Published online Feb 27, 2020. doi: 10.4254/wjh.v12.i2.64
Peer-review started: November 14, 2019
First decision: December 12, 2019
Revised: December 19, 2019
Accepted: January 1, 2020
Article in press: January 1, 2020
Published online: February 27, 2020
Processing time: 104 Days and 19.2 Hours
Benign recurrent intrahepatic cholestasis is a genetic disorder with recurrent cholestatic jaundice due to ATP8B1 and ABCB11 gene mutations encoding for hepato-canalicular transporters. Herein, we firstly provide the evidence that a nonsense variant of ATP8B1 gene (c.1558A>T) in heterozygous form is involved in BRIC pathogenesis.
A 29-year-old male showed severe jaundice and laboratory tests consistent with intrahepatic cholestasis despite normal gamma-glutamyltranspeptidase. Acute and chronic liver diseases with viral, metabolic and autoimmune etiology were excluded. Normal intra/extra-hepatic bile ducts were demonstrated by magnetic resonance. Liver biopsy showed: Cholestasis in the centrilobular and intermediate zones with bile plugs and intra-hepatocyte pigment, Kupffer’s cell activation/hyperplasia and preserved biliary ducts. Being satisfied benign recurrent intrahepatic cholestasis diagnostic criteria, ATP8B1 and ABCB11 gene analysis was performed. Surprisingly, we found a novel nonsense variant of ATP8B1 gene (c.1558A>T) in heterozygosis. The variant was confirmed by Sanger sequencing following a standard protocol and tested for familial segregation, showing a maternal inheritance. Immunohistochemistry confirmed a significant reduction of mutated gene related protein (familial intrahepatic cholestasis 1). The patient was treated with ursodeoxycholic acid 15 mg/kg per day and colestyramine 8 g daily with total bilirubin decrease and normalization at the 6th and 12th mo.
A genetic abnormality, different from those already known, could be involved in familial intrahepatic cholestatic disorders and/or pro-cholestatic genetic predisposition, thus encouraging further mutation detection in this field.
Core tip: Benign recurrent intrahepatic cholestasis is a rare genetic disorder characterized by recurrent jaundice due to the mutation of the ATP8B1/ABCB11 genes encoding for hepato-canalicular transporters. The original finding, which characterizes the case we observed, is the association of a novel nonsense variant of ATP8B1 gene (c.1558A>T) in a heterozygous condition with hepato-canalicular transporter protein deficiency. Indeed, the disorder has been described until now as an autosomal recessive one, whereas, in this case, the patient expressed the disease despite having only one mutated allele of ATP8B1 gene.
- Citation: Piazzolla M, Castellaneta N, Novelli A, Agolini E, Cocciadiferro D, Resta L, Duda L, Barone M, Ierardi E, Di Leo A. Nonsense variant of ATP8B1 gene in heterozygosis and benign recurrent intrahepatic cholestasis: A case report and review of literature. World J Hepatol 2020; 12(2): 64-71
- URL: https://www.wjgnet.com/1948-5182/full/v12/i2/64.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i2.64
Benign recurrent intrahepatic cholestasis (BRIC) is a rare genetic disorder characterized by recurrent episodes of cholestatic jaundice that undergo spontaneous resolution within a period lasting from few weeks to some months without an evolution towards chronic liver disease[1]. The first case of BRIC was described by Summerskill et al[2] in 1956. Its exact prevalence remains unknown, but estimated incidence is approximately 1 in 50000 to 100000 people worldwide. BRIC is an autosomal recessive disorder. Two subtypes have been described according to associated gene mutations. BRIC-1 is associated with a mutation in the ATP8B1 gene (chromosome 18q21-22) that encodes a protein called familial intrahepatic cholestasis 1 (FIC1)[3]. BRIC-2 is caused by a mutation in the ABCB11 gene (chromosome 2q24) encoding bile salt export pump protein[4]. Consequent impaired function of these hepato-canalicular transporters, responsible for the secretion of bile components, induces an intrahepatic cholestasis. Mutations in ATP8B1 and ABCB11 genes are also present in progressive familial intrahepatic cholestasis (PFIC - type 1 and 2, respectively). Despite both are due to mutations in the same gene, phenotypes of BRIC and PFIC differ, since PFIC is characterized by a progressive liver damage up to end-stage liver disease[5].
BRIC diagnostic criteria have been proposed by Luketic et al[1]; at the moment, they represent the basics in the diagnosis of the disorder. They include: (1) At least two episodes of jaundice separated by a symptom-free interval lasting several months to years; (2) Laboratory values consistent with intrahepatic cholestasis; (3) Gamma-glutamyltranspeptidase (GGT) either normal or only minimally elevated; (4) Severe pruritus secondary to cholestasis; (5) Liver histology demonstrating centrilobular cholestasis; (6) Normal intra- and extra-hepatic bile ducts by cholangiography (endoscopic retrograde cholangiopancreatography, magnetic resonance cholangiography); and (7) Absence of factors known to be associated with cholestasis (i.e., drugs, pregnancy)[1].
On these bases, we believe of interest to report the case of a young adult patient with clinical, biochemical and histological features of BRIC, where we have, at the best of our knowledge, firstly provided the evidence of a heterozygous pathogenic nonsense variant in the ATP8B1 gene (c.1558A>T).
A 29-year-old male was admitted into our Gastroenterology Unit for jaundice, severe pruritus, dark colored urine, clay colored stool and weight loss.
Patient symptoms started one month before the admission in our Unit. He had undergone two previous hospitalizations without clinical benefit. No infections or fever were reported.
The patient had a free previous medical history. In the past, he had never suffered from jaundice.
There was no history of drug exposure, blood transfusions or alcohol intake. Family history was negative for similar complaints. Mother had never reported intrahepatic cholestasis of pregnancy during his two pregnancies.
On physical examination, the patient showed an evident jaundice, widespread scratch marks over the whole body, hepatomegaly (1 cm below the right costal margin) without clinical signs of liver failure (ascites, edema, encephalopathy).
Laboratory investigations showed low hemoglobin (10 g/dL) and mean corpuscular volume (78.1 fl) with normal white blood cell (6330/microL) and platelet (295000/microL) count. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were normal. Markers of cholestasis showed normal GGT, alkaline phosphatase (ALP) 2.9 times upper normal limit and total bilirubin value of 32.70 mg/dL (conjugated fraction: 28.59 mg/dL). Markers of hepatic parenchymal activity showed hypoalbuminemia (1.9 g/dL) with normal values of international normalized ratio of prothrombin time (0.94), Partial thromboplastin time (ratio: 1.03; normal: < 1.20), coagulation factor V, ammonium, cholinesterase, blood sugar and cholesterol levels. Other altered biochemical tests were: low vitamin D level (14 ng/mL; normal: 30-100 ng/mL), mild elevation of amylase and lipase (1.1 times and 1.4 times upper normal limit, respectively), reduced renal function test (estimated Glomerular filtration rate - eGFR 67 mL/min), reduced serum potassium (3.1 mmol/L).
Serological investigations excluded viral, metabolic and autoimmune causes of acute and chronic liver diseases. Negative results were found for the following parameters: IgM antibodies to hepatitis A virus, hepatitis B surface antigen, hepatitis B virus DNA, hepatitis C virus RNA, antibodies to hepatitis C virus, serum levels of iron, transferrin, and ferritin, serum copper and ceruloplasmin levels, alpha1-antitrypsin, anti-nuclear, anti-smooth muscle, anti-liver kidney microsomal anti-mitochondrial antibodies, serum protein electrophoresis and immunoglobulins (IgG, IgA, IgM).
Ultrasonographic abdominal investigation revealed mild hepatomegaly with normal echogenicity. Magnetic resonance cholangiopancreatography showed normal intra- and extra-hepatic biliary tree and pancreatic ductal system.
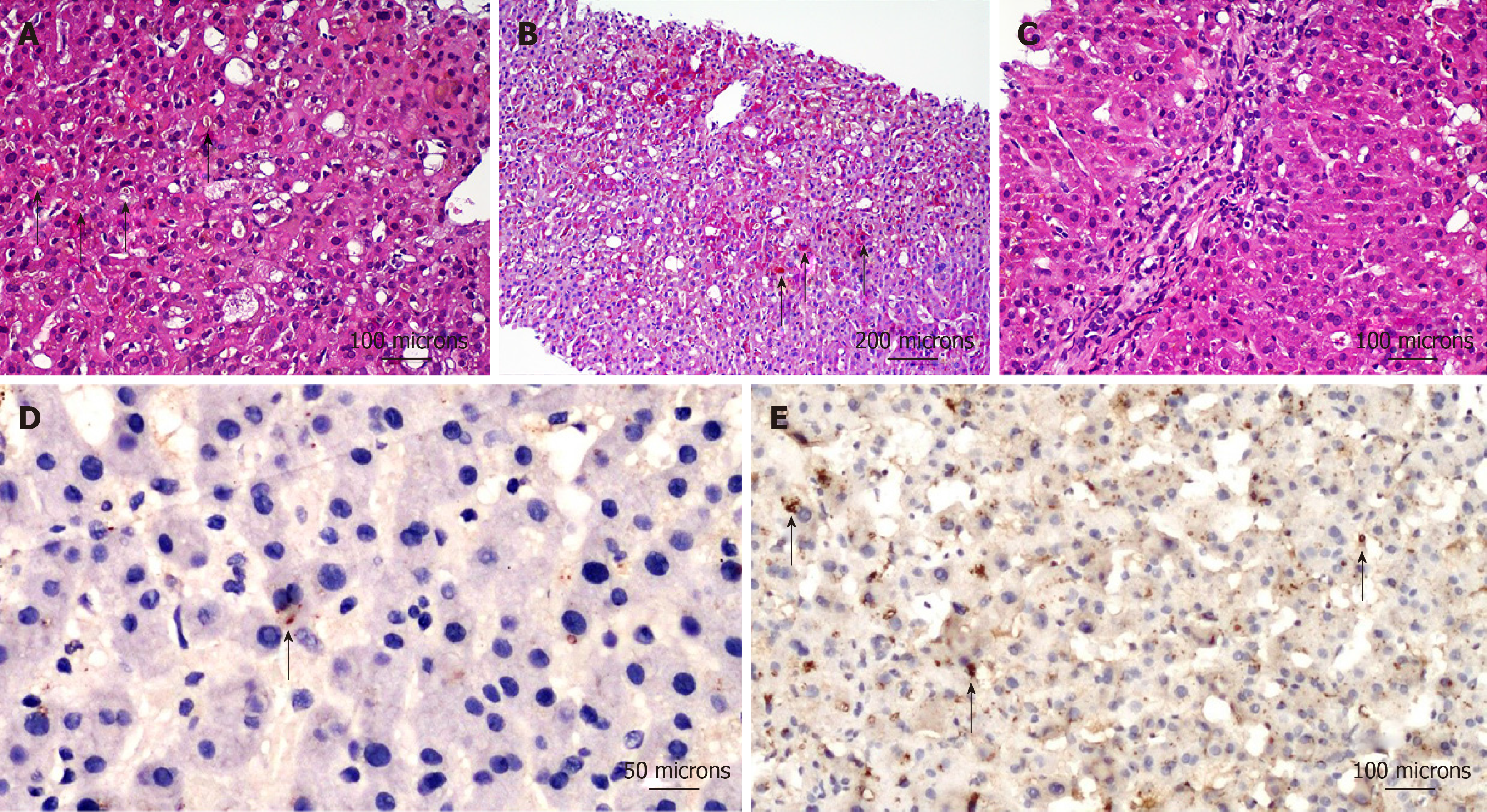
Liver biopsy showed: severe cholestasis in the centrilobular hepatocytes, involvement of intermediate zone with evidence of bile plugs as well as intra-hepatocyte bile pigment (Figure 1A), activation and hyperplasia of Kupffer’s cells (Figure 1B) and preserved biliary ducts in portal areas (Figure 1C).
Being satisfied BRIC diagnostic criteria, ATP8B1 and ABCB11 gene analysis was performed. After obtaining the informed consent for the genetic analyses, targeted enrichment and panel parallel sequencing were performed on genomic DNA extracted from circulating leukocytes of the affected subject. Patient library preparation and targeted re-sequencing were performed by using the NimbleGenSeqCap Target Enrichment kit (Roche, Pleasanton, CA, United States) on a NextSeq550 (Illumina Inc, San Diego, CA, United States) platform, according to the manufacture’s protocol. The BaseSpace pipeline (Illumina, https://basespace.illumina.com/) and the TGex software LifeMap Sciences (http://tgex.genecards.org/) were used for the variant calling and annotating variants, respectively. Sequencing data have been aligned to the hg19 human reference genome. The variants were analyzed in silico by using Scale-Invariant Feature Transform and Polymorphism Phenotyping v2 (http://genetics.bwh.harvard.edu/pph2) for the prediction of deleterious non-synonymous SNVs for human diseases. Based on the guidelines of the American College of Medical Genetics and Genomics[6], a minimum mean depth coverage of 30 × was considered suitable for analysis. Variants were examined for coverage and Qscore (minimum threshold of 30), and visualized by the Integrative Genome Viewer. Clinical investigations and genetic analyses were approved by the institutional scientific board of the institutes involved, and were conducted in accordance with the Helsinki Declaration.
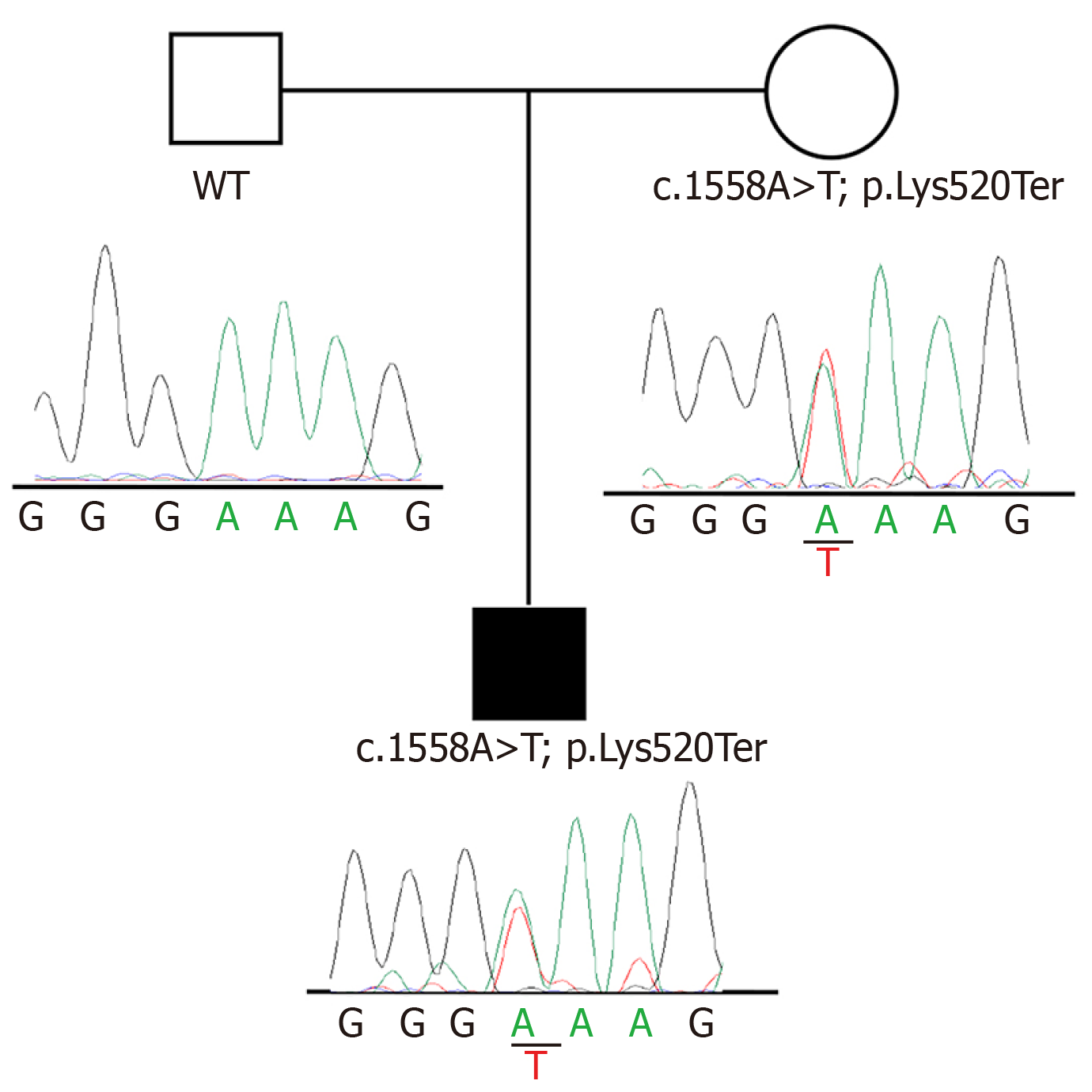
Sequencing analysis identified a heterozygous nonsense variant, c.1558A>T, in exon14 of ATP8B1 gene (NM_005603). This variant has never been described before, and is not present in dbSNP, Exome Variant Server, or ExAC databases. The finding was confirmed by Sanger sequencing following a standard protocol (BigDye Terminator v3.1 Cycle Sequencing Kit, Applied Biosystems by Life Technologies) and tested for familial segregation, showing a maternal inheritance. According to the American College of Medical Genetics and Genomics criteria, the c.1558A>T (p.Lys520Ter) variant can be classified as likely pathogenic. Figure 2 reports pedigrees and electropherograms of ATP8B1 c.1558A>T variant, which were identified in the patient family.
To confirm the pathogenetic role of observed mutation, immunohistochemical study was performed with anti-ATP8B1 Antibody (rabbit polyclonal antibody - HPA018674, 1:20 working dilution, Atlas Antibodies, Stockholm, Sweden). This antibody has a specific affinity for the protein codified by ATP8B1 gene (https://www.atlasantibodies.com/products/antibodies/primary-antibodies/triple-a-polyclonals/atp8b1-antibody-hpa018674/). The protein expression in the liver of the patient with c.1558A>T mutation was significantly reduced (Figure 1D) as compared to that of a healthy control (Figure 1E).
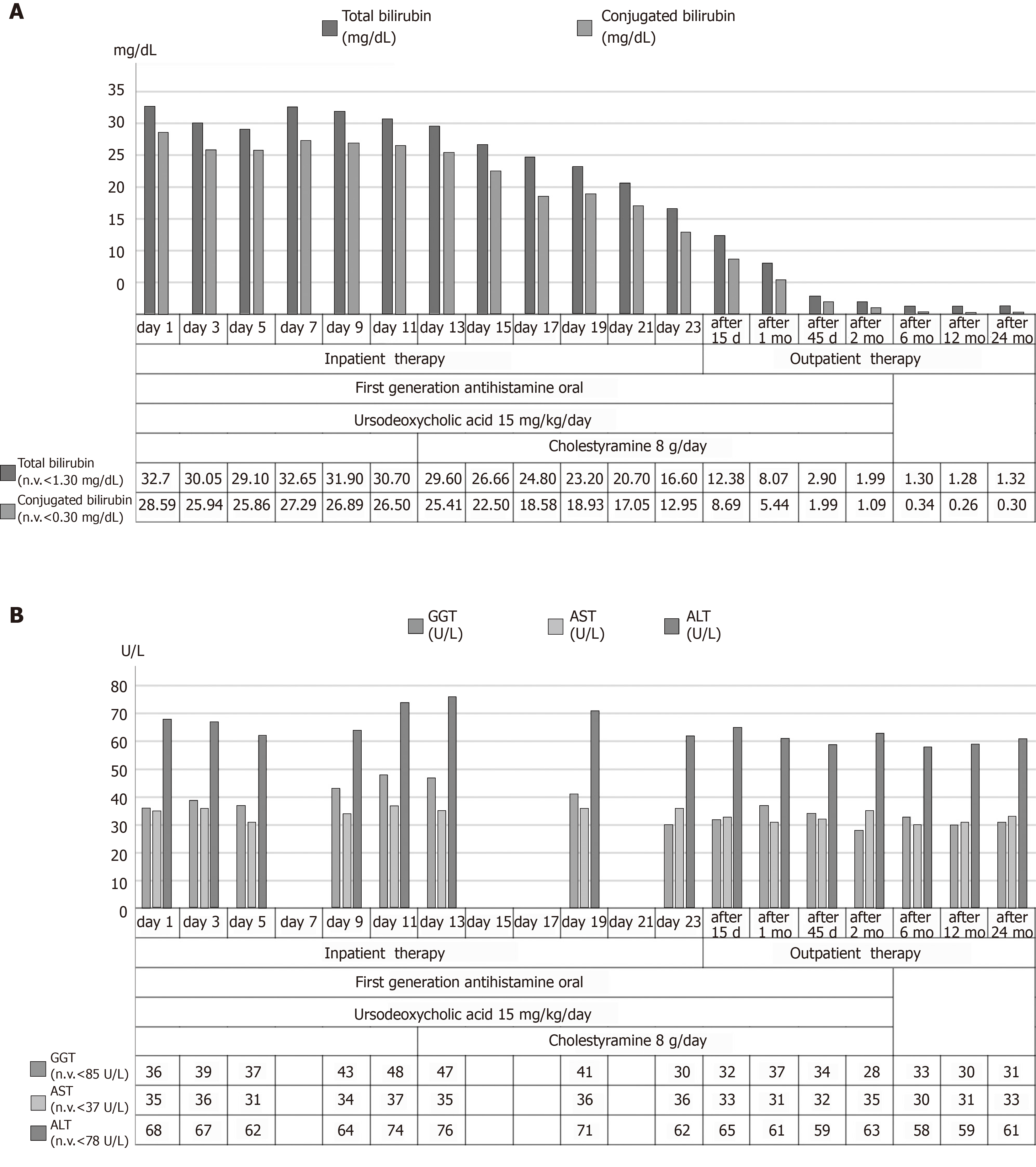
The patient was treated with ursodeoxycholic acid 300 mg thrice daily (15 mg/kg per day) and colestyramine 4 g twice daily with a clinical improvement. After 15 d, total bilirubin decreased to 12.38 mg/dL which was near to normal values after 2 mo of therapy (1.99 mg/dL) with a complete normalization at the 6th and 12th mon.
A 24-mo follow up did not develop any further episode of jaundice; laboratory investigations showed normal values of hemoglobin (13 g/dL), AST, ALT, GGT, ALP and total bilirubin (1.32 mg/dL) at this time. Figure 3 summarizes the course of most significant laboratory tests and a trend chart with the therapeutic process.
BRIC is a self-limiting disorder. There is no progression to cirrhosis: liver histology remains normal during remission. Therefore, it does not cause chronic liver disease. The knowledge of this entity is crucial as an early recognition can suggest the optimal prognostic and therapeutic approach.
The first report of BRIC dates back to 1956. Its estimated incidence is approximately 1 in 50000 to 100000 people worldwide[1]. It is due to an autosomal recessive disorder even if two subtypes are known according to respective associated gene mutations, i.e., BRIC-1 (mutation in the ATP8B1 gene)[3] and BRIC-2 (mutation in the ABCB11 gene)[4]. These mutations induce an impaired function of hepato-canalicular transporters for the secretion of bile components with a consequent intrahepatic cholestasis. Mutations in ATP8B1 and ABCB11 genes are also involved in a PFIC - type 1 and 2 characterized by a liver damage up to end-stage liver disease[5]. BRIC diagnostic criteria proposed by Luketic and Shiffman represent the basics in the diagnosis of the disorder[1]. Indeed, rare cases of BRIC that subsequently transitioned to a persistent progressive form of the disease (PFIC) have been described, thus suggesting the possibility of a clinical continuum among the different phenotypic expressions due to gene defect[7]. Since both diseases (BRIC and PFIC) are referred to ATP8B1 and ABCB11 deficiency, as described by van der Woerd et al[5], the central role of genetic abnormalities is evident and molecular analysis may be useful both to predict the disease phenotype and determine a tailored treatment strategy.
BRIC clinical, biochemical and histological pattern of our patient fully agreed with the criteria of Luketic and Shiffman[1]. The finding of the nonsense variant (c.1558A>T), in exon14 of ATP8B1 gene, associated with immunohistochemical demonstration of FIC1 deficiency, confirmed our diagnostic hypothesis. At the best of our knowledge, the mutation we found (c.1558A>T) has not been described in the scientific literature and international databases (ClinVar, Human Gene Mutation Database, Leiden Open Variation Database). Indeed, even if BRIC is an autosomal recessive disease, a single heterozygous variant in the ATP8B1 gene has been found in a single case of this disorder[8]. This unexpected finding has induced to hypothesize that a counter allele could be present but not detected, since some mutations (such as large deletions) may be undetectable by common methods or involve not analyzed regions (untranslated regions, upstream regulatory sequences, introns)[9]. On the other hand, the state of art demonstrates that the most common ATP8B1 mutation identified in European BRIC patients (I661T) shows an incomplete penetrance in homozygous form, while its compound heterozygous form has been even associated to a case of PFIC-1[9]. In addition, mutation type or location correlate with disease severity[9].
In this multifaceted setting of hepato-canalicular transporter genetic disorders, an apparently autosomal dominant transmission in a family with BRIC has been recently reported[10]. These data suggest the likehood of an additional unmapped locus for autosomal recessive BRIC and PFIC[11]. To support this possibility, biallelic MYO5B mutations has been recently reported in patients with isolated low-GGT cholestasis[12]. This finding suggests that patients lacking mutations in both ATP8B1 and ABCB11 genes should be screened for additional gene mutations involved in bile secretion and transport pathways[13]. Indeed, at this regard, a recent systematic review highlighted the “expanded PFIC disorders” thus suggesting the possible involvement of genes other than already known ones, such as those related to TJP2, NR1H4 (FXR protein), MYO5B, USP53 and LSR defects, being these genes are all involved in bile transport physiology[14].
Differently from the other sequence variants of ATP8B1 gene found in heterozygous form, we observed a “nonsense” mutation able to induce the insertion of a premature stop codon (p.Lys520Ter) and impair the production of FIC1. Additionally, this variant has never been described at the best of our knowledge, being not present in dbSNP, Exome Variant Server, or ExAC databases. Finally, a mechanistic study of its biological effect was performed by immunohistochemistry for gene codified protein deficiency and unquestionably confirmed that FIC1 level was severely lacking[15].
In this context, the mutation found in our patient perfectly fits with what known about the complex genetic scenario. Additionally, the nonsense variant was confirmed by Sanger sequencing and tested for familial segregation, showing a maternal inheritance, thus supporting the known familiarity of the disorder.
FIC refers to a heterogeneous group of autosomal recessive liver disorders, which occur worldwide. In all cases of FIC, a wide range of variations in clinical phenotypes has been observed, possibly due to the variability of the mutations and type of segregation, which have even been sporadically found as compound heterozygosity. Molecular analysis is mandatory for a correct and complete diagnosis and our case provides the novelty that the finding of a single nonsense heterozygous mutation does not exclude an inherited disorder. In this case, however, immunostaining for the corresponding encoded protein is recommended to confirm a cause-effect relationship. Therefore, the genetic heterogeneity and the complexity of the mechanisms related to the biliary flow suggest that inherited abnormalities other than those already known, could be not only at the basis of the spectrum of familial intrahepatic cholestatic disorders, but also of specific pro-cholestatic genetic predisposition, thus encouraging further investigations in this field.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Deneau M, Li XH S-Editor: Ma RY L-Editor: A E-Editor: Xing YX
| 1. | Luketic VA, Shiffman ML. Benign recurrent intrahepatic cholestasis. Clin Liver Dis. 2004;8:133-149, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Summerskill WH, Walshe JM. Benign recurrent intrahepatic "obstructive" jaundice. Lancet. 1959;2:686-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 100] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Bull LN, van Eijk MJ, Pawlikowska L, DeYoung JA, Juijn JA, Liao M, Klomp LW, Lomri N, Berger R, Scharschmidt BF, Knisely AS, Houwen RH, Freimer NB. A gene encoding a P-type ATPase mutated in two forms of hereditary cholestasis. Nat Genet. 1998;18:219-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 505] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 4. | van Mil SW, van der Woerd WL, van der Brugge G, Sturm E, Jansen PL, Bull LN, van den Berg IE, Berger R, Houwen RH, Klomp LW. Benign recurrent intrahepatic cholestasis type 2 is caused by mutations in ABCB11. Gastroenterology. 2004;127:379-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 220] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 5. | van der Woerd WL, van Mil SW, Stapelbroek JM, Klomp LW, van de Graaf SF, Houwen RH. Familial cholestasis: progressive familial intrahepatic cholestasis, benign recurrent intrahepatic cholestasis and intrahepatic cholestasis of pregnancy. Best Pract Res Clin Gastroenterol. 2010;24:541-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL; ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19696] [Cited by in RCA: 22474] [Article Influence: 2247.4] [Reference Citation Analysis (0)] |
| 7. | van Ooteghem NA, Klomp LW, van Berge-Henegouwen GP, Houwen RH. Benign recurrent intrahepatic cholestasis progressing to progressive familial intrahepatic cholestasis: low GGT cholestasis is a clinical continuum. J Hepatol. 2002;36:439-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Lee YS, Kim MJ, Ki CS, Lee YM, Lee Y, Choe YH. Benign Recurrent Intrahepatic Cholestasis with a Single Heterozygote Mutation in the ATP8B1 Gene. Pediatr Gastroenterol Hepatol Nutr. 2012;15:122-126. [DOI] [Full Text] |
| 9. | Klomp LW, Vargas JC, van Mil SW, Pawlikowska L, Strautnieks SS, van Eijk MJ, Juijn JA, Pabón-Peña C, Smith LB, DeYoung JA, Byrne JA, Gombert J, van der Brugge G, Berger R, Jankowska I, Pawlowska J, Villa E, Knisely AS, Thompson RJ, Freimer NB, Houwen RH, Bull LN. Characterization of mutations in ATP8B1 associated with hereditary cholestasis. Hepatology. 2004;40:27-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 188] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | Floreani A, Molaro M, Mottes M, Sangalli A, Baragiotta A, Roda A, Naccarato R, Clementi M. Autosomal dominant benign recurrent intrahepatic cholestasis (BRIC) unlinked to 18q21 and 2q24. Am J Med Genet. 2000;95:450-453. [PubMed] [DOI] [Full Text] |
| 11. | Vitale G, Gitto S, Vukotic R, Raimondi F, Andreone P. Familial intrahepatic cholestasis: New and wide perspectives. Dig Liver Dis. 2019;51:922-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (4)] |
| 12. | Qiu YL, Gong JY, Feng JY, Wang RX, Han J, Liu T, Lu Y, Li LT, Zhang MH, Sheps JA, Wang NL, Yan YY, Li JQ, Chen L, Borchers CH, Sipos B, Knisely AS, Ling V, Xing QH, Wang JS. Defects in myosin VB are associated with a spectrum of previously undiagnosed low γ-glutamyltransferase cholestasis. Hepatology. 2017;65:1655-1669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 13. | Sticova E, Jirsa M, Pawłowska J. New Insights in Genetic Cholestasis: From Molecular Mechanisms to Clinical Implications. Can J Gastroenterol Hepatol. 2018;2018:2313675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 14. | Henkel SA, Squires JH, Ayers M, Ganoza A, Mckiernan P, Squires JE. Expanding etiology of progressive familial intrahepatic cholestasis. World J Hepatol. 2019;11:450-463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (3)] |
| 15. | Giovannoni I, Callea F, Bellacchio E, Torre G, De Ville De Goyet J, Francalanci P. Genetics and Molecular Modeling of New Mutations of Familial Intrahepatic Cholestasis in a Single Italian Center. PLoS One. 2015;10:e0145021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |