Published online Dec 27, 2020. doi: 10.4254/wjh.v12.i12.1349
Peer-review started: July 28, 2020
First decision: September 17, 2020
Revised: September 29, 2020
Accepted: October 21, 2020
Article in press: October 21, 2020
Published online: December 27, 2020
Processing time: 142 Days and 7.9 Hours
Lenvatinib has been shown to be noninferior to sorafenib regarding prognosis and recurrence rate in patients with unresectable hepatocellular carcinoma (HCC) who have not received prior systemic chemotherapy. In patients treated with lenvatinib, 40% of cases achieved sufficient tumor reduction to make potential surgery possible. However, the outcomes of such surgery are unknown. We report a successful case of hepatic resection for recurrent HCC after lenvatinib treatment.
A 69-year-old man underwent right anterior sectionectomy for HCC in segment 8 of the liver. Ten months later, he was found to have an intrahepatic HCC recurrence that grew rapidly to 10 cm in diameter with sternal bone metastases. After confirming partial response to lenvatinib administration for 2 mo, a second hepatectomy was performed. Pathological examination showed that 80% of the tumor was necrotic. The patient did not develop any adverse effects under lenvatinib treatment. He was discharged at 25 d after surgery. Radiation therapy for bone metastases continued to be given under lenvatinib, and the patient has remained alive for 1 year after the second hepatectomy.
The prognosis of patients with recurrent HCC may be improved by liver resection combined with prior lenvatinib therapy.
Core Tip: We report a case of intrahepatic hepatocellular carcinoma recurrence with rapid growth and sternal bone metastases after initial resection. A second surgery was successfully achieved after lenvatinib administration induced tumor shrinkage. Because the patient had bone metastases, multidisciplinary therapy, postoperative radiation therapy, and lenvatinib administration were performed, and the patient remains alive at 1 year postoperatively. In this case, lenvatinib induced a partial response for rapid growth of recurrent hepatocellular carcinoma with bone metastases, and conversion to surgery was successfully achieved for the purpose of controlling the intrahepatic lesion for the first time.
- Citation: Yokoo H, Takahashi H, Hagiwara M, Iwata H, Imai K, Saito Y, Matsuno N, Furukawa H. Successful hepatic resection for recurrent hepatocellular carcinoma after lenvatinib treatment: A case report. World J Hepatol 2020; 12(12): 1349-1357
- URL: https://www.wjgnet.com/1948-5182/full/v12/i12/1349.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i12.1349
The treatment strategy for hepatocellular carcinoma (HCC) has been established in various guidelines, including the Clinical Practice Guidelines for Hepatocellular Carcinoma in Japan[1], Barcelona Clinic Liver Cancer Guidelines[2], American Association for the Study of the Liver Diseases Guidelines[3], and European Association for the Study of the Liver European Organization for Research and Treatment of Cancer Guidelines[4]. Hepatectomy is a potential curative treatment for early-stage HCC. However, the indications for liver resection are limited by tumor progression and liver function, and many cases are not eligible for resection. Transarterial chemoembolization(TACE) is commonly used for these unresectable cases, but the outcomes, especially of chemotherapy, have not been satisfactory compared with liver resection until recently[5,6].
Against this background, the systemic chemotherapy landscape for HCC changed more than a decade ago, with sorafenib demonstrating a survival benefit in the first-line setting and becoming the first systemic therapy to receive approval for HCC. More recently, regorafenib and ramucirumab have been approved in the second-line setting after sorafenib, and lenvatinib has emerged in the first-line setting after a positive phase 3 study[7-11]. The advent of these effective molecular targeted agents has allowed multidisciplinary treatments combined with chemotherapy and liver resection for HCC.
Regarding colorectal cancer, there are many reports on postoperative adjuvant therapy to prevent recurrence after resection and preoperative neoadjuvant chemotherapy for colorectal cancer and its liver metastases[12-15]. Thus, conversion therapy in colorectal cancer has become a routine practice. Although conversion therapy for HCC has not yet been established, lenvatinib is expected to be a possible candidate agent because it exhibited a tumor-reducing effect in 40% of cases in the REFLECT study[14]. There is a possibility that patients with unresectable or recurrent HCC can be converted to surgery after lenvatinib treatment. In the present case, lenvatinib induced a partial response (PR) for rapid growth of recurrent HCC with bone metastases, and conversion to surgery was successfully achieved for the purpose of controlling the intrahepatic lesion.
The patient complained of general malaise.
A 69-year-old man was referred to our facility with a diagnosis of recurrent HCC. He had undergone right anterior sectionectomy for two tumors in segment 8 at another hospital 10 mo previously. The pathological diagnosis of the two tumors was moderately differentiated HCC and poorly differentiated HCC, respectively, without vascular invasion. The pathological stage was stage III according to the Union for International Cancer Control 8th edition.
The patient had undergone appendectomy for appendicitis at 27 years of age.
The patient’s father had experienced lung cancer, and his sister had suffered from breast cancer.
On physical examination, the patient had a temperature of 36.6 °C, heart rate of 76 bpm, respiratory rate of 16 breaths/min, blood pressure of 143/86 mmHg, and oxygen saturation in room air of 98%. The patient’s weight was 59 kg. He had no jaundice or anemia in the bulbar conjunctiva. There was an operative scar for an inverted L-shaped incision on the abdomen from the previous liver resection. No ascites and encephalopathy were detected.
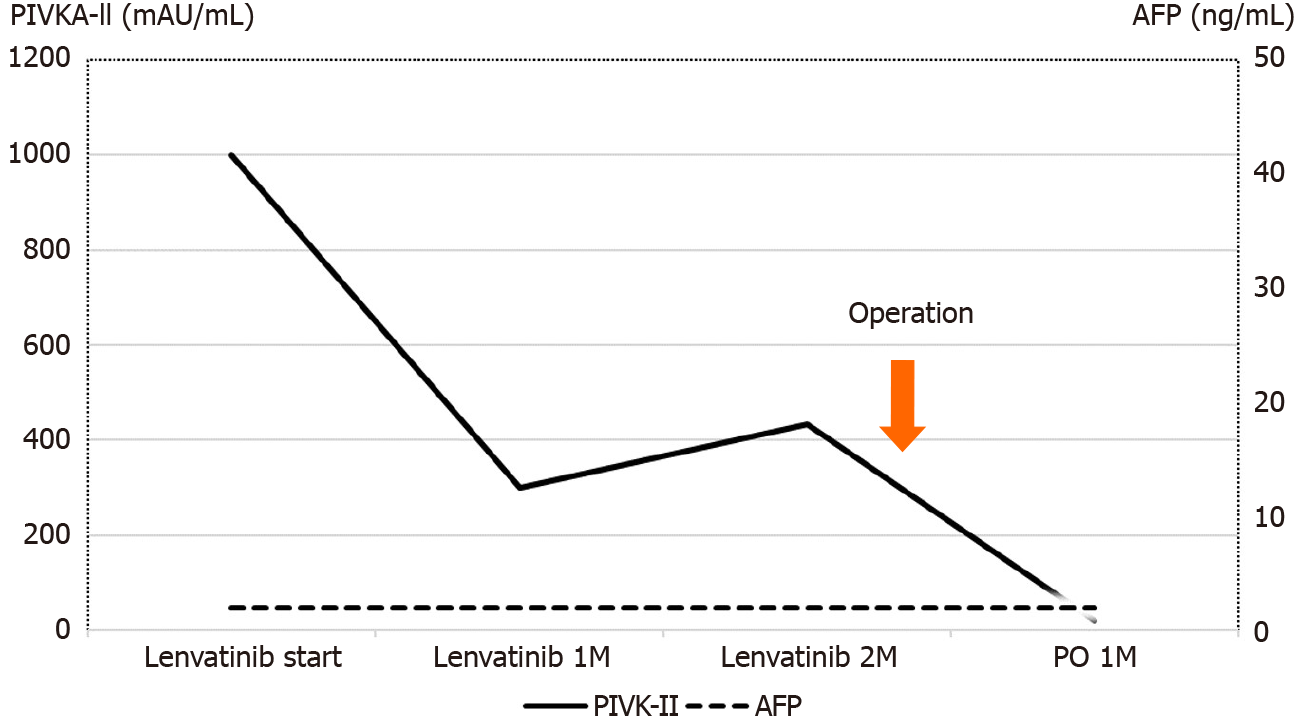
The preoperative liver functional reserve was good. Laboratory data revealed serum albumin of 3.5 g/dL, total bilirubin of 0.9 mg/dL, prothrombin time of 93%, and indocyanine green retention rate at 15 min of 5.2%. The Child–Pugh score/ classification was 5/A and the albumin-bilirubin (ALBI) score/modified ALBI grade was -2.19/2b. The alpha fetoprotein value was within the normal range, while the protein-induced vitamin K absence or antagonist II value was elevated at 998 mAU/mL.
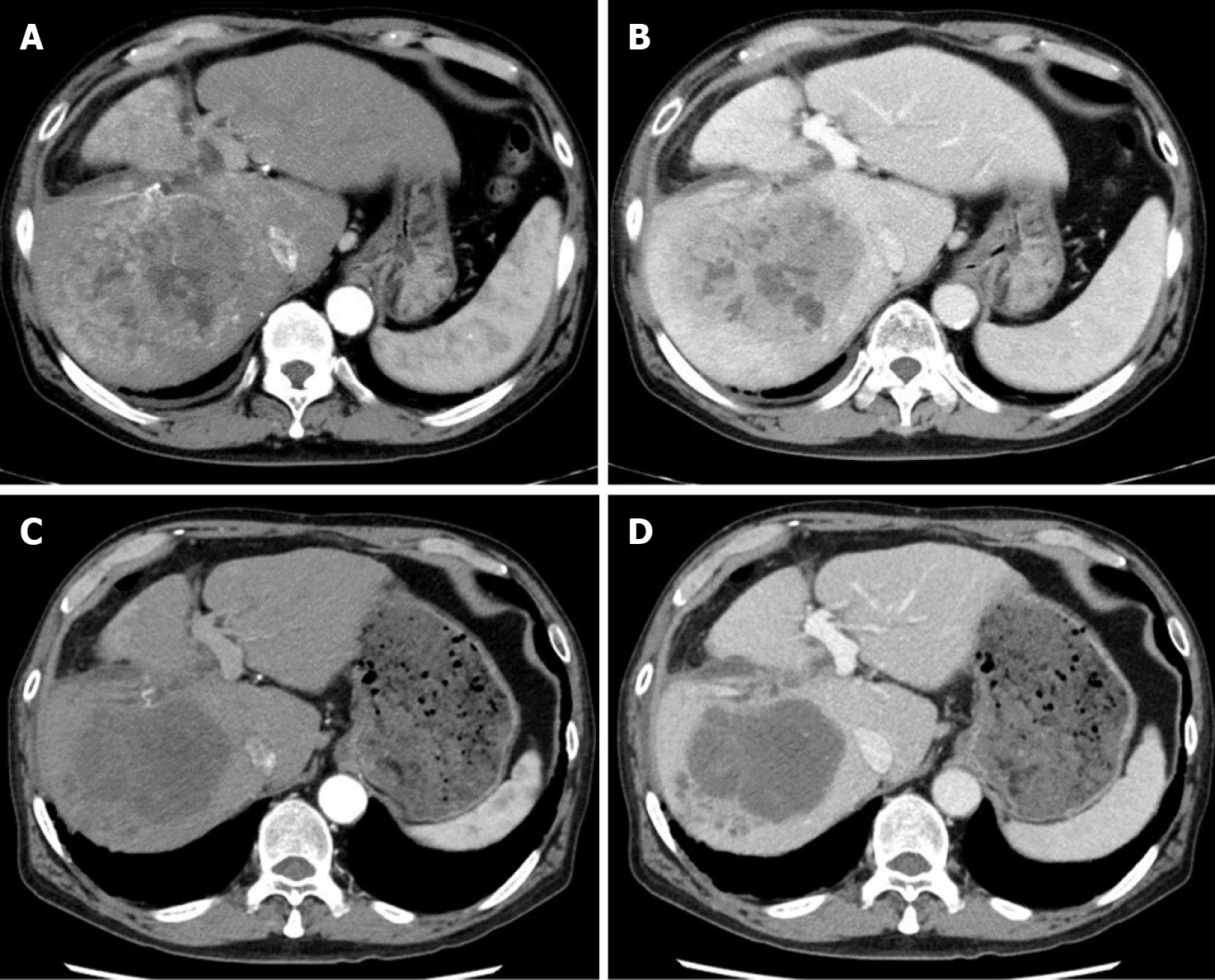
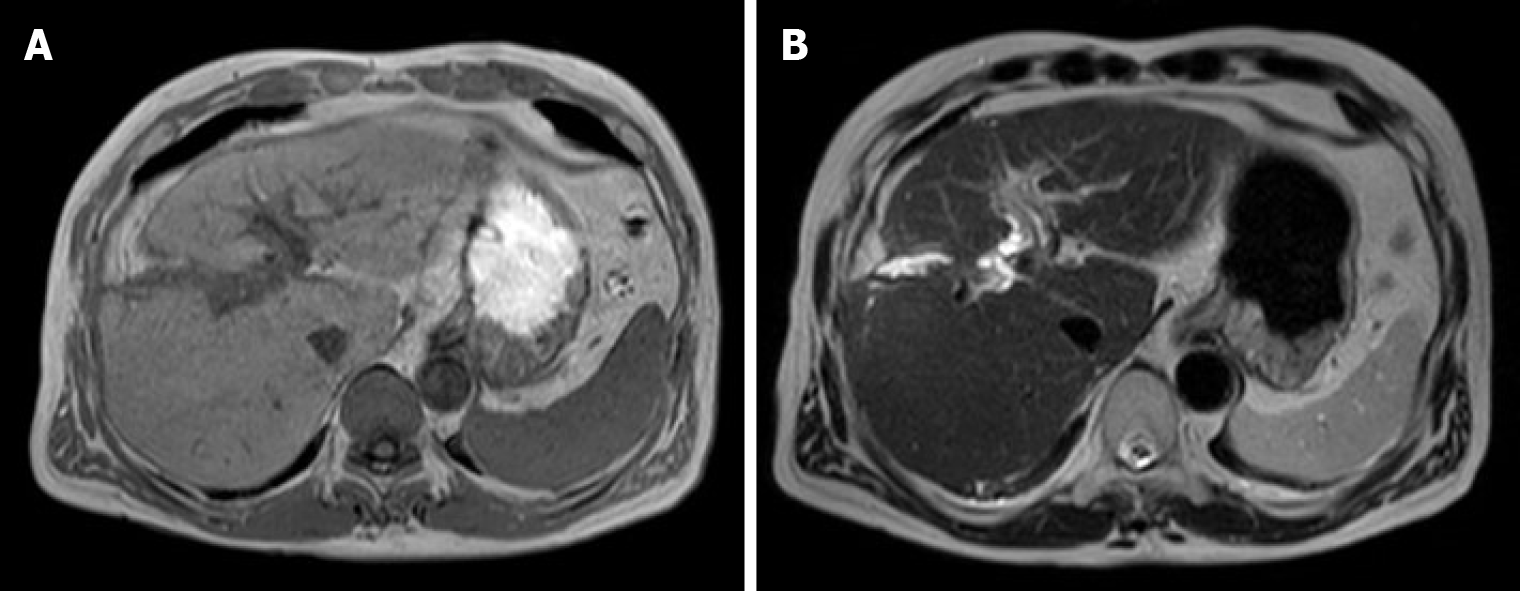
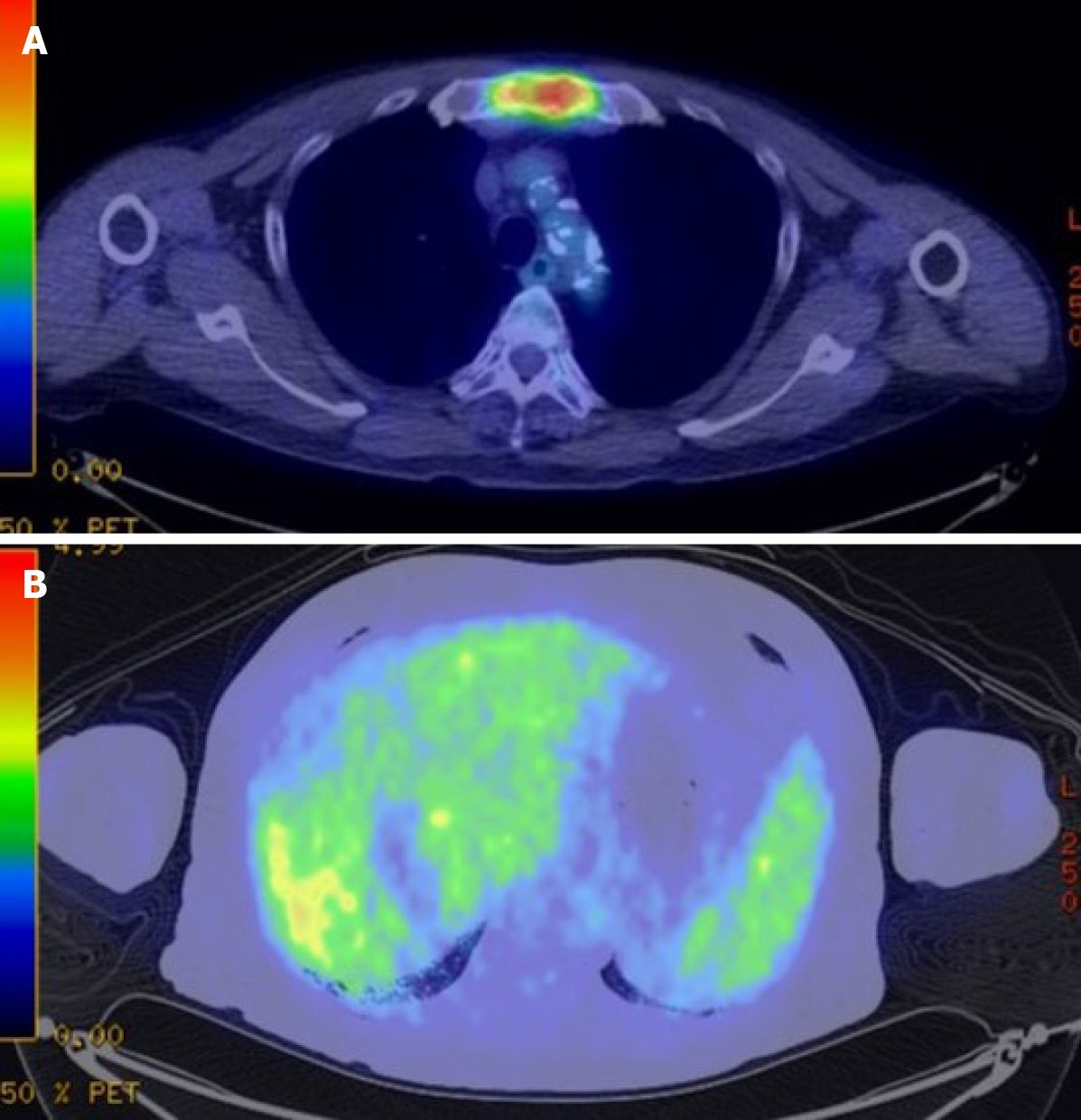
A computed tomography scan of the abdomen demonstrated a heterogeneous contrast-enhanced mass of 10 cm in size in segment 7, which had rapidly increased in 5 mo (Figure 1A and 1B). The magnetic resonance imaging (MRI) images at 5 mo before the second operation are shown in Figure 2 (A: T1 image; B: T2 image). There was no evidence of the recurrent tumor on these MRI images. A positron emission tomography examination showed elevated standardized uptake volume in the sternum and slight uptake in the liver (Figure 3A and 3B). The patient was diagnosed as recurrent HCC in segment 7 of the liver with metastasis in the sternum.
As a treatment strategy, we should administer lenvatinib at a dose of 8 mg because the patient weighed less than 60 kg to suppress the rapidly increasing intrahepatic lesion, and if the intrahepatic lesion exhibited shrinkage, we should surgically remove the lesion. The reason for choosing lenvatinib was that in addition to the intrahepatic recurrence, there was a sternal metastasis. Therefore, we considered that systemic therapy would be better than transcatheter therapy, such as B-TACE. The reason for not choosing surgical resection without prior lenvatinib was that the site of recurrence was rapidly increasing, and it was thought that new lesions might appear in other parts of the liver immediately after surgical resection. In addition, after surgical treatment, we should perform postoperative radiation therapy and treatment using molecular targeted drugs for the metastatic bone lesions.
Computed tomography imaging after 1 mo of lenvatinib administration revealed that 90% of the tumor had lost the contrast effect. The response evaluation was PR, though close to complete response by the modified Response Evaluation Criteria in Solid Tumors[16] (Figure 1C and 1D). Protein-induced vitamin K absence or antagonist II decreased to 434 mAU/mL at 2 mo after the treatment (Figure 4).
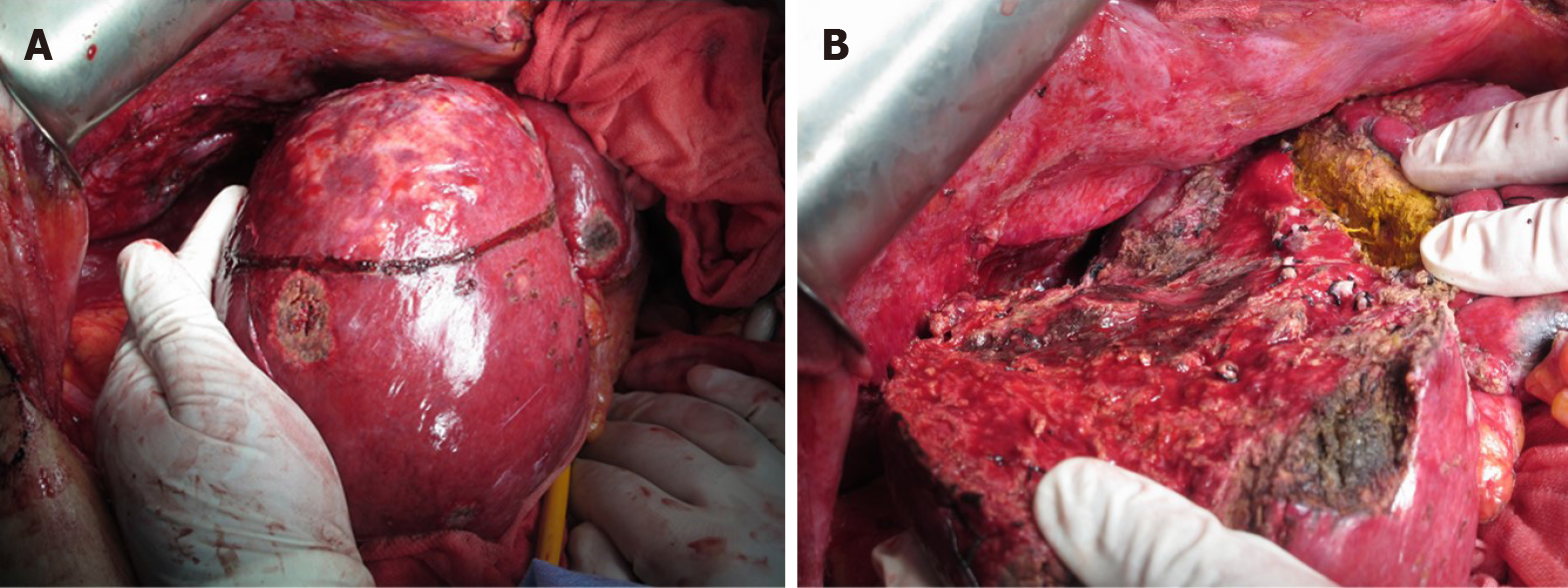
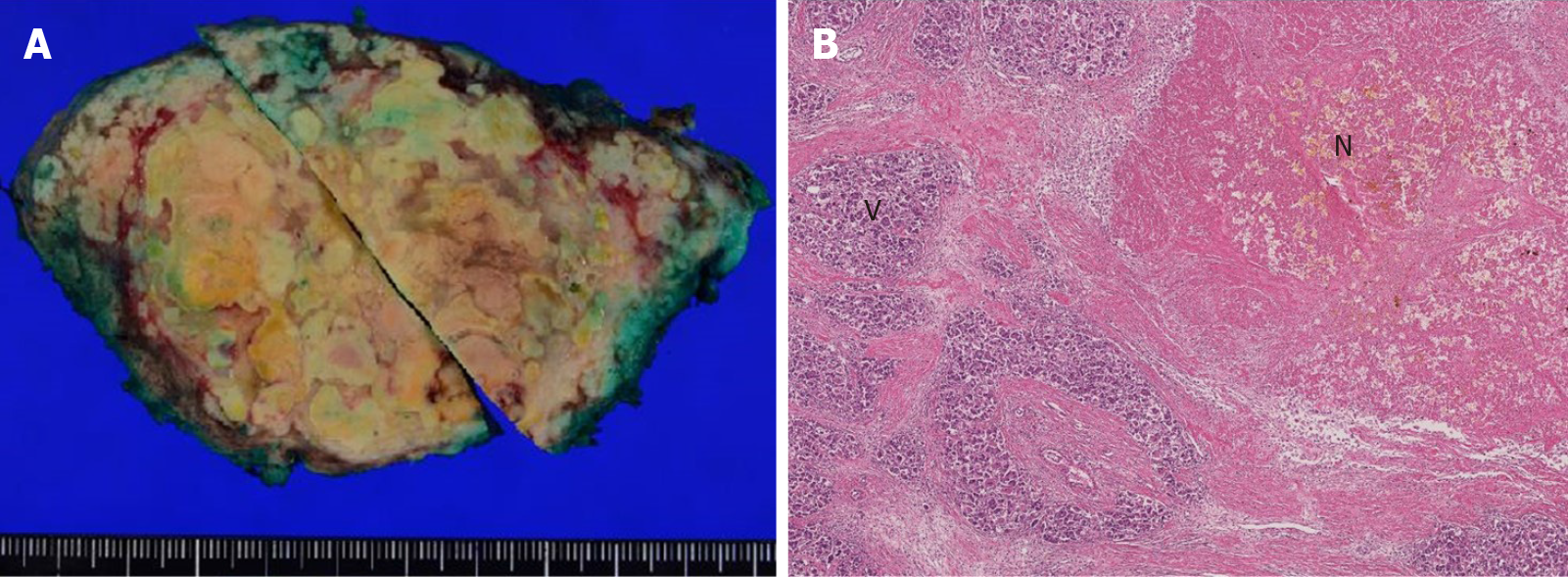
Because the intrahepatic recurrent tumor was reduced by administration of lenvatinib, hepatectomy of segment 7 was performed, with blood loss of 3913 mL and operation time of 545 min. Segments 5 and 6 were successfully preserved without bile duct injury (Figure 5). Histopathological findings showed that 80% of the tumor was necrotic, and the resected margin of the liver had no cancer component (Figure 6). The reason for choosing surgical resection after lenvatinib was that long-term administration of lenvatinib may result in decreased liver function such as decreased albumin, which can make surgery impossible. Early surgery was selected because it was unknown whether lenvatinib would result in a complete response, and it was better to aim for complete removal of the tumor by surgery.
No serious complications, such as bile leakage, were observed. The protein-induced vitamin K absence or antagonist II level decreased to the normal range after surgery (Figure 3). The patient was discharged on day 25 after the operation. Radiation therapy for bone metastases was performed by dividing 30 Gy irradiation into ten applications. At 1 mo after the end of radiotherapy, there was a small intrahepatic recurrence, and lenvatinib was immediately administered. PR was obtained, and the patient has remained alive on administration for 1 year after the second hepatectomy.
Here, we report a case in which lenvatinib was administered to a rapidly growing recurrent HCC, and conversion to hepatectomy was achieved after PR was obtained. There are previous case reports describing conversion to surgery for HCC after molecular targeted drug therapy with sorafenib and regorafenib[17,18]. The present case report describing conversion to surgery for recurrent HCC after lenvatinib treatment appears to be the first case.
Numerous papers have been published on conversion to surgery for colorectal liver metastases[19,20]. However, conversion therapy for HCC has not been established. Lenvatinib is expected to be a possible candidate agent and provides a new treatment perspective for recurrent HCC. To date, most reports have described cases of unresectable or recurrent HCC that were converted after local treatment, such as TACE and hepatic arterial infusion chemotherapy (HAIC), or after conventional anticancer drug treatment[21,22]. For example, in a report from the MD Anderson Cancer Center[23], the chemotherapy regimen was cisplatin, interferon α-1b, doxorubicin, and 5-fluorouracil, but the doses and administration schedules had two types (conventional and modified). The study compared and examined the resection rate (conversion rate to curative surgery) and survival rate between the two regimens. In the results, the 3-yr survival rates were 70% for modified chemotherapy plus surgery (n = 11) compared with 20% for modified chemotherapy alone (n = 22) and 60% for conventional chemotherapy plus surgery (n = 8) compared with 5% for conventional chemotherapy alone (n = 76). Therefore, the cases with conversion to surgery showed better survival rates for both the conventional and modified regimens. There are various other reports on conversion to surgery. Hamaoka et al[22] investigated the safety of hepatectomy and overall survival (OS) in 52 patients with unresectable advanced HCC treated with HAIC combined with three-dimensional chemoradiation therapy for portal vein tumor thrombus and compared the resection group with the nonresection group. As a result, OS was significantly higher in the resection group than in the nonresection group. A multivariate analysis identified conversion to surgery as an independent factor influencing OS.
A recent report described a case of HCC with hepatic vein tumor thrombosis protruding into the inferior vena cava that was successfully treated by surgery after second-line chemotherapy with regorafenib[24]. Administration of sorafenib was initially started for this case, but 5 wk later the lesion had increased in size. Therefore, regorafenib was subsequently given as second-line therapy and administered for 12 mo. After shrinkage of the inferior vena cava-hepatic vein tumor thrombus, conversion to surgery was successful and a better prognosis was achieved. Meanwhile, Sato et al[25] described a patient with initially unresectable HCC and an arterioportal shunt who underwent conversion hepatectomy after multidisciplinary treatment including lenvatinib[25].
Thus, it is expected that the number of conversion cases will increase with the advent of molecular targeted drugs thereby contributing to improvement in the prognosis of HCC. Similar to previous reports, our patient obtained PR for recurrent HCC after lenvatinib administration and conversion to surgery was achieved. Therefore, it is anticipated that survival can be prolonged by continuing treatment with lenvatinib for bone metastases only.
The limitation is that the administration period for the molecular targeted drug before surgery and the timing of conversion to surgery are unknown. Therefore, it is necessary to collect more cases and perform further studies to clarify these points.
In conclusion, we have reported a case of recurrent HCC in which PR was obtained and conversion to surgery was achieved after lenvatinib administration. We believe that even for unresectable advanced and recurrent HCC, hepatectomy can be performed after administration of molecular targeted therapeutic drugs such as lenvatinib and can thus contribute to improvement of the prognosis.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Eso Y, Tuo BG S-Editor: Gao CC L-Editor: Filipodia P-Editor: Liu JH
| 1. | Kokudo N, Takemura N, Hasegawa K, Takayama T, Kubo S, Shimada M, Nagano H, Hatano E, Izumi N, Kaneko S, Kudo M, Iijima H, Genda T, Tateishi R, Torimura T, Igaki H, Kobayashi S, Sakurai H, Murakami T, Watadani T, Matsuyama Y. Clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol Res. 2019;49:1109-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 394] [Article Influence: 65.7] [Reference Citation Analysis (0)] |
| 2. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2870] [Article Influence: 110.4] [Reference Citation Analysis (1)] |
| 3. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3014] [Article Influence: 430.6] [Reference Citation Analysis (3)] |
| 4. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6032] [Article Influence: 861.7] [Reference Citation Analysis (3)] |
| 5. | Luo J, Guo RP, Lai EC, Zhang YJ, Lau WY, Chen MS, Shi M. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol. 2011;18:413-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 273] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 6. | Eun JR, Lee HJ, Moon HJ, Kim TN, Kim JW, Chang JC. Hepatic arterial infusion chemotherapy using high-dose 5-fluorouracil and cisplatin with or without interferon-alpha for the treatment of advanced hepatocellular carcinoma with portal vein tumor thrombosis. Scand J Gastroenterol. 2009;44:1477-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 7. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10249] [Article Influence: 602.9] [Reference Citation Analysis (2)] |
| 8. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4646] [Article Influence: 273.3] [Reference Citation Analysis (0)] |
| 9. | Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G; RESORCE Investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2160] [Cited by in RCA: 2706] [Article Influence: 338.3] [Reference Citation Analysis (0)] |
| 10. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib vs sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3128] [Cited by in RCA: 3813] [Article Influence: 544.7] [Reference Citation Analysis (1)] |
| 11. | Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, Assenat E, Brandi G, Pracht M, Lim HY, Rau KM, Motomura K, Ohno I, Merle P, Daniele B, Shin DB, Gerken G, Borg C, Hiriart JB, Okusaka T, Morimoto M, Hsu Y, Abada PB, Kudo M; REACH-2 study investigators. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1027] [Cited by in RCA: 1243] [Article Influence: 207.2] [Reference Citation Analysis (0)] |
| 12. | Mitry E, Fields AL, Bleiberg H, Labianca R, Portier G, Tu D, Nitti D, Torri V, Elias D, O'Callaghan C, Langer B, Martignoni G, Bouché O, Lazorthes F, Van Cutsem E, Bedenne L, Moore MJ, Rougier P. Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: a pooled analysis of two randomized trials. J Clin Oncol. 2008;26:4906-4911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 410] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 13. | Hasegawa K, Saiura A, Takayama T, Miyagawa S, Yamamoto J, Ijichi M, Teruya M, Yoshimi F, Kawasaki S, Koyama H, Oba M, Takahashi M, Mizunuma N, Matsuyama Y, Watanabe T, Makuuchi M, Kokudo N. Adjuvant Oral Uracil-Tegafur with Leucovorin for Colorectal Cancer Liver Metastases: A Randomized Controlled Trial. PLoS One. 2016;11:e0162400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 14. | Foubert F, Matysiak-Budnik T, Touchefeu Y. Options for metastatic colorectal cancer beyond the second line of treatment. Dig Liver Dis. 2014;46:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzén F, Cassidy J. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2302] [Cited by in RCA: 2267] [Article Influence: 133.4] [Reference Citation Analysis (0)] |
| 16. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3353] [Cited by in RCA: 3292] [Article Influence: 219.5] [Reference Citation Analysis (36)] |
| 17. | Nakamura K, Beppu T, Hayashi H, Okabe H, Imai K, Nitta H, Chikamoto A, Ishiko T, Sasaki M, Baba H. Recurrence-free survival of a hepatocellular carcinoma patient with tumor thrombosis of the inferior vena cava after treatment with sorafenib and hepatic resection. Int Surg. 2015;100:908-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Barbier L, Fuks D, Pessaux P, Muscari F, Le Treut YP, Faivre S, Belghiti J. Safety of liver resection for hepatocellular carcinoma after sorafenib therapy: a multicenter case-matched study. Ann Surg Oncol. 2013;20:3603-3609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Tomasello G, Petrelli F, Ghidini M, Russo A, Passalacqua R, Barni S. FOLFOXIRI Plus Bevacizumab as Conversion Therapy for Patients With Initially Unresectable Metastatic Colorectal Cancer: A Systematic Review and Pooled Analysis. JAMA Oncol. 2017;3:e170278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 149] [Article Influence: 18.6] [Reference Citation Analysis (1)] |
| 20. | Morine Y, Ikemoto T, Iwahashi S, Saito YU, Yamada S, Takasu C, Higashijima J, Imura S, Shimada M. Clinical Impact of FOLFOXIRI Aiming for Conversion Surgery in Unresectable Multiple Colorectal Liver Metastasis. Anticancer Res. 2019;39:5089-5096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Kishi Y, Saiura A, Yamamoto J, Koga R, Seki M, Morimura R, Yoshioka R, Kokudo N, Yamaguchi T. Preoperative transarterial chemoembolization for hepatocellular carcinoma. Hepatogastroenterology. 2012;59:2295-2299. [PubMed] |
| 22. | Hamaoka M, Kobayashi T, Kuroda S, Iwako H, Okimoto S, Kimura T, Aikata H, Nagata Y, Chayama K, Ohdan H. Hepatectomy after down-staging of hepatocellular carcinoma with portal vein tumor thrombus using chemoradiotherapy: A retrospective cohort study. Int J Surg. 2017;44:223-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 23. | Kaseb AO, Shindoh J, Patt YZ, Roses RE, Zimmitti G, Lozano RD, Hassan MM, Hassabo HM, Curley SA, Aloia TA, Abbruzzese JL, Vauthey JN. Modified cisplatin/interferon α-2b/doxorubicin/5-fluorouracil (PIAF) chemotherapy in patients with no hepatitis or cirrhosis is associated with improved response rate, resectability, and survival of initially unresectable hepatocellular carcinoma. Cancer. 2013;119:3334-3342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Takeda K, Tsurumaru Y, Yamamoto Y, Araki K, Kogure Y, Mori K, Nakagawa K, Shimizu T, Matsuda G, Niino H, Sekido H, Kobayashi S, Morimoto M, Kunisaki C, Endo I. Treatment of hepatocellular carcinoma with hepatic vein tumor thrombosis protruding into the inferior vena cava by conversion surgery following chemotherapy with regorafenib: a case report. Clin J Gastroenterol. 2020;13:428-433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Sato N, Beppu T, Kinoshita K, Yuki H, Suyama K, Chiyonaga S, Motohara T, Komohara Y, Hara A, Akahoshi S. Conversion Hepatectomy for Huge Hepatocellular Carcinoma With Arterioportal Shunt After Chemoembolization and Lenvatinib Therapy. Anticancer Res. 2019;39:5695-5701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |