive aldehydes are produced from the metabolism process such as malondialdehyde-acetaldehyde (MAA), 4-hydroxy-2-nonenal (HNE) and lipid hydroperoxides which can bind to proteins to produce protein adducts[8].
These protein adducts are capable of provoking an immune response. In vitro experiments showed that the viability of antigen-presenting cells, lymphocytes, and hepatocytes was decreased on incubation with an MAA hen egg lysosome adduct[9]. Circulating antibodies against MAA protein adducts were increased in patients with ALD and AH and correlated with the severity of liver injury[9].
The second major pathway to metabolise ethanol is the microsomal ethanol oxidizing system (MEOS), which involves an NADPH-requiring enzyme, the cytochrome P450 enzyme CYP2E1[10], which is induced by chronic alcohol exposure[11,12]. The increase of CYP2E1 after alcohol intake is due to stabilization of CYP2E1 rather than to a de novo synthesis[11]. The MEOS pathway metabolises ethanol to acetaldehyde by converting NADPH+ and O2 to NADP and H2O resulting in the generation of reactive oxygen species (ROS). CYP2E1 plays a role in lipid peroxidation, protein oxidation, and protein nitration (Figure 1)[11]. It is also known to promote hepatic carcinogenesis by oxidizing DNA in alcohol-exposed rodents[13].
Ethanol metabolism through CYP2E1 not only produces acetaldehyde but also generates ROS including H2O2, hydroxyl (OH-) and carbon centered OH- (Figure 1)[14]. These ROS may be neutralized by a potent antioxidant defense system[14]. However, chronic alcohol consumption disrupts this system; depletion of mitochondrial glutathione (GSH) is observed in patients with alcohol dependence[15], which impairs hepatocyte tolerance to tumour necrosis factor alpha (TNF-α) resulting in an increased likelihood of cell death[16]. ROS increases and activates c-Jun N-terminal kinase (JNK) with consecutive expression of the activator protein 1 (AP-1) transcription factor leading to cellular hyper-regeneration, and lipid peroxidation. Lipid peroxidation products such as malondialdehyde and HNE are generated. HNE can bind to adenosine and cytosine forming highly carcinogenic exocyclic etheno DNA adducts[17]. These DNA adducts have been identified in the livers of patients with ALD and other types of liver disease associated with inflammation and oxidative stress like viral hepatitis[18].
The other two most prevalent DNA adducts are N2-ethyldeoxyguanosine (N2-Et-dG), and 1,N(2)-propano-2′-deoxyguanosine (PdG). N2-Et-dG is detectable in livers of alcohol-exposed mice and leukocytes of human alcohol misusers[19]. PdG, on the other hand, is distinguished by its genotoxic and mutagenic effects which impair DNA replication, thereby triggering cell death. These two major acetaldehyde-DNA adducts also promote carcinogenesis by initiating replication errors and mutations in oncogenes/onco-suppressor genes[19].
A third minor pathway for ethanol metabolism involves catalase, a peroxisomal enzyme (Figure 1)[20], which requires the presence of H2O2, a breakdown product of fatty acids. Catalase located in the peroxisomes of the hepatocyte plays only a minimal role in alcohol metabolism due to low hepatic production of H2O2. Under normal conditions, ADH metabolizes about 75%-80% of the ethanol entering the liver and MEOS the remainder. Hepatic ADH and hepatic catalase activities remain unchanged following chronic alcohol consumption, whereas hepatic MEOS activity strikingly increases and is responsible for the enhanced alcohol metabolism found after chronic alcohol consumption[11,12].
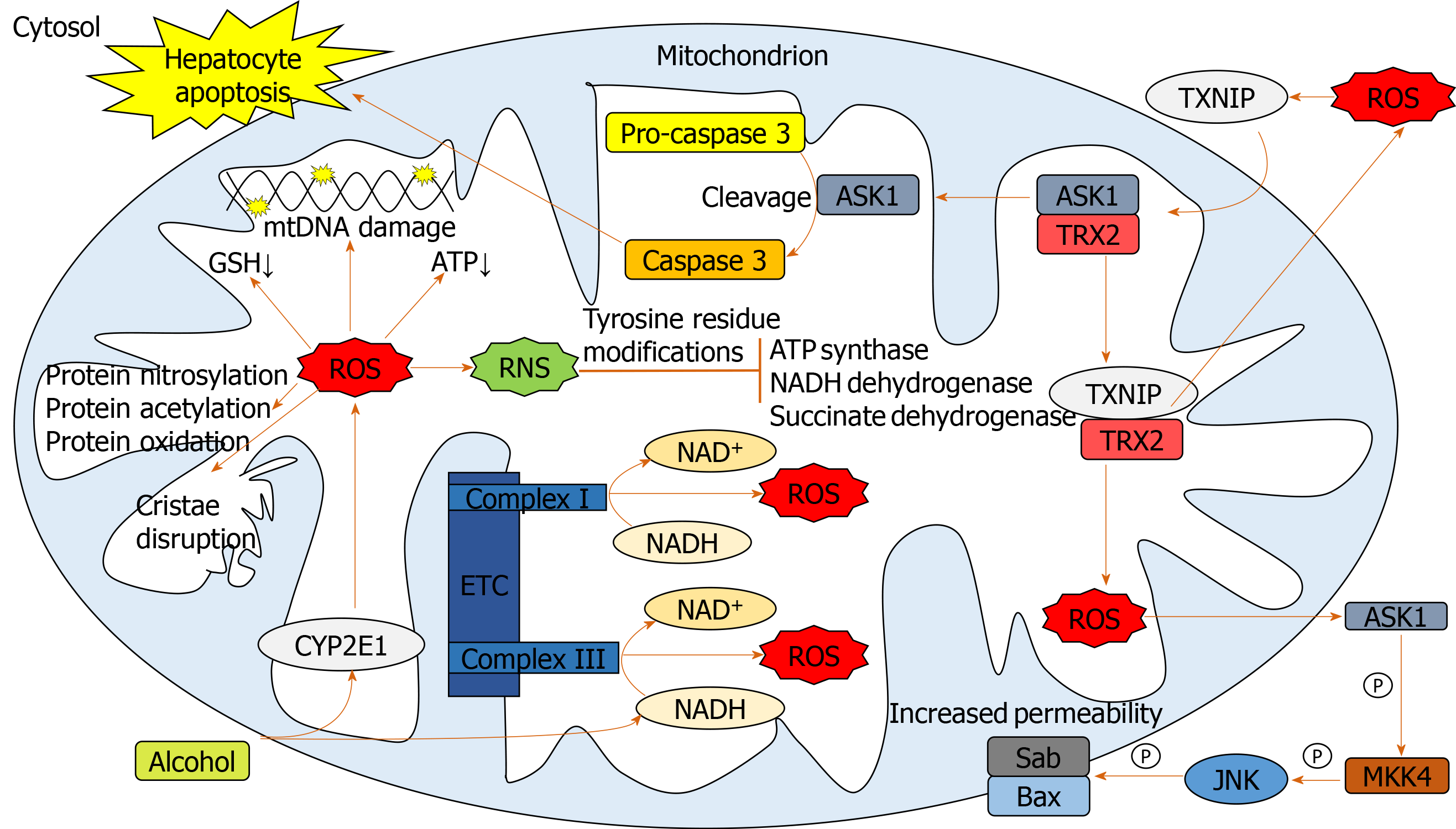
Chronic alcohol consumption results in structural and functional abnormalities in hepatic mitochondria, including enlarged morphology[21,22], mitochondrial DNA (mtDNA) damage[23], reductions in hepatic ATP levels[24] and mitochondrial protein synthesis[25] (Figure 2). This can result in hepatocellular apoptosis and associated necrosis[26]. Chronic alcohol metabolism and associated mitochondrial dysfunction has been implicated in increasing ROS production and accumulation in hepatic mitochondria.
In humans, in vivo measurement of ROS is complicated due to their rapid reactions with surrounding molecules[27]. Surrogate measures of mitochondrial-derived ROS include urinary isoprostane levels[28,29], NADH delivery to the respiratory chain[30], lipid peroxidation[31,32] and HNE levels and associated adducts[17,33]. The greatest indicator of ROS overproduction is the increase in hepatic CYP2E1 levels[32,34-37]. The respiratory chain has also been implicated in mitochondrial ROS overproduction in response to chronic alcohol consumption. Excessive levels of reducing equivalents (e.g., NADH), produced by alcohol and ADH entering the mitochondrial respiratory chain, lead to electron transport chain reduction, facilitating superoxide anion formation[38,25].
Cell death can be triggered through ROS-induced release of apoptosis signal-regulating kinase 1 (ASK1) (a member of the mitogen-activated protein kinase [MAPK] family), resulting in the cleavage of pro-caspase-3 to active caspase-3, which promotes cellular apoptosis[39-41]. Additionally, cytosolic ASK1 activates MAPK kinase 4 and JNK resulting in increased mitochondrial permeability, mediated by SAB protein, and thus hepatocyte cell death[40,41](Figure 2).
Reactive nitrogen species (RNS) also contribute to mitochondrial damage[22,38]. Alcohol-mediated overproduction of the superoxide anion can result in the generation of RNS, such as peroxynitrite, via interaction with nitric oxide, culminating in mitochondrial protein damage[25]. Numerous mitochondrial-localized enzymes involved in respiration and cellular energetic processes are inactivated in this way, including NADH dehydrogenase, succinate dehydrogenase, cytochrome c reductase and ATP synthase[42].
To limit oxidative damage following alcohol consumption, hepatic mitochondria have various adaptive mechanisms to prevent functional and structural impairments. Uncoupling proteins (UCPs), specifically UCPs 1-3, reduce ROS production by the uncoupling of mitochondrial oxidative phosphorylation[43], a process observed in patients with non-alcoholic fatty liver disease (NAFLD)[44]. Furthermore, there is mitochondrial upregulation of enzymatic antioxidants catalase, glutathione transferase and heme oxygenase-1 and a marked increase in GSH[45,46]. However, mitochondrial GSH depletion was observed in patients with alcohol dependence and ALD[15,16] suggesting that chronic alcohol exposure downregulates GSH expression.
Manganese-dependent superoxide dismutase (MnSOD) detoxifies mitochondrial superoxide[47], but its response to alcohol is poorly documented. Increased mitochondrial localization of MnSOD was associated with more severe forms of ALD[48], which may be mediated by increased hydroxyl radical generation[22]. Thus, overexpression of MnSOD may be hepatotoxic rather than hepatoprotective.
S-adenosylmethionine (SAMe) has been implicated in regulating mitochondrial function, following alcohol consumption in a variety of animal models[49]. SAMe binds and inactivates the catalytic activity of CYP2E1[50], limiting alcohol-dependent increases in mitochondrial production of superoxide[49]. SAMe also increases synthesis and availability of glutathione[51] and maintains mitochondrial respiration rate and mtDNA integrity[38]. Although greater SAMe levels have been observed in the serum of ALD patients compared to healthy subjects[52], a reduction in hepatic SAMe levels was observed in patients with AH[53], suggesting the acute inflammatory state leads to hepatic SAMe depletion. SAMe has been evaluated as a treatment for AH in a recent phase 2 randomized controlled clinical trial. SAMe with prednisolone improved 6-mo survival compared to prednisolone treatment alone[54]. Although these preliminary results are encouraging, a definitive study has yet to be undertaken.
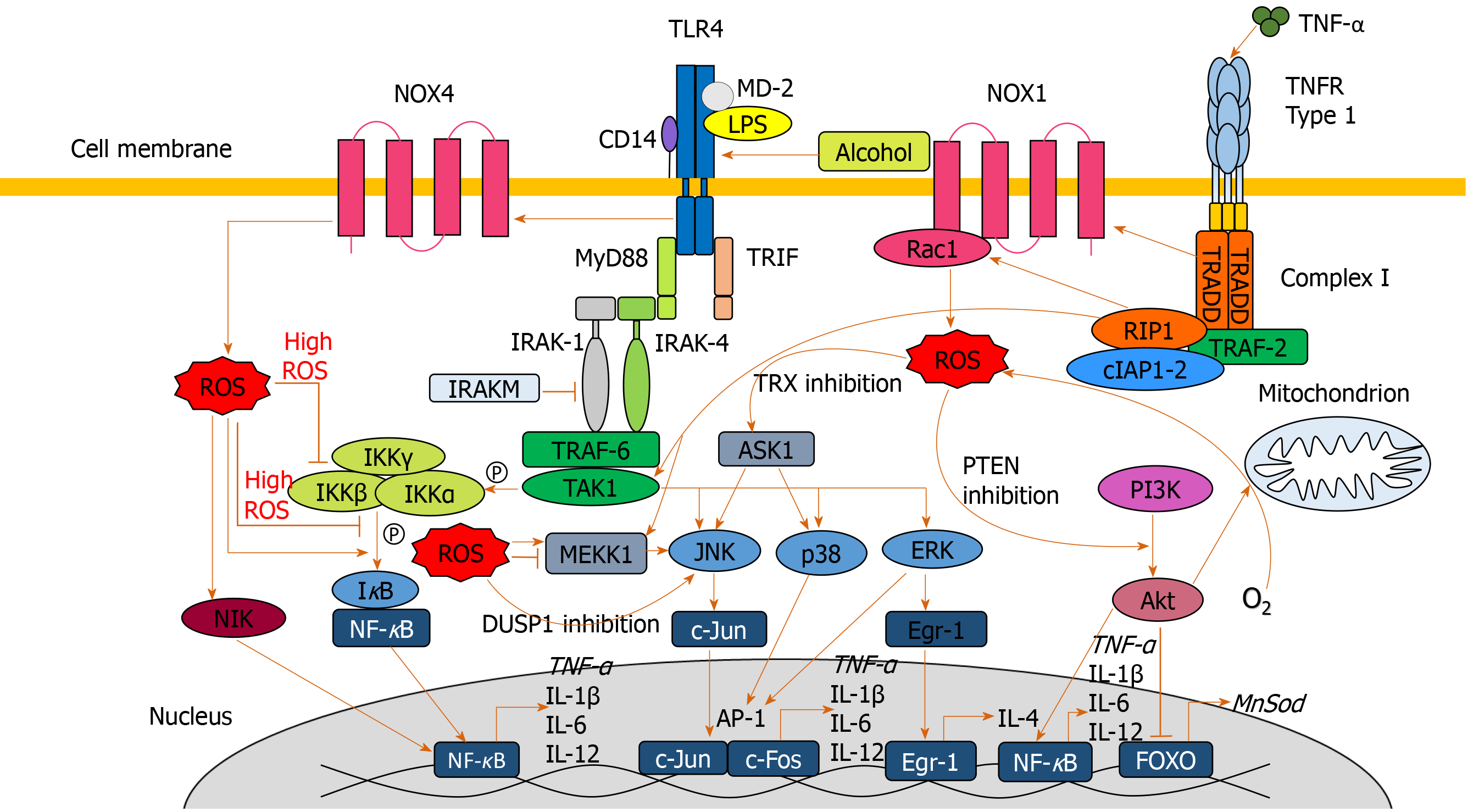
Lipopolysaccharide (LPS) plays a key role in the pathogenesis of ALD, with higher circulating LPS levels in alcohol dependent patients[55,56]. In AH, LPS predicts organ failure, mortality[57] and infection[58]. Alcohol exposure increases gut permeability, mediating translocation of LPS from the lumen of the intestine to the portal vein into the liver[55]. LPS binds to Toll-like receptor 4 (TLR4) expressed on a wide variety of immune and parenchymal cells including Kupffer cells, hepatocytes, endothelial cells and hepatic stellate cells, initiating one of the primary signaling cascades associated with liver damage[59,60]. LPS-mediated cell signaling results in transcription of pro-inflammatory genes through nuclear factor-κB (NF-κB) and interferon regulatory factor 3 DNA binding[59,61].
Upon LPS stimulation of the TLR4 complex, NADPH oxidase (NOX) 4 interacts with the COOH-terminal region of TLR4 resulting in ROS generation in neutrophils and monocytes[62,63], which directly activates NF-κB[62,64]. ROS-mediated activation and potential regulation of NF-κB activity occurs by several mechanisms: IκBα phosphorylation; S-glutathionylation of IKKβ; disruption of IκB ubiquitination and degradation; NF-κB inducing kinase (NIK) activation and phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) stimulation[65] (Figure 3). ROS both negatively and positively regulates NF-κB, with oxidative stress in the early phase being a positive regulator, compared to a negative regulator in the late phase[66]. Diphenyliodonium (DPI), an inhibitor of NOX, used as a pre-treatment in alcohol-fed rats, results in normalized ROS production, and inhibition of TNF-α production in Kupffer cells[59,67]. Treatment of alcohol-fed rats with the antioxidant dilinoleoyl-phosphatidylcholine, also inhibited TNF-α production in Kupffer cells and LPS-induced NF-κB activation[68].
Diphenyliodonium and dilinoleoyl-phosphatidylcholine reduce extracellular signal-regulated protein kinase (ERK)1/2 activation[67,68]. LPS-induced activation of ERK1/2 results in transcription of early growth response protein 1 (Egr-1), involved in binding to the TNF-α promoter and increasing TNF-α expression[59]. Egr-1 deficient mice are protected from chronic alcohol-induced liver injury in association with decreased TNF-α messenger RNA (mRNA) levels[69].
LPS activates other MAPKs including p38 and JNK[59], involved in TNF-α production[70]. p38 has been implicated in maintaining the stability of TNF-α mRNA[59,71]. In response to acute alcohol exposure, the JNK pathway has been associated with increased hepatic mitochondrial ROS production[72], increased JNK phosphorylation and AP-1 binding in monocytes[73]. ROS is likely to activate JNK through interaction with upstream MEKK1[65] and by inactivating JNK inhibitor dual specificity protein phosphatase 1[40,74]. ROS have also been associated with activation of cytosolic ASK1[40] (Figure 3). Clinical trials of ASK1 inhibitors as a treatment for inflammatory liver disease are ongoing with a suggestion of reduced fibrosis in patients with NAFLD[75] but no efficacy seen in AH[76].
ROS-mediated S-glutathionylation results in decreased expression of downstream antioxidants such as MnSOD, catalase and Sestrin3 via the PI3K/AKT pathway[77]. Akt has also been implicated in increasing oxygen consumption, resulting in elevated mitochondrial generation of H2O2, facilitating further oxidative damage[78,79].
The net result of these alcohol-induced cell signaling pathways is the increased production of pro-inflammatory cytokines through upregulation of transcription factors such as AP-1 and NFκB. TNF-α, a key pro-inflammatory cytokine, is highly elevated in patients with ALD and AH[80-82], with observed TNF-α gene expression increasing in ALD patients[83]. TNF-α induces apoptosis through interaction with TNF-α receptor 1 (TNFR1), initiating a cell-death cascade via activation of caspases[84]. In ALD, TNF-α-induces mitochondrial peroxidation[55], which is worsened following depletion of GSH[15,85].
TNF-α exacerbates oxidative damage and inflammation via a positive feedback loop. Through association with TNFR1, TNF-α stimulates the association of complex I[86], which culminates in MAPK activation (JNK, p38 and ERK). Complex I also directly contributes to ROS accumulation through generation of superoxide, capable of causing further oxidative damage and eventual TNF-α, perpetuating the cycle[40,87,88].
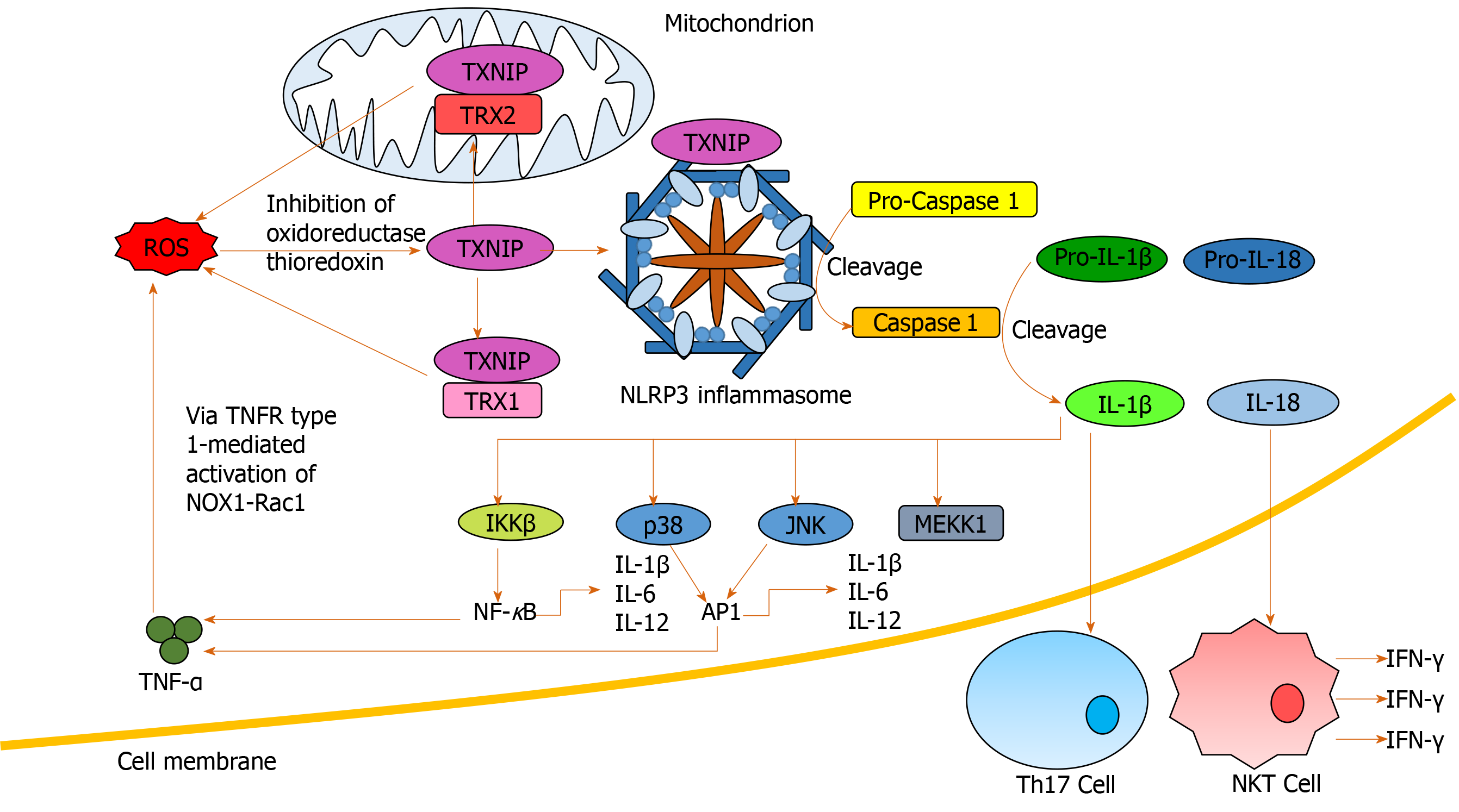
Soluble inflammatory mediators including interleukins have been implicated in ALD[60,89,90] and are associated with outcome in patients with AH[91]. Elevated serum IL-6 levels have recently been identified as a predictor of mortality in severe AH patients[64]. Hepatic upregulation of IL-6 and IL-1β in ALD, results in the differentiation of naïve CD4+ cells into IL-17-producing T-helper 17 cells (Th17) (Figure 4), resulting in elevated hepatic and serum levels of IL-17 observed in ALD patients[64,92]. IL-17 has a multitude of pro-inflammatory downstream effects, including inducing neutrophil recruitment to the liver; stimulating IL-8 and CXCL1 production by hepatic stellate cells[93] and CXCL4, 5 and 6 expression[92,93]. IL-6 and interferon (IFN)-γ are involved in JAK/STAT activation promoting hepatic regeneration[59,94]. Conversely, despite upregulation of IL-6 in ALD patients, downregulation of STAT activation has been observed in human monocytes with chronic alcohol exposure[95].
Inflammasomes propagate IL-1β and IL-18 signals, important in the regulation of hepatic inflammation[94]. ROS mediates IL-1β and IL-18 signaling via inflammasome NLRP3 activation[96,97] and inhibition of antioxidant molecules[41] (Figure 4). Increased production of IL-1β is critical in Th17 differentiation[64,92,98], while IL-18 activates natural killer T-cells (NKTs) to produce IFN-γ[99]. Anti-IL-18 antibodies reduce activation of NF-κB and AP-1, inflammation, liver damage and mortality in animal models[99,100]. IL-1β has also been identified as an activator of MAPKs, including p38, JNK, MEKK1 and IKKβ, involved in mediating upregulation of itself and other pro-inflammatory cytokines[101], creating another positive feedback loop.
Trace elements are a group of naturally occurring minerals that are nutritionally fundamental to basic cellular and immunological functions[102]. An essential role of the these molecules, including zinc, copper, selenium and manganese, is to act as cofactors of anti-oxidant enzymes, making their role imperative in the context of oxidative stress[103,104]. Manganese, copper and zinc are part of the SOD enzyme group that catalyze the breakdown of highly reactive superoxide radicals to H2O2 or O2-. Selenium is a component of the active site of glutathione peroxides (GPx), the main function of which is the neutralization of hydrogen peroxide[105]. These enzyme systems are crucial in counterbalancing the oxidative stress state and are impaired in chronic liver disease[106].
Reduced serum levels of trace elements have been confirmed in patients with liver disease, including ALD, and correlate with severity[107-110]. Decreased zinc is associated with liver cirrhosis in alcohol dependent individuals[111] and reduced serum levels of zinc, copper and iron have been observed when compared with healthy controls[112]. Zinc is a crucial trace element involved in multiple cellular and metabolic pathways[113] as well as acting as a cofactor for ALDH. Deficiency or abnormality in zinc function is implicated multiple pathologies, including liver disease (both acute and chronic)[114,115] and is associated with immune dysfunction evidenced by increased inflammation and aberrant immune cell activation[116]. Zinc deficiency in endothelial cells results in increased oxidative stress and decreased inflammatory regulation which is corrected or partially ameliorated by zinc supplementation[117,118]. In alcohol-fed mice, zinc deficiency worsens the balance between hepatic pro- and antioxidant enzymes[119] and is associated with accumulation of ROS in gut epithelial cells and disruption of tight junctions[120]. Given zinc’s influence on antioxidant responses, gut integrity and immune function, a trial of zinc supplementation to improve clinical outcomes in patients with ALD cirrhosis is ongoing (NCT02072746). Preliminary reports suggest zinc supplementation is associated with a reduction in liver inflammation and improvement in immune function[121].
Antioxidant therapy may also have a benefit in the treatment of AH. An antioxidant cocktail (including zinc and selenium) in combination with steroids for the treatment of severe AH correlated with a significant reduction in serum biomarkers, improved short-term prognosis and reduced length of stay in hospital[122]. However, a subsequent study of a complex regimen of N-acetylcysteine (NAC) followed by antioxidant therapy, alone or in conjunction with steroids, reduced renal injury but resulted in no survival benefit over 6 mo[121]. Another clinical trial of steroids combined with NAC in AH showed reduced infection rate but not mortality at 6 mo[123]. Antioxidants have also been shown to have a protective effect in patients with NAFLD by reducing serum levels of alanine transaminase (ALT) and spleen size, a finding that likely correlates with an improvement of fatty infiltration[122]. These findings suggest that targeting or counterbalancing oxidative stress in ALD patients may improve patient outcomes.
Lifestyle and environmental factors can modify gene expression without altering the DNA sequence, which gets transmitted to the next generation of cells after mitotic division, termed epigenetics[123]. Epigenetic regulation includes both DNA and histone protein modifications as well as action through non-coding micro RNAs[123]. DNA methylation is the most abundant epigenetic modification that directly affects the function of a gene in eukaryotes[124]. Acetylation and deacetylation are modifications in histone proteins carried out by two enzyme families, histone deacetylases (HDACs) and histone acetyl transferase (HAT)[124]. Histone modifying enzymes contribute to the activation or inactivation of transcription by catalyzing the unfolding or further compaction, respectively, of chromatin structure[124].
Excessive ROS is involved in epigenetic gene activation or silencing by changing DNA methylation levels[125]. ROS production induces alterations in DNA methylation patterns and global histone acetylation, which then lead to aberrant gene expression, and may contribute to the process of carcinogenesis[124]. The reduction of global histone acetylation in short term oxidative stress might be due to an immediate increase of class I/II HDAC activity by an unknown mechanism[126,127]. Class III HDAC (Sirtuin NAD+-dependent family of protein deacetylases) has been hypothesized to be upregulated under oxidative stress because NAD+ levels increase in the mitochondria under oxidative stress conditions but direct evidence is lacking[126].
Alcohol consumption increases gene-selective acetylation of histone H3 at lysine 9 (H3K9), levels of enzymes mediating histone acetylation, and results in a generalized increase in DNA methylation[126,127]. These epigenetic-mediated effects of alcohol consumption regulate the inflammatory response, through key pro-inflammatory cytokines, such as TNF-α, which is silenced by H3K9 methylation and activated by H3K9 acetylation[128]. In a macrophage cell line, alcohol treatment resulted in global increased histone H3 and H4 acetylation and specifically increased acetylation of pro-inflammatory gene histones[129].
Oxidative stress itself is an important regulator of epigenetic processes by inhibition of HDAC expression[130]. This takes place via activation of PI3Kδ, a signalling molecule controlling many inflammatory signalling pathways[131]. Drugs that inhibit PI3Kδ (e.g., theophylline, nortriptyline and specific inhibitors) reduce oxidative stress in in vitro and in vivo models of lung disease[132]. In patients with AH, there is in vitro evidence that theophylline can enhance response to corticosteroid treatment, which may be mediated by its epigenetic effects[133]. Targeting epigenetic regulation has recently been shown to have a beneficial effect in patients with AH; a novel sulphated oxysterol, DUR-928, was well tolerated and improved liver biochemistry in a small phase 2 clinical trial in AH[134].
Activation of the transcription factor Nrf2 is central to cellular defence against ROS[135]. Its negative regulator, kelch-like ECM-associated protein 1 (Keap1), promotes proteasomal degradation of Nrf2. ROS decouples Nrf2 from Keap1, allowing it to translocate to the nucleus to bind to antioxidant response elements (AREs), initiating a range of antioxidant processes[135,136]. Both Nrf2 and Keap1 expression are influenced by epigenetics with evidence of DNA hypermethylation in the Nrf2 promoter[135,136] and Keap1 promoter[137]. Histone acetylation and deacetylation also modify ARE-dependent gene expression with Class 1 HDACs reducing Nrf2[138]. Conversely, HDAC inhibitors restore Nrf2 expression and antioxidant responses. Targeting epigenetic regulation of Nrf2/Keap1 to ameliorate oxidative stress induced inhibition of antioxidant responses is an appealing strategy[138]. However, much of this work has been performed in cancer cell lines and needs further investigation in the context of ALD.
An improved understanding of the detailed mechanisms by which oxidative stress influences liver damage in patients with ALD may yield new targets for therapy. Current data from pre-clinical and clinical studies suggest potential new avenues for therapy of ALD.
Chronic alcohol consumption results in significant mitochondrial ROS generation leading to morphological and functional changes. Preventing ROS generation may ameliorate this process. Pre-clinical and early phase clinical studies have shown promise of this approach with SAMe. A systematic review and meta-analysis of 11 randomized controlled trials of SAMe treatment for chronic liver disease concluded that it improved liver biochemistry (bilirubin and AST) and had a good safety profile but did not affect mortality[139]. Long term SAMe treatment in patients with ALD does not appear to be clinically effective with no reduction in adverse events or mortality in the two included studies performed in patients with ALD[140,141]. However, short term treatment of the acute mitochondrial stress seen in AH may be a better strategy for the use of SAMe. A phase 2 clinical trial of SAMe with prednisolone for the treatment of severe AH demonstrated improved response rate measured by Lille score and a reduction in hepatorenal syndrome[54]. However, there was no statistically significant difference in 28-d mortality. It may yet prove to be an effective adjunct to anti-inflammatory therapy for AH.
UCPs are strongly associated with mitochondrial stress in ALD. Overexpression of UCP2 reduces apoptosis and oxidative stress in vitro[142]. Hepatocellular downregulated mitochondrial carrier protein (HDMCP) expression induced uncoupling and reduced steatosis in an animal model of NAFLD[143]. However, such an approach may promote hepatocyte necrosis and increase the risk of hepatocellular carcinoma[144]. Further studies in this area are required to determine whether targeting UCPs would be a beneficial therapeutic strategy.
NAC, an antioxidant therapy that provides cysteine for glutathione synthesis, has been tested in patients with AH. Although initial trials did not demonstrate a survival benefit[145,146]. a more recent study of NAC in combination with prednisolone, showed a reduction in infective events and 1-month mortality[147]. Therefore, NAC has been suggested for the treatment of AH in clinical practice guidelines, with the caveat that a definitive randomized controlled trial is still required[148].
Deficiency of key trace elements is associated with oxidative stress, which is ameliorated by supplementation. Antioxidant therapy including zinc and other trace elements has shown clinical benefit in patients with AH[122]. However, interpretation is hampered by use of a variety of antioxidants at differing concentrations and durations[145,146]. A trial of long-term zinc supplementation in ALD patients has demonstrated improvements in short-term immune function[149] with long-term clinical outcomes due to be reported shortly. Improved understanding of the role of trace elements in ALD and the optimal formulation and duration of treatment is required.
Oxidative stress reduces HDAC expression via PI3Kδ activation resulting in increased expression of pro-inflammatory genes. Studies targeting HDACs have yet to be performed in patients with ALD. Although in vitro studies suggest an antioxidant effect of HDAC inhibition with upregulation of Nrf2 expression[138], HDAC inhibitors approved for use in the treatment of cancer induce cell cycle arrest, apoptosis and oxidative stress in cancer cells which overexpress HDAC[150]. The effect of HDAC inhibitors in the context of ALD requires careful in vitro confirmation before clinical translation. However, targeting PI3Kδ is a more appealing strategy with evidence from the respiratory field that specific inhibitors reduce oxidative stress in vitro and in vivo[132].
Alcohol is a major global healthcare and economic burden and is a growing cause of chronic liver disease. However, there are currently no effective therapies to treat ALD. Oxidative stress is involved in multiple aspects of ALD pathogenesis (Figure 5). Chronic alcohol consumption results in the saturation of the ADH pathway and increased CYP2E1-mediated alcohol metabolism. This leads to the generation of reactive species including MAA, HNE, lipid hydroperoxides, RNS and ROS, which cause hepatic damage via lipid and protein peroxidation, adduct formation and cellular hyper-regulation. Similar damage occurs in hepatic mitochondria with ROS inducing structural and functional damage. ROS cause oxidative damage through multiple mechanisms: Promoting cell death via protein mediators, increasing and sustaining the upregulation of pro-inflammatory mediators, as well as inducing multiple epigenetic modifications.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Conti CB, Fujii J S-Editor: Yan JP L-Editor: A E-Editor: Wang LL
| 1. | Pimpin L, Cortez-Pinto H, Negro F, Corbould E, Lazarus JV, Webber L, Sheron N; EASL HEPAHEALTH Steering Committee. Burden of liver disease in Europe: Epidemiology and analysis of risk factors to identify prevention policies. J Hepatol. 2018;69:718-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 475] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 2. | Bhattacharya A. Which cost of alcohol? What should we compare it against? Addiction. 2017;112:559-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Anderson P, Baumberg B. Alcohol in Europe-Public Health Perspective: Report summary. Drugs Educ Prev Policy. 2006;13:483-488. [RCA] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Ohashi K, Pimienta M, Seki E. Alcoholic liver disease: A current molecular and clinical perspective. Liver Res. 2018;2:161-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 5. | Philips CA, Augustine P, Yerol PK, Rajesh S, Mahadevan P. Severe alcoholic hepatitis: current perspectives. Hepat Med. 2019;11:97-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Bennett K, Enki DG, Thursz M, Cramp ME, Dhanda AD. Systematic review with meta-analysis: high mortality in patients with non-severe alcoholic hepatitis. Aliment Pharmacol Ther. 2019;50:249-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Li S, Tan HY, Wang N, Zhang ZJ, Lao L, Wong CW, Feng Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int J Mol Sci. 2015;16:26087-26124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1267] [Cited by in RCA: 1088] [Article Influence: 108.8] [Reference Citation Analysis (1)] |
| 8. | Cederbaum AI, Lu Y, Wu D. Role of oxidative stress in alcohol-induced liver injury. Arch Toxicol. 2009;83:519-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 446] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 9. | Willis MS, Klassen LW, Tuma DJ, Sorrell MF, Thiele GM. Adduction of soluble proteins with malondialdehyde-acetaldehyde (MAA) induces antibody production and enhances T-cell proliferation. Alcohol Clin Exp Res. 2002;26:94-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 223] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 10. | Lieber CS, Rubin E, DeCarli LM. Hepatic microsomal ethanol oxidizing system (MEOS): differentiation from alcohol dehydrogenase and NADPH oxidase. Biochem Biophys Res Commun. 1970;40:858-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Pérez MJ, Cederbaum AI. Metallothionein 2A induction by zinc protects HEPG2 cells against CYP2E1-dependent toxicity. Free Radic Biol Med. 2003;34:443-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Weltman MD, Farrell GC, Hall P, Ingelman-Sundberg M, Liddle C. Hepatic cytochrome P450 2E1 is increased in patients with nonalcoholic steatohepatitis. Hepatology. 1998;27:128-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 432] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 13. | Lieber CS, Seitz HK, Garro AJ, Worner TM. Alcohol-related diseases and carcinogenesis. Cancer Res. 1979;39:2863-2886. [PubMed] |
| 14. | Seitz HK, Stickel F. Risk factors and mechanisms of hepatocarcinogenesis with special emphasis on alcohol and oxidative stress. Biol Chem. 2006;387:349-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 204] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 15. | Albano E. Oxidative mechanisms in the pathogenesis of alcoholic liver disease. Mol Aspects Med. 2008;29:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 219] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 16. | Fernandez-Checa JC, Kaplowitz N. Hepatic mitochondrial glutathione: transport and role in disease and toxicity. Toxicol Appl Pharmacol. 2005;204:263-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 198] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 17. | Setshedi M, Wands JR, Monte SM. Acetaldehyde adducts in alcoholic liver disease. Oxid Med Cell Longev. 2010;3:178-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 237] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 18. | Nair J, Srivatanakul P, Haas C, Jedpiyawongse A, Khuhaprema T, Seitz HK, Bartsch H. High urinary excretion of lipid peroxidation-derived DNA damage in patients with cancer-prone liver diseases. Mutat Res. 2010;683:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 228] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 19. | Abdelmegeed MA, Choi Y, Ha SK, Song BJ. Cytochrome P450-2E1 promotes aging-related hepatic steatosis, apoptosis and fibrosis through increased nitroxidative stress. Free Radic Biol Med. 2016;91:188-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Handler JA, Thurman RG. Hepatic ethanol metabolism is mediated predominantly by catalase-H2O2 in the fasted state. FEBS Lett. 1988;238:139-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Nassir F, Ibdah JA. Role of mitochondria in alcoholic liver disease. World J Gastroenterol. 2014;20:2136-2142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 89] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 22. | Das SK, Vasudevan DM. Alcohol-induced oxidative stress. Life Sci. 2007;81:177-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 607] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 23. | Venkatraman A, Landar A, Davis AJ, Chamlee L, Sanderson T, Kim H, Page G, Pompilius M, Ballinger S, Darley-Usmar V, Bailey SM. Modification of the mitochondrial proteome in response to the stress of ethanol-dependent hepatotoxicity. J Biol Chem. 2004;279:22092-22101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 139] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 24. | Grattagliano I, Russmann S, Diogo C, Bonfrate L, Oliveira PJ, Wang DQ, Portincasa P. Mitochondria in chronic liver disease. Curr Drug Targets. 2011;12:879-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 25. | García-Ruiz C, Kaplowitz N, Fernandez-Checa JC. Role of Mitochondria in Alcoholic Liver Disease. Curr Pathobiol Rep. 2013;1:159-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Hoek JB, Cahill A, Pastorino JG. Alcohol and mitochondria: a dysfunctional relationship. Gastroenterology. 2002;122:2049-2063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 391] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 27. | Beier JI, McClain CJ. Mechanisms and cell signaling in alcoholic liver disease. Biol Chem. 2010;391:1249-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 28. | Meagher EA, Barry OP, Burke A, Lucey MR, Lawson JA, Rokach J, FitzGerald GA. Alcohol-induced generation of lipid peroxidation products in humans. J Clin Invest. 1999;104:805-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 181] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 29. | Morrow JD. The isoprostanes: their quantification as an index of oxidant stress status in vivo. Drug Metab Rev. 2000;32:377-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 149] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Mantena SK, King AL, Andringa KK, Landar A, Darley-Usmar V, Bailey SM. Novel interactions of mitochondria and reactive oxygen/nitrogen species in alcohol mediated liver disease. World J Gastroenterol. 2007;13:4967-4973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Ekström G, Ingelman-Sundberg M. Rat liver microsomal NADPH-supported oxidase activity and lipid peroxidation dependent on ethanol-inducible cytochrome P-450 (P-450IIE1). Biochem Pharmacol. 1989;38:1313-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 375] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 32. | Seth D, Haber PS, Syn WK, Diehl AM, Day CP. Pathogenesis of alcohol-induced liver disease: classical concepts and recent advances. J Gastroenterol Hepatol. 2011;26:1089-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (1)] |
| 33. | Roede JR, Jones DP. Reactive species and mitochondrial dysfunction: mechanistic significance of 4-hydroxynonenal. Environ Mol Mutagen. 2010;51:380-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 34. | Niemelä O, Parkkila S, Juvonen RO, Viitala K, Gelboin HV, Pasanen M. Cytochromes P450 2A6, 2E1, and 3A and production of protein-aldehyde adducts in the liver of patients with alcoholic and non-alcoholic liver diseases. J Hepatol. 2000;33:893-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 125] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 35. | Takahashi T, Lasker JM, Rosman AS, Lieber CS. Induction of cytochrome P-4502E1 in the human liver by ethanol is caused by a corresponding increase in encoding messenger RNA. Hepatology. 1993;17:236-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | Yun JW, Son MJ, Abdelmegeed MA, Banerjee A, Morgan TR, Yoo SH, Song BJ. Binge alcohol promotes hypoxic liver injury through a CYP2E1-HIF-1α-dependent apoptosis pathway in mice and humans. Free Radic Biol Med. 2014;77:183-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 37. | Adam-Vizi V. Production of reactive oxygen species in brain mitochondria: contribution by electron transport chain and non-electron transport chain sources. Antioxid Redox Signal. 2005;7:1140-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 335] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 38. | Manzo-Avalos S, Saavedra-Molina A. Cellular and mitochondrial effects of alcohol consumption. Int J Environ Res Public Health. 2010;7:4281-4304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 183] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 39. | Harijith A, Ebenezer DL, Natarajan V. Reactive oxygen species at the crossroads of inflammasome and inflammation. Front Physiol. 2014;5:352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 341] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 40. | Seki E, Brenner DA, Karin M. A liver full of JNK: signaling in regulation of cell function and disease pathogenesis, and clinical approaches. Gastroenterology. 2012;143:307-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 408] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 41. | Gunawan BK, Liu ZX, Han D, Hanawa N, Gaarde WA, Kaplowitz N. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131:165-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 367] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 42. | Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4983] [Cited by in RCA: 4453] [Article Influence: 247.4] [Reference Citation Analysis (0)] |
| 43. | Mailloux RJ, Harper ME. Uncoupling proteins and the control of mitochondrial reactive oxygen species production. Free Radic Biol Med. 2011;51:1106-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 410] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 44. | Chavin KD, Yang S, Lin HZ, Chatham J, Chacko VP, Hoek JB, Walajtys-Rode E, Rashid A, Chen CH, Huang CC, Wu TC, Lane MD, Diehl AM. Obesity induces expression of uncoupling protein-2 in hepatocytes and promotes liver ATP depletion. J Biol Chem. 1999;274:5692-5700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 326] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 45. | Cederbaum AI. Cytochrome P450 2E1-dependent oxidant stress and upregulation of anti-oxidant defense in liver cells. J Gastroenterol Hepatol. 2006;21 Suppl 3:S22-S25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 46. | Marí M, Cederbaum AI. CYP2E1 overexpression in HepG2 cells induces glutathione synthesis by transcriptional activation of gamma-glutamylcysteine synthetase. J Biol Chem. 2000;275:15563-15571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 99] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 47. | Candas D, Li JJ. MnSOD in oxidative stress response-potential regulation via mitochondrial protein influx. Antioxid Redox Signal. 2014;20:1599-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 468] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 48. | Degoul F, Sutton A, Mansouri A, Cepanec C, Degott C, Fromenty B, Beaugrand M, Valla D, Pessayre D. Homozygosity for alanine in the mitochondrial targeting sequence of superoxide dismutase and risk for severe alcoholic liver disease. Gastroenterology. 2001;120:1468-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 78] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 49. | Andringa KK, King AL, Eccleston HB, Mantena SK, Landar A, Jhala NC, Dickinson DA, Squadrito GL, Bailey SM. Analysis of the liver mitochondrial proteome in response to ethanol and S-adenosylmethionine treatments: novel molecular targets of disease and hepatoprotection. Am J Physiol Gastrointest Liver Physiol. 2010;298:G732-G745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 50. | Cederbaum AI. Hepatoprotective effects of S-adenosyl-L-methionine against alcohol- and cytochrome P450 2E1-induced liver injury. World J Gastroenterol. 2010;16:1366-1376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 68] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 51. | Fernández-Checa JC, Colell A, García-Ruiz C. S-Adenosyl-L-methionine and mitochondrial reduced glutathione depletion in alcoholic liver disease. Alcohol. 2002;27:179-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 52. | Mudd SH, Wagner C, Luka Z, Stabler SP, Allen RH, Schroer R, Wood T, Wang J, Wong LJ. Two patients with hepatic mtDNA depletion syndromes and marked elevations of S-adenosylmethionine and methionine. Mol Genet Metab. 2012;105:228-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 53. | Lee TD, Sadda MR, Mendler MH, Bottiglieri T, Kanel G, Mato JM, Lu SC. Abnormal hepatic methionine and glutathione metabolism in patients with alcoholic hepatitis. Alcohol Clin Exp Res. 2004;28:173-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 116] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 54. | Tkachenko P, Maevskaya M, Pavlov A, Komkova I, Pavlov C, Ivashkin V. Prednisolone plus S-adenosil-L-methionine in severe alcoholic hepatitis. Hepatol Int. 2016;10:983-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 55. | Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360:2758-2769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 693] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 56. | Fujimoto M, Uemura M, Nakatani Y, Tsujita S, Hoppo K, Tamagawa T, Kitano H, Kikukawa M, Ann T, Ishii Y, Kojima H, Sakurai S, Tanaka R, Namisaki T, Noguchi R, Higashino T, Kikuchi E, Nishimura K, Takaya A, Fukui H. Plasma endotoxin and serum cytokine levels in patients with alcoholic hepatitis: relation to severity of liver disturbance. Alcohol Clin Exp Res. 2000;24:48S-54S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 112] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 57. | Michelena J, Altamirano J, Abraldes JG, Affò S, Morales-Ibanez O, Sancho-Bru P, Dominguez M, García-Pagán JC, Fernández J, Arroyo V, Ginès P, Louvet A, Mathurin P, Mehal WZ, Caballería J, Bataller R. Systemic inflammatory response and serum lipopolysaccharide levels predict multiple organ failure and death in alcoholic hepatitis. Hepatology. 2015;62:762-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 219] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 58. | Vergis N, Atkinson SR, Knapp S, Maurice J, Allison M, Austin A, Forrest EH, Masson S, McCune A, Patch D, Richardson P, Gleeson D, Ryder SD, Wright M, Thursz MR. In Patients With Severe Alcoholic Hepatitis, Prednisolone Increases Susceptibility to Infection and Infection-Related Mortality, and Is Associated With High Circulating Levels of Bacterial DNA. Gastroenterology. 2017;152:1068-1077.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 151] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 59. | Mandrekar P, Szabo G. Signalling pathways in alcohol-induced liver inflammation. J Hepatol. 2009;50:1258-1266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 396] [Cited by in RCA: 392] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 60. | Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology. 2008;48:322-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 551] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 61. | Li P, He K, Li J, Liu Z, Gong J. The role of Kupffer cells in hepatic diseases. Mol Immunol. 2017;85:222-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 164] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 62. | Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol. 2004;173:3589-3593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 527] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 63. | Petrasek J, Mandrekar P, Szabo G. Toll-like receptors in the pathogenesis of alcoholic liver disease. Gastroenterol Res Pract. 2010;2010:710381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 64. | Shasthry SM, Sarin SK. New treatment options for alcoholic hepatitis. World J Gastroenterol. 2016;22:3892-3906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 65. | Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu Y, Dong W. ROS and ROS-Mediated Cellular Signaling. Oxid Med Cell Longev. 2016;2016:4350965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 740] [Cited by in RCA: 1252] [Article Influence: 139.1] [Reference Citation Analysis (0)] |
| 66. | Mu W, Liu LZ. Reactive Oxygen Species Signaling in Cancer Development. React Oxyg Species. 2017;4:251-265. [DOI] [Full Text] |
| 67. | Thakur V, Pritchard MT, McMullen MR, Wang Q, Nagy LE. Chronic ethanol feeding increases activation of NADPH oxidase by lipopolysaccharide in rat Kupffer cells: role of increased reactive oxygen in LPS-stimulated ERK1/2 activation and TNF-alpha production. J Leukoc Biol. 2006;79:1348-1356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 170] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 68. | Cao Q, Mak KM, Lieber CS. Dilinoleoylphosphatidylcholine decreases acetaldehyde-induced TNF-alpha generation in Kupffer cells of ethanol-fed rats. Biochem Biophys Res Commun. 2002;299:459-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 69. | McMullen MR, Pritchard MT, Wang Q, Millward CA, Croniger CM, Nagy LE. Early growth response-1 transcription factor is essential for ethanol-induced fatty liver injury in mice. Gastroenterology. 2005;128:2066-2076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 99] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 70. | Yao J, Mackman N, Edgington TS, Fan ST. Lipopolysaccharide induction of the tumor necrosis factor-alpha promoter in human monocytic cells. Regulation by Egr-1, c-Jun, and NF-kappaB transcription factors. J Biol Chem. 1997;272:17795-17801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 379] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 71. | Kishore R, McMullen MR, Nagy LE. Stabilization of tumor necrosis factor alpha mRNA by chronic ethanol: role of A + U-rich elements and p38 mitogen-activated protein kinase signaling pathway. J Biol Chem. 2001;276:41930-41937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 72. | Win S, Than TA, Zhang J, Oo C, Min RWM, Kaplowitz N. New insights into the role and mechanism of c-Jun-N-terminal kinase signaling in the pathobiology of liver diseases. Hepatology. 2018;67:2013-2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 134] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 73. | Oak S, Mandrekar P, Catalano D, Kodys K, Szabo G. TLR2- and TLR4-mediated signals determine attenuation or augmentation of inflammation by acute alcohol in monocytes. J Immunol. 2006;176:7628-7635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 74. | Chang Q, Zhang Y, Beezhold KJ, Bhatia D, Zhao H, Chen J, Castranova V, Shi X, Chen F. Sustained JNK1 activation is associated with altered histone H3 methylations in human liver cancer. J Hepatol. 2009;50:323-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 75. | Loomba R, Lawitz E, Mantry PS, Jayakumar S, Caldwell SH, Arnold H, Diehl AM, Djedjos CS, Han L, Myers RP, Subramanian GM, McHutchison JG, Goodman ZD, Afdhal NH, Charlton MR; GS-US-384-1497 Investigators. The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: A randomized, phase 2 trial. Hepatology. 2018;67:549-559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 350] [Cited by in RCA: 440] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 76. | Mathurin P, Dufour J-F, Bzowej NH, Shiffman ML, Arterburn S, Nguyen T, Billin A, Chung C, Subramanian M, Myers RP, Szabo G, Thevenot T, Cramp ME, Ryder SD, Tilg H, Moreno C, Thursz MR, Agarwal K. Selonsertib in combination with prednisolone for the treatment of severe alcoholic hepatitis: a phase 2 randomized controlled trial. Hepatology. 2018;68; 8A-9A. [DOI] [Full Text] |
| 77. | Nogueira V, Hay N. Molecular pathways: reactive oxygen species homeostasis in cancer cells and implications for cancer therapy. Clin Cancer Res. 2013;19:4309-4314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 389] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 78. | Zhang Y, Du Y, Le W, Wang K, Kieffer N, Zhang J. Redox control of the survival of healthy and diseased cells. Antioxid Redox Signal. 2011;15:2867-2908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 79. | Zhao Y, Hu X, Liu Y, Dong S, Wen Z, He W, Zhang S, Huang Q, Shi M. ROS signaling under metabolic stress: cross-talk between AMPK and AKT pathway. Mol Cancer. 2017;16:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 294] [Cited by in RCA: 518] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 80. | Latvala J, Hietala J, Koivisto H, Järvi K, Anttila P, Niemelä O. Immune Responses to Ethanol Metabolites and Cytokine Profiles Differentiate Alcoholics with or without Liver Disease. Am J Gastroenterol. 2005;100:1303-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 81. | Bird GL, Sheron N, Goka AK, Alexander GJ, Williams RS. Increased plasma tumor necrosis factor in severe alcoholic hepatitis. Ann Intern Med. 1990;112:917-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 326] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 82. | Szabo G, Bala S. Alcoholic liver disease and the gut-liver axis. World J Gastroenterol. 2010;16:1321-1329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 273] [Cited by in RCA: 289] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 83. | Colmenero J, Bataller R, Sancho-Bru P, Bellot P, Miquel R, Moreno M, Jares P, Bosch J, Arroyo V, Caballería J, Ginès P. Hepatic expression of candidate genes in patients with alcoholic hepatitis: correlation with disease severity. Gastroenterology. 2007;132:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 84. | McVicker BL, Tuma DJ, Casey CA. Effect of ethanol on pro-apoptotic mechanisms in polarized hepatic cells. World J Gastroenterol. 2007;13:4960-4966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 85. | Neuman MG, Shear NH, Bellentani S, Tiribelli C. Role of cytokines in ethanol-induced cytotoxicity in vitro in Hep G2 cells. Gastroenterology. 1998;115:157-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 97] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 86. | Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1941] [Cited by in RCA: 2105] [Article Influence: 95.7] [Reference Citation Analysis (0)] |
| 87. | Kim YS, Morgan MJ, Choksi S, Liu ZG. TNF-induced activation of the Nox1 NADPH oxidase and its role in the induction of necrotic cell death. Mol Cell. 2007;26:675-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 425] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 88. | Vanden Berghe T, Declercq W, Vandenabeele P. NADPH oxidases: new players in TNF-induced necrotic cell death. Mol Cell. 2007;26:769-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 89. | Minayoshi Y, Maeda H, Yanagisawa H, Hamasaki K, Mizuta Y, Nishida K, Kinoshita R, Enoki Y, Imafuku T, Chuang VTG, Koga T, Fujiwara Y, Takeya M, Sonoda K, Wakayama T, Taguchi K, Ishima Y, Ishida T, Iwakiri Y, Tanaka M, Sasaki Y, Watanabe H, Otagiri M, Maruyama T. Development of Kupffer cell targeting type-I interferon for the treatment of hepatitis via inducing anti-inflammatory and immunomodulatory actions. Drug Deliv. 2018;25:1067-1077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 90. | Sun B, Karin M. NF-kappaB signaling, liver disease and hepatoprotective agents. Oncogene. 2008;27:6228-6244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 244] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 91. | Dhanda AD, Yates E, Schewitz-Bowers LP, Lait PJ, Lee RWJ, Cramp ME. Ex Vivo T Cell Cytokine Expression Predicts Survival in Patients with Severe Alcoholic Hepatitis. Gut Liver. 2020;14:265-268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 92. | Lemmers A, Moreno C, Gustot T, Maréchal R, Degré D, Demetter P, de Nadai P, Geerts A, Quertinmont E, Vercruysse V, Le Moine O, Devière J. The interleukin-17 pathway is involved in human alcoholic liver disease. Hepatology. 2009;49:646-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 306] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 93. | Orman ES, Odena G, Bataller R. Alcoholic liver disease: pathogenesis, management, and novel targets for therapy. J Gastroenterol Hepatol. 2013;28 Suppl 1:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 179] [Article Influence: 14.9] [Reference Citation Analysis (2)] |
| 94. | Kerr IM, Costa-Pereira AP, Lillemeier BF, Strobl B. Of JAKs, STATs, blind watchmakers, jeeps and trains. FEBS Lett. 2003;546:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 95. | Norkina O, Dolganiuc A, Catalano D, Kodys K, Mandrekar P, Syed A, Efros M, Szabo G. Acute alcohol intake induces SOCS1 and SOCS3 and inhibits cytokine-induced STAT1 and STAT3 signaling in human monocytes. Alcohol Clin Exp Res. 2008;32:1565-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 96. | Tilg H, Moschen AR, Szabo G. Interleukin-1 and inflammasomes in alcoholic liver disease/acute alcoholic hepatitis and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology. 2016;64:955-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 254] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 97. | Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10:210-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1162] [Cited by in RCA: 1424] [Article Influence: 94.9] [Reference Citation Analysis (0)] |
| 98. | Revu S, Wu J, Henkel M, Rittenhouse N, Menk A, Delgoffe GM, Poholek AC, McGeachy MJ. IL-23 and IL-1β Drive Human Th17 Cell Differentiation and Metabolic Reprogramming in Absence of CD28 Costimulation. Cell Rep. 2018;22:2642-2653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 154] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 99. | Szabo G, Csak T. Inflammasomes in liver diseases. J Hepatol. 2012;57:642-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 404] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 100. | Takeuchi D, Yoshidome H, Kato A, Ito H, Kimura F, Shimizu H, Ohtsuka M, Morita Y, Miyazaki M. Interleukin 18 causes hepatic ischemia/reperfusion injury by suppressing anti-inflammatory cytokine expression in mice. Hepatology. 2004;39:699-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 101. | Sakai A, Han J, Cato AC, Akira S, Li JD. Glucocorticoids synergize with IL-1beta to induce TLR2 expression via MAP Kinase Phosphatase-1-dependent dual Inhibition of MAPK JNK and p38 in epithelial cells. BMC Mol Biol. 2004;5:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 102. | Wintergerst ES, Maggini S, Hornig DH. Contribution of selected vitamins and trace elements to immune function. Ann Nutr Metab. 2007;51:301-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 399] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 103. | Zhu H, Jia Z, Misra H, Li YR. Oxidative stress and redox signaling mechanisms of alcoholic liver disease: updated experimental and clinical evidence. J Dig Dis. 2012;13:133-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 104. | Shah D, Mahajan N, Sah S, Nath SK, Paudyal B. Oxidative stress and its biomarkers in systemic lupus erythematosus. J Biomed Sci. 2014;21:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 150] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 105. | Wołonciej M, Milewska E, Roszkowska-Jakimiec W. Trace elements as an activator of antioxidant enzymes. Postepy Hig Med Dosw (Online). 2016;70:1483-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 106. | Czuczejko J, Zachara BA, Staubach-Topczewska E, Halota W, Kedziora J. Selenium, glutathione and glutathione peroxidases in blood of patients with chronic liver diseases. Acta Biochim Pol. 2003;50:1147-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 107. | Arakawa Y, Moriyama M, Arakawa Y. Liver cirrhosis and metabolism (sugar, protein, fat and trace elements). Hepatol Res. 2004;30S:46-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 108. | Nangliya V, Sharma A, Yadav D, Sunder S, Nijhawan S, Mishra S. Study of trace elements in liver cirrhosis patients and their role in prognosis of disease. Biol Trace Elem Res. 2015;165:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 109. | Burk RF, Hill KE, Motley AK, Byrne DW, Norsworthy BK. Selenium deficiency occurs in some patients with moderate-to-severe cirrhosis and can be corrected by administration of selenate but not selenomethionine: a randomized controlled trial. Am J Clin Nutr. 2015;102:1126-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 110. | Prystupa A, Błażewicz A, Kiciński P, Sak JJ, Niedziałek J, Załuska W. Serum Concentrations of Selected Heavy Metals in Patients with Alcoholic Liver Cirrhosis from the Lublin Region in Eastern Poland. Int J Environ Res Public Health. 2016;13:582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 111. | Huang MC, Chen CH, Peng FC, Tang SH, Chen CC. Alterations in oxidative stress status during early alcohol withdrawal in alcoholic patients. J Formos Med Assoc. 2009;108:560-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 112. | Saribal D, Hocaoglu-Emre FS, Karaman F, Mırsal H, Akyolcu MC. Trace Element Levels and Oxidant/Antioxidant Status in Patients with Alcohol Abuse. Biol Trace Elem Res. 2020;193:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 113. | Grüngreiff K, Reinhold D, Wedemeyer H. The role of zinc in liver cirrhosis. Ann Hepatol. 2016;15:7-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 117] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 114. | Bode JC, Hanisch P, Henning H, Koenig W, Richter FW, Bode C. Hepatic zinc content in patients with various stages of alcoholic liver disease and in patients with chronic active and chronic persistent hepatitis. Hepatology. 1988;8:1605-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 79] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 115. | Tapiero H, Tew KD. Trace elements in human physiology and pathology: zinc and metallothioneins. Biomed Pharmacother. 2003;57:399-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 624] [Cited by in RCA: 537] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 116. | Wong CP, Rinaldi NA, Ho E. Zinc deficiency enhanced inflammatory response by increasing immune cell activation and inducing IL6 promoter demethylation. Mol Nutr Food Res. 2015;59:991-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 127] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 117. | Hennig B, Meerarani P, Toborek M, McClain CJ. Antioxidant-like properties of zinc in activated endothelial cells. J Am Coll Nutr. 1999;18:152-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 101] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 118. | Shen H, Arzuaga X, Toborek M, Hennig B. Zinc nutritional status modulates expression of ahr-responsive p450 enzymes in vascular endothelial cells. Environ Toxicol Pharmacol. 2008;25:197-201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 119. | Zhong W, Zhao Y, Sun X, Song Z, McClain CJ, Zhou Z. Dietary zinc deficiency exaggerates ethanol-induced liver injury in mice: involvement of intrahepatic and extrahepatic factors. PLoS One. 2013;8:e76522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 120. | Zhong W, McClain CJ, Cave M, Kang YJ, Zhou Z. The role of zinc deficiency in alcohol-induced intestinal barrier dysfunction. Am J Physiol Gastrointest Liver Physiol. 2010;298:G625-G633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 171] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 121. | Mohammad MK, Song M, Falkner K, McClain C, Cave M. Zinc Sulfate for Alcoholic Cirrhosis (ZAC) Clinical Trial - Interim Analysis of Fiver Injury/Inflammation Biomarkers. Hepatology. 2014;60:794A. [DOI] [Full Text] |
| 122. | Wenzel G, Kuklinski B, Rühlmann C, Ehrhardt D. [Alcohol-induced toxic hepatitis--a "free radical" associated disease. Lowering fatality by adjuvant antioxidant therapy]. Z Gesamte Inn Med. 1993;48:490-496. [PubMed] |
| 123. | Pár A, Pár G. [Alcoholic liver disease: the roles of genetic-epigenetic factors and the effect of abstinence]. Orv Hetil. 2019;160:524-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 124. | Wu Q, Ni X. ROS-mediated DNA methylation pattern alterations in carcinogenesis. Curr Drug Targets. 2015;16:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 215] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 125. | Afanas'ev I. New nucleophilic mechanisms of ros-dependent epigenetic modifications: comparison of aging and cancer. Aging Dis. 2014;5:52-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 126. | Boccuto L, Abenavoli L. Genetic and Epigenetic Profile of Patients With Alcoholic Liver Disease. Ann Hepatol. 2017;16:490-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 127. | Meroni M, Longo M, Rametta R, Dongiovanni P. Genetic and Epigenetic Modifiers of Alcoholic Liver Disease. Int J Mol Sci. 2018;19:3857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 128. | Zahs A, Curtis BJ, Waldschmidt TJ, Brown LA, Gauthier TW, Choudhry MA, Kovacs EJ, Bird MD. Alcohol and epigenetic changes: summary of the 2011 Alcohol and Immunology Research Interest Group (AIRIG) meeting. Alcohol. 2012;46:783-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 129. | Kendrick SF, O'Boyle G, Mann J, Zeybel M, Palmer J, Jones DE, Day CP. Acetate, the key modulator of inflammatory responses in acute alcoholic hepatitis. Hepatology. 2010;51:1988-1997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 130. | To Y, Ito K, Kizawa Y, Failla M, Ito M, Kusama T, Elliott WM, Hogg JC, Adcock IM, Barnes PJ. Targeting phosphoinositide-3-kinase-delta with theophylline reverses corticosteroid insensitivity in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182:897-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 276] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 131. | Ito K, Caramori G, Adcock IM. Therapeutic potential of phosphatidylinositol 3-kinase inhibitors in inflammatory respiratory disease. J Pharmacol Exp Ther. 2007;321:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 122] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 132. | Barnes PJ. Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2013;131:636-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 521] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 133. | Kendrick SF, Henderson E, Palmer J, Jones DE, Day CP. Theophylline improves steroid sensitivity in acute alcoholic hepatitis. Hepatology. 2010;52:126-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 134. | Hassanein T, Stein LL, Flamm SL, Martin P, Cave MC, Blevins C, Scott, D, Krebs W, Lin W. Safety and efficacy of DUR-928: a potential new therapy for acute alcoholic hepatitis. Hepatology. 2019;70:1477A-1501A. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 135. | Yu S, Khor TO, Cheung KL, Li W, Wu TY, Huang Y, Foster BA, Kan YW, Kong AN. Nrf2 expression is regulated by epigenetic mechanisms in prostate cancer of TRAMP mice. PLoS One. 2010;5:e8579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 178] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 136. | Khor TO, Fuentes F, Shu L, Paredes-Gonzalez X, Yang AY, Liu Y, Smiraglia DJ, Yegnasubramanian S, Nelson WG, Kong AN. Epigenetic DNA methylation of antioxidative stress regulator NRF2 in human prostate cancer. Cancer Prev Res (Phila). 2014;7:1186-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 137. | Wang D, Ma Y, Yang X, Xu X, Zhao Y, Zhu Z, Wang X, Deng H, Li C, Gao F, Tong J, Yamanaka K, An Y. Hypermethylation of the Keap1 gene inactivates its function, promotes Nrf2 nuclear accumulation, and is involved in arsenite-induced human keratinocyte transformation. Free Radic Biol Med. 2015;89:209-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 138. | Correa F, Mallard C, Nilsson M, Sandberg M. Activated microglia decrease histone acetylation and Nrf2-inducible anti-oxidant defence in astrocytes: restoring effects of inhibitors of HDACs, p38 MAPK and GSK3β. Neurobiol Dis. 2011;44:142-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 139. | Guo T, Chang L, Xiao Y, Liu Q. S-adenosyl-L-methionine for the treatment of chronic liver disease: a systematic review and meta-analysis. PLoS One. 2015;10:e0122124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 140. | Medici V, Virata MC, Peerson JM, Stabler SP, French SW, Gregory JF, Albanese A, Bowlus CL, Devaraj S, Panacek EA, Richards JR, Halsted CH. S-adenosyl-L-methionine treatment for alcoholic liver disease: a double-blinded, randomized, placebo-controlled trial. Alcohol Clin Exp Res. 2011;35:1960-1965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 141. | Mato JM, Cámara J, Fernández de Paz J, Caballería L, Coll S, Caballero A, García-Buey L, Beltrán J, Benita V, Caballería J, Solà R, Moreno-Otero R, Barrao F, Martín-Duce A, Correa JA, Parés A, Barrao E, García-Magaz I, Puerta JL, Moreno J, Boissard G, Ortiz P, Rodés J. S-adenosylmethionine in alcoholic liver cirrhosis: a randomized, placebo-controlled, double-blind, multicenter clinical trial. J Hepatol. 1999;30:1081-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 300] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 142. | Collins P, Jones C, Choudhury S, Damelin L, Hodgson H. Increased expression of uncoupling protein 2 in HepG2 cells attenuates oxidative damage and apoptosis. Liver Int. 2005;25:880-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 143. | Jin X, Yang YD, Chen K, Lv ZY, Zheng L, Liu YP, Chen SH, Yu CH, Jiang XY, Zhang CY, Li YM. HDMCP uncouples yeast mitochondrial respiration and alleviates steatosis in L02 and hepG2 cells by decreasing ATP and H2O2 levels: a novel mechanism for NAFLD. J Hepatol. 2009;50:1019-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 144. | Cortez-Pinto H, Machado MV. Uncoupling proteins and non-alcoholic fatty liver disease. J Hepatol. 2009;50:857-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 145. | Phillips M, Curtis H, Portmann B, Donaldson N, Bomford A, O'Grady J. Antioxidants versus corticosteroids in the treatment of severe alcoholic hepatitis--a randomised clinical trial. J Hepatol. 2006;44:784-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 144] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 146. | Stewart S, Prince M, Bassendine M, Hudson M, James O, Jones D, Record C, Day CP. A randomized trial of antioxidant therapy alone or with corticosteroids in acute alcoholic hepatitis. J Hepatol. 2007;47:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 121] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 147. | Nguyen-Khac E, Thevenot T, Piquet MA, Benferhat S, Goria O, Chatelain D, Tramier B, Dewaele F, Ghrib S, Rudler M, Carbonell N, Tossou H, Bental A, Bernard-Chabert B, Dupas JL; AAH-NAC Study Group. Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis. N Engl J Med. 2011;365:1781-1789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 294] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 148. | European Association for the Study of the Liver; Thursz M, Gual A, Lackner C, Mathurin P, Moreno C, Spahr L, Sterneck M, Cortez-Pinto H. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of alcohol-related liver disease. J Hepatol. 2018;69:154-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 585] [Article Influence: 83.6] [Reference Citation Analysis (0)] |
| 149. | McClain C, Vatsalya V, Cave M. Role of Zinc in the Development/Progression of Alcoholic Liver Disease. Curr Treat Options Gastroenterol. 2017;15:285-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 150. | Singh AK, Bishayee A, Pandey AK. Targeting Histone Deacetylases with Natural and Synthetic Agents: An Emerging Anticancer Strategy. Nutrients. 2018;10:731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 156] [Article Influence: 22.3] [Reference Citation Analysis (0)] |