Published online Dec 27, 2020. doi: 10.4254/wjh.v12.i12.1239
Peer-review started: June 30, 2020
First decision: August 9, 2020
Revised: August 14, 2020
Accepted: October 9, 2020
Article in press: October 9, 2020
Published online: December 27, 2020
Processing time: 170 Days and 13.1 Hours
Hepatocellular carcinoma (HCC) represents the most common primitive liver malignancy. A relevant concern involves the lack of agreement on staging systems, prognostic scores, and treatment allocation algorithms.
To compare the survival rates among already developed prognostic scores.
We retrospectively evaluated 140 patients with HCC diagnosed between February 2006 and November 2017. Patients were categorized according to 15 prognostic scoring systems and estimated median survivals were compared with those available from the current medical literature.
The median overall survival of the cohort of patients was 35 (17; 67) mo, and it was statistically different in relation to treatment choice, ultrasound surveillance, and serum alpha-fetoprotein. The Italian Liver Cancer (ITA.LI.CA) tumor staging system performed best in predicting survival according to stage allocation among all 15 evaluated prognostic scores. Using the ITA.LI.CA prognostic system, 28.6%, 40.7%, 22.1%, and 8.6% of patients fell within stages 0-1, 2-3, 4-5 and > 5 respectively. The median survival was 57.9 mo for stages 0-1, 43 mo for stages 2-3, 21.7 mo for stages 4-5, and 10.4 mo for stage > 5. The 1-, 3-, and 5-year survival rates were respectively 95%, 65%, and 20%, for stages 0-1; 94.7%, 43.9% and 26.3% for stages 2-3; 71%, 25.8% and 16.1% for stages 4-5; and 50%, 16.7% and 8.3% for stage > 5. At the same time, although statistically significant in prognostic stratification, the most commonly used Barcelona Clinic Liver Cancer system showed one of the most relevant differences in median survival, especially for stages A and C, when compared to the medical literature. In fact, 10.7%, 59.3%, 27.1%, 1.4%, and 0% of patients were stratified into stages 0, A, B, C, and D respectively. The median survival was > 81.1 mo for stage 0, 44.9 mo for stage A, 21.3 mo for stage B, and 3.1 mo for stage C. The 1-, 3-, and 5-year survival rates were respectively 86.7%, 60%, and 46.7% for stage 0; 91.6%, 50.6%, and 20.5% for stage A; 73.7%, 23.7% and 13.2% for stage B; and 2%, 0% and 0% for stage C.
Survival analysis shows excellent prognostic ability of the ITA.LI.CA scoring system compared to other staging systems.
Core Tip: Italian Liver Cancer tumor staging system seems a promising prognostic score system with a good applicability and reproducibility for patients with hepatocellular carcinoma.
- Citation: Campigotto M, Giuffrè M, Colombo A, Visintin A, Aversano A, Budel M, Masutti F, Abazia C, Crocé LS. Comparison between hepatocellular carcinoma prognostic scores: A 10-year single-center experience and brief review of the current literature. World J Hepatol 2020; 12(12): 1239-1257
- URL: https://www.wjgnet.com/1948-5182/full/v12/i12/1239.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i12.1239
Hepatocellular carcinoma (HCC) is the sixth most common malignancy worldwide, and the third cause of cancer-related mortality[1]. It is the second most frequent liver malignancy following liver metastasis and the most frequent primitive liver neoplasm, accounting for more than 850000 new diagnoses each year and more than 800000 deaths[2]. Incidence and death rates are increasing steadily (about 2%-3% per year)[3,4]. HCC usually arises in patients affected by liver cirrhosis, regardless of the etiology[5,6]. As chronic liver disease represents the leading risk factor for developing HCC, ultrasound surveillance in this condition is crucial to increase early detection rates and improve the overall survival in treated patients[7,8]. Current unmet clinical needs involve proper staging, prognosis, and treatment allocation of HCC patients. Both the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver and the European Organization for Research and Treatment of Cancer recommend staging systems that take into account tumor stage, liver function, and physical status in the form of the Barcelona Clinic Liver Cancer (BCLC) staging classification[9-13]. Also, patients’ characteristics, features of the nodules, and liver function drive the choice of treatment, which might be curative (e.g., liver resection, liver transplantation, radiofrequency ablation, microwave ablation, percutaneous ethanol injection) or merely palliative (transarterial chemoembolization/radioembolization, or specific protein kinases inhibitors such as sorafenib or lenvatinib). However, since the clinical management for HCC can be challenging, treatment should be defined and individualized by a multidisciplinary team composed of hepatologists, hepatobiliary surgeons, interventional radiologists, surgical and medical oncologists.
In the last 30 years, several staging systems have been proposed for the prognosis stratification and treatment choices of HCC. The tumor node metastasis[14,15] system does not take into account patient characteristics (e.g., liver function tests), thus not allowing for an appropriate prognostic stratification, especially for patients with large tumors[16,17]; therefore, other systems have been developed. For example, the BCLC is the most widely accepted and used in clinical practice although many others in past times (i.e. Okuda Staging System[18], Cancer of the Liver Italian Program (CLIP) staging system[19], Groupe d’Etude et de Traitement du Carcinome Hépatocellulaire [GRETCH] staging system[18,20]) and more recently (i.e. Japanese Integrated Staging [JIS] score[21], Tokyo Scoring System, Hong Kong Liver Cancer [HKLC] classification[22,23], Model to Estimate Survival in Ambulatory HCC patients [MESIAH] staging score[24,25], albumin-bilirubin [ALBI] grading system[26], ALBI-based BCLC staging system, ALBI-T score[27], model to estimate survival for HCC patients [MESH] scoring system[28], NIACE score system[29] and Italian Liver Cancer Group [ITA.LI.CA] score system) allowed physicians allocate all possible presentations of HCC cases. In addition, other scores aimed toward driving treatment procedures have been developed to improve and provide more effective and customized therapy for specific groups of patients; consensus on their use, however, is still to be reached. Meaningful examples are represented by the needle and syringe program (NSP) scoring system[30], hepatoma arterial-embolization (HAP) scoring system[31], the Selection for Transarterial chemoembolization Treatment (STATE) scoring system and START strategy[32] and tumor size and number, baseline alpha-fetoprotein (AFP), Child-Pugh and objective radiological response (SNACOR) staging system[33]. The main features of the above-mentioned scoring system are reported in Table 1.
| Score system | Parameters taken into account | Classes/ levels | 1-, 2-, 3-, or 5-yr survival rates/median survival | Ref. |
| BCLC | Tumor size; presence of metastasis; portal hypertension; Child Pugh score; total bilirubin; performance status | Stage 0 (very early HCC); stage A (early HCC, subdivided into A1-A4); stage B (Intermediate HCC); stage C (advanced HCC); stage D (end-stage HCC) | 5-yr survival rates: 50%-70% for BCLC 0-A;2-yr survival rates: 63% for BCLC B; 1-yr survival rates: 82%, 44% and 11% for BCLC B, C and D respectively | Llovet et al[34], Mazzaferro et al[35], Weinmann et al[36], Barman et al[37], Yopp et al[38] |
| Okuda staging system | Tumor size (tumor > 50% of the liver; presence of ascites; albumin < 3 g/dL; bilirubin > 3 mg/dL | Stage I (0 factors); stage II (1-2 factors); stage 3 (3-4 factors) | 1-yr survival rates: 57% for stage 1, 20% for stage 2 and 3% for stage 3 respectively | Maida et al[18] |
| CLIP staging system | Tumor size; tumor morphology (uninodular, < 50%; multinodular, < 50%; massive or > 50%); Child-Turcotte-Pugh score; alpha-fetoprotein levels (< or ≥ 400 ng/mL); presence of portal vein thrombosis | One point each parameter (total score ranging from 0 to 6) | 1-yr survival rates: 86% for CLIP 0, 76% for CLIP 1, 57% for CLIP 2, 38% for CLIP 3, 22% for CLIP 4, 9% for CLIP 5 and 0% for CLIP 6 respectively; 2-yr survival rates: 69% for CLIP 0, 53% for CLIP 1, 25% for CLIP 2, 7% for CLIP 3, 10% for CLIP 4 respectively; 3-yr survival rates: 58% for CLIP 0, 39% for CLIP 1, 15% for CLIP 2, 6% for CLIP 3, 5% for CLIP 4 | [19] |
| GRETCH staging system | Serum bilirubin; alkaline phosphatase; alpha-fetoprotein; evidence of portal obstruction; Karnofsky score | Stage A (low risk); stage B (intermediate risk); stage C (high risk) | 1-yr survival rates are 79%, 31% and 4% for stage A, B and C, respectively | Maida et al[18], Cammà et al[20] |
| Japanese integrated staging score | LCSGJ TNM (presence of single mass; dimension < 2 cm absence of vessel invasion); Child-Pugh score | Total JIS score is the sum of LCSGJ TNM (I to IV are assigned 0 to 3 points) and Child Turcotte-Pugh score (A, B or C are assigned 0, 1 or 2 points) | 2-yr survival rates are 94.5%, 88.9% 78.2%, 52.7%, 30.3% and 15.3% for JIS 0 to JIS 5 | Kudo et al[21] |
| Tokyo scoring system | Serum albumin; serum bilirubin; tumor size; number of nodules, each of which is attributed a score | Total Tokyo score is the sum of: 0 points for serum albumin levels > 3.5 g/dL, serum bilirubin levels < 1 mg/dL, tumor size < 2 cm and ≤ 3 tumors; 1 point for serum albumin levels 2.8-3.5 g/dL, serum; bilirubin levels 1-2 mg/dL and tumor size 2-5 cm; 2 points for serum albumin levels < 2.8 g/dL, serum; bilirubin levels > 2 mg/dL, tumor size > 5 cm and > 3 tumors. | 1-yr survival rates: 100% for score 0, 97.6% for score 1, 94.2% for score 2, 84.6% for score 3, 73.8% for score 4-6; 2-yr survival rates: 98.1% for score 0, 90.5% for score 1, 81.7% for score 2, 70.5% for score 3, 52.4% for score 4-6; 3-yr survival rates: 96.2% for score 0, 90.5% for score 1, 63.5% for score 2, 47.4% for score 3, 33.3 % for score 4-6; 5-yr survival rates: 52.8% for score 0, 37.3% for score 1, 27.9% for score 2, 19.2% for score 3, 16.7 % for score 4-6 | Tateishi et al[54] |
| MESIAH staging score | Tumor size; number of nodules; vascular invasion; extrahepatic metastasis; age; serum albumin; AFP levels; MELD score | Each of the parameters is assigned a specific coefficient. | Along with the score is provided a tailored probability of survival at 1, 3, 6, 12, 24 and 36 mo | Kinoshita et al[24], Choi et al[25] |
| ALBI grading system | Serum bilirubin (µmol/L); serum albumin (g/L). | ALBI grade 1 corresponds to a score ≤ -2.60.ALBI grade 2 corresponds to a score > -2.60 and ≤ -1.39.ALBI grade 3 corresponds to a score > -1.39. | In European patients, the median survivals reported in the study were 24.7 mo for ALBI grade 1, 11.4 mo for ALBI grade 2 and 4.9 mo for ALBI grade 3. | Ogasawara et al[26] |
| ALBI-based BCLC staging system | The procedure to calculate the BCLC stage stays the same, but, instead of Child-Turcotte-Pugh grade A, B and C, ALBI grade 1, 2 and 3 are employed respectively. | An ALBI score 1 can be present in BCLC stage 0, A, B and C; ALBI score 2 can be present in BCLC stage A, B and C; ALBI score 3 is related to BCLC stage D | 1-yr survival rates: 91.3% for ALBI- based BCLC 0, 85.8% for ALBI- based BCLC stage A, 72.6% for ALBI- based BCLC stage B, 32.9% for ALBI- based BCLC Stage C, 26.6% for ALBI- based BCLC stage D. 2-yr survival rates: 79.7% for ALBI- based BCLC 0, 69.2% for ALBI- based BCLC stage A, 46% for ALBI- based BCLC stage B, 14.5% for ALBI- based BCLC Stage C, 15.1% for ALBI- based BCLC stage D. 3-yr survival rates: 71.5% for ALBI- based BCLC 0, 69.2% for ALBI- based BCLC stage A, 26.4% for ALBI- based BCLC stage B, 7.2% for ALBI- based BCLC Stage C, 15.1% for ALBI- based BCLC stage D. 5-yr survival rates: 50% for ALBI- based BCLC 0, 30.1% for ALBI- based BCLC stage A, 10.2% for ALBI- based BCLC stage B, 2.9% for ALBI- based BCLC Stage C, 2% for ALBI- based BCLC stage D. | Chan et al[45] |
| ALBI-T score | ALBI grade; LCSGJ TNM staging system | The final score, ranging from 0 to 5, is obtained by adding the ALBI grade to the TNM stage and then subtracting 2 | The reported median survival were 137.7 mo for ALBI-T score 0, 83.2 mo for ALBI-T score 1, 53.4 mo for ALBI-T score 2, 27.4 mo for ALBI-T score 3, 5 mo for ALBI-T score 4 and 1.4 mo for ALBI-T score 5 | Hiraoka et al[27] |
| MESH scoring system | Tumor burden (within/beyond Milan criteria); vascular invasion; metastasis; Child-Pugh score; Performance Status; serum AFP; ALP | The sum of the points obtained in the various sections leads to the final MESH score (ranging from 0 to 6). | 1-yr survival rates: 89.5% for MESH 0, 82.5% for MESH 1, 74% for MESH 2, 45.2% for MESH 3, 21.4% for MESH 4, 5.7% for MESH 5, 0% for MESH 6; 2-yr survival rates: 72.9% for MESH 0, 52.8% for MESH 1, 74% for MESH 2, 49.4% for MESH 3, 12.8% for MESH 4, 3.7% for MESH 5; 3-yr survival rates: 53.3% for MESH 0, 52.8% for MESH 1, 36% for MESH 2, 14.8% for MESH 3, 8.2% for MESH 4, 1.4% for MESH 5; 5-yr survival rates: 38.6% for MESH 0, 28% for MESH 1, 14.9% for MESH 2, 5.1% for MESH 3, 3.5% for MESH 4, 0% for MESH 5 | Liu et al[28] |
| NIACE score system | Number of nodules (N); infiltrative HCC (I); serum AFP levels (A); Child-Turcotte-Pugh grade (C); ECOG PS (E) | The sum of the points obtained in the various sections leads to the final NIACE score (ranging from 0 to 7). | The reported median survivals are 44 mo for NIACE 0, 22 mo for NIACE 1, 20 mo for NIACE 1.5, 14 mo for NIACE 2.5, 9 mo for NIACE 3, 7 mo for NIACE 4, 4 mo for NIACE 4.5, 4 mo for NIACE 5.5, 3 mo for NIACE 6 and 3 mo for NIACE 7 | Adhoute et al[29] |
| ITA.LI.CA score system | Tumor burden (assessed via the ITA.LI.CA tumor staging); performance status test; Child-Pugh score; AFP concentration | Each is assigned an amount of points that finally contribute to the total prognostic score (from 0, best prognosis, to 13, worst prognosis) | The median survival was reported to be 61 mo for patients in quartile 1 (ITA.LI.CA score ≤ 1), 38 mo for patients in quartile 2 (ITA.LI.CA score 2-3), 23 mo for patients in quartile 3 (ITA.LI.CA score 4-5) and 8 mo for patients in quartile 4 (ITA.LI.CA score > 5) | Farinati et al[46], Yoo et al[47], Borzio et al[48] |
| NSP scoring system | Tumor number (N); tumor size (S); prothrombin time (P) | The sum of the points obtained in the various sections leads to the final NSP score. Using a threshold score of 1 allows to identify 2 subgroups with different prognosis | 1-yr survival rates are 88.4% for NSP ≤ 1 and 62.7% for NSP > 1; 3-yr survival rates are 57% for NSP ≤ 1 and 16.9% for NSP > 1; 5-yr survival rates are 30.2% for NSP ≤ 1 and 20.4% for NSP > 1 | Zhang et al[30] |
| HAP scoring system | Serum levels of albumin; serum AFP; bilirubin; maximum tumor diameter; 1 point is assigned for serum albumin levels < 3.6 g/dL, serum AFP > 400 ng/dL, serum bilirubin > 0.99 mg/dL (17 mmol/L) and for a maximum tumor diameter > 7 cm | HAP A (low risk) for a total score 0, -HAP B (intermediate risk) for a total score 1; HAP C (high risk) for a total score 2; HAP D (very high risk) for a total score > 2 | 1-yr survival rates: 64.7% for HAP A, 50% for HAP B, 38.5% for HAP C, 25% for HAP D; 2-yr survival rates: 17.6% for HAP A. 10.3% for HAP B, 10.3% for HAP C, 10% for HAP D | Kadalayil et al[31] |
| STATE scoring system and START strategy | Up-to-7 criteria; serum albumin level; C reactive protein values. A neoplasia within Up-to-7 criteria is assigned 0 points, while a neoplasia beyond the criteria subtracts 12 points.C reactive protein values < 1 mg/dL are attributed 0 points, whereas values ≥ 1 mg/dL subtract 12 points | 2 groups of patients presenting different prognosis were identified: STATE score < 18 and ≥ 18 | Median survival of 20.5 mo for patients with a STATE score ≥ 18. Median survival of 6.1 mo for patients with a score < 18 | Hucke et al[32] |
| SNACOR staging system | Tumor size (S); tumor number (N); baseline AFP (A); Child-Turcotte-Pugh class (C); objective radiological response (OR). No points are assigned for tumors < 5 cm, a number of tumors < 4, a baseline AFP < 400 ng/mL, a Child-Turcotte-Pugh class A and for complete response or partial response after TACE. 1 point is assigned for tumors ≥ 5 cm and for a Child-Turcotte-Pugh class B; 2 points are assigned for a number of tumors ≥ 4; 3 points are assigned for a baseline AFP ≥ 400 ng/ml and for stable disease or progressive disease after TACE | The final SNACOR score is the sum of the points obtained for the previous features and ranges from 0 to 10 | 1-yr survival rates: 80.9% for SNACOR 0-2, 69.4% for SNACOR 3-6, 40% for SNACOR 7-10; 2-yr survival rates: 55.3% for SNACOR 0-2, 38.9% for SNACOR 3-6, 20% for SNACOR 7-10; 3-yr survival rates: 42.6% for SNACOR 0-2, 26.4% for SNACOR 3-6, 6.7% for SNACOR 7-10; 5-yr survival rates: 24.5% for SNACOR 0-2, 16%% for SNACOR 3-6, 3.3% for SNACOR 7-10 | Mähringer-Kunz et al[33] |
Proposed in 1999 and updated in 2003, the BCLC staging classification analyzes tumor size, presence of metastasis, portal hypertension, Child-Turcotte-Pugh score, total bilirubin and performance status, stratifying patients into five groups: Stage 0 (very early HCC), stage A (early HCC) which is divided into four subgroups A1-A4; stage B (intermediate HCC); stage C (advanced HCC); stage D (end-stage HCC). The recommended therapy changes according to the stage: Surgical resection is indicated from stage 0 to A2, liver transplant or local ablation procedures from stage A2 to A4, transarterial chemoembolization (TACE) for stage B, sorafenib for stage C, and supportive care for stage D[34-36]. The median survival for the various stages is over 60 mo for BCLC 0-A, 20 mo for BCLC B, 11 mo for BCLC C and less than 3 mo for BCLC D. Despite its widespread application, the BCLC staging classification has some limitations, especially the strictness in treatment recommendation and the fact that it includes considerably heterogeneous populations in the same stage (principally stage B and C)[37,38]. Because of the heterogeneity of patients in the intermediate stage (B) of BCLC, several authors have attempted to create subclassifications within this stage to provide more precise prognostic information and allow a more tailored therapeutic approach. In 2012, Bolondi et al[39] proposed a four-class substaging from B1 to B4, based on characteristics such as Child-Turcotte-Pugh score, beyond Milan and up-to-7 criteria, Eastern Cooperative Oncology Group (ECOG) PS and portal vein thrombosis[35], thus modifying treatment approach according to BCLC scheme[39]. In 2014, the staging system proposed by Bolondi et al[39] was validated in an Asian population-based study. A year later, the Japanese Society of Transcatheter Hepatic Arterial Embolization (JSTHAE) proposed an alternative subclassification of BCLC stage B, based only on Child-Turcotte-Pugh score and the 4-of-7 cm criterion (total of ≤ 4 tumor nodule, with maximum diameter ≤ 7 cm)[40-42]. During the same year, researchers from the Kindai University developed other substaging criteria, which appear to perform appropriately; however, external validation is needed[43]. Another subclassification for intermediate HCC based on the one proposed by Bolondi et al[39] was designed by a Taiwanese group in 2015; however, it has not been validated in Western cohorts of patients. In 2016, a study was conducted to assess whether the ALBI grade could substitute the Child-Turcotte-Pugh score in the BCLC staging system. Concerning the prediction of the clinical outcome of HCC, the ALBI grade performed similarly to the Child-Turcotte-Pugh score when integrated into the BCLC staging system[44,45]. A few months later, the ITA.LI.CA study group developed and validated its own prognostic system, trying to overcome the shortcomings of previous scores. In particular, 5183 Italian HCC patients (mainly hepatitis C virus infected patients with good performance status and compensated cirrhosis) from the ITA.LI.CA dataset were included in the analysis for internal validation, while other 2651 patients from Taipei (mainly chronic hepatitis B virus infected patients) were recruited for external validation to test the general application of the system. The ITA.LI.CA prognostic system features parameters such as tumor burden (assessed via the ITA.LI.CA tumor staging), performance status test, Child-Turcotte-Pugh score and AFP concentration, and each is assigned a number of points that finally contribute to the total prognostic score (from 0, best prognosis, to 13, worst prognosis). The ITA.LI.CA tumor staging system, taking into account features such as the diameter of the largest nodule, the number of nodules, vascular invasion or metastasis, classifies patients in stages: 0 (very early), A (early), B (intermediate, divided into B1, B2, and B3) and C (advanced). The median survival was reported to be 61 mo for patients with ITA.LI.CA score ≤ 1, 38 mo for patients with ITA.LI.CA scores 2-3, 23 mo for patients with scores 4-5 and 8 mo for patients with more than 5 points. In the validation cohorts, the ITA.LI.CA score proved to have the best discriminatory ability among other staging systems such as BCLC, CLIP, JIS, HKLC, and MESIAH[46]. Compared to the BCLC classification, the ITA.LI.CA prognostic system allows a more thorough analysis of tumor burden, subclassifying intermediate patients into three groups (B1, B2, B3) rather than grouping them as stage B. Furthermore, the ITA.LI.CA prognostic system differentiates patients with intrahepatic or extrahepatic metastasis, who studies proved to have different prognosis[47]. Finally, external and independent validation studies proved ITA.LI.CA to offer the best predictive ability in terms of calibration, discriminatory ability, and monotonicity of gradients in both treated and untreated patients[13,48].
A total of 140 patients diagnosed with HCC and treated at our Liver Clinic (University Hospital of Trieste) between February 2006 and November 2017, were retrospectively enrolled. Follow-up was censored on June 30, 2018. The following variables were analyzed before the first active treatment: Gender, age, etiology of liver disease, presence of portal vein thrombosis and ascites, Child-Turcotte-Pugh classification, Model for End-Stage Liver Disease score, Karnofsky score, and ECOG PS score. Laboratory tests conducted featured serum levels of albumin, total and direct bilirubin, aspartate aminotransferase and alanine aminotransferase, alkaline phosphatase, gamma-glutamyl transferase, total proteins, creatinine, hemoglobin, sodium, potassium, white blood cells, red blood cells, platelets, international normalized ratio and activated partial thromboplastin time. The diagnosis of HCC was based on typical imaging features of HCC in computed tomography or magnetic resonance imaging. Liver biopsy was the technique of choice for diagnosing in case previous imaging studies did not allow diagnostic certainty. Imaging was further employed to obtain information on the number of lesions, tumor diameter, presence of metastasis, and Milan and up-to-7 criteria fulfillment. Depending on their characteristics, patients underwent different therapeutic procedures: Surgical resection, radiofrequency ablation, transarterial chemoembolization (TACE), systemic therapy (sorafenib), or supportive care. Patients were then classified according to different prognostic systems, namely ITA.LI.CA, BCLC (and its subclassifications), CLIP, JIS, HKLC, Tokyo score, Okuda, GRETCH, NIACE, MESH, ALBI (and scores derived from it), HAP, STATE, SNACOR, NSP.
Continuous variables were reported as median (interquartile range) according to the results of the Shapiro-Wilk test. Discrete variables were reported as number (percentage). Between-group comparisons of discrete variables were per- formed using Pearson’s Chi-square test and those of continuous variables using Mann-Whitney U test. The overall survival was defined as the time from the date of diagnosis of HCC to the date of death or data censoring (June 30, 2018). Kaplan-Meier survival curves were employed to estimate the median overall survival, and the log-rank test was used to compare differences in survival. All statistical analyses were performed using SPSS version 20 (IBM Corp., Armonk, NY, United States).
Patients’ clinical, laboratory, radiological characteristics and treatment choice are summarized in Table 2. The median overall survival was 35 (17; 67) mo, and it was statistically different in relation to treatment choice, ultrasound surveillance and serum AFP (Table 2).
| Feature of interest | Study population, n = 140 | Intergroup statistical significance |
| Gender | ||
| Male | 109 (77.9%) | |
| Female | 31 (22.1%) | |
| Age at diagnosis, yr | 71.6 (65.6; 75.6) | |
| Liver disease etiology | ||
| Viral | 36 (25.7%) | |
| Alcoholic | 30 (21.4%) | |
| Metabolic | 19 (13.6%) | |
| Mixed | 55 (39.3%) | |
| Laboratory parameters at diagnosis | ||
| Albumin, g/dL | 1.12 (0.94-2.23) | |
| INR | 1.12 (0.94-2.23) | |
| Total bilirubin, mg/dL | 1.06 (0.37-14.47) | |
| AST, UI/L | 41 (11-511) | |
| ALT, UI/L | 32 (7-336) | |
| ALP, UI/L | 99 (40-529) | |
| GGT, UI/L | 69 (11-473) | |
| Total serum proteins, g/dL | 7.3 (5.1-8.9) | |
| AFP, ng/mL | 9.3 (5-110) | |
| Creatinine, mg/dL | 0.89 (0.5-2.99) | |
| White blood cells, × 103 cells/µL | 5.04 (1.51-12.18) | |
| Red blood cells, × 106 cells/µL | 4.34 (2.85-6.78) | |
| Hemoglobin, g/dL | 13.5 (8.7-17.8) | |
| Platelets, × 103platelets/µL | 113 (29-346) | |
| Sodium, mmol/L | 139 (128-145) | |
| Potassium, mmol/L | 4.24 (3.40-6.15) | |
| Clinical characteristics at diagnosis | ||
| Ascites | 11 (7.9%) | |
| Portal hypertension | 64 (45.7%) | |
| Hepatic encephalopathy | 10 (7.1%) | |
| Portal vein thrombosis | 10 (7.1%) | |
| Metastasis | 2 (2.4%) | |
| Child-Turcotte-Pugh | ||
| Class A | 116 (82.9%) | |
| Class B | 22 (15.7%) | |
| Class C | 2 (1.4%) | |
| MELD score | 9 (6-25) | |
| Karnofsky score | ||
| 100 | 136 (97.1%) | |
| 90 | 3 (2.1%) | |
| 80 | 0 (0%) | |
| 70 | 1 (0.7%) | |
| < 70 | 0 (0%) | |
| ECOG PS | ||
| 0 | 137 (97.9%) | |
| 1 | 3 (2.1%) | |
| > 1 | 0 (0%) | |
| Number of nodules at diagnosis | ||
| 1 | 91 (65%) | |
| 2 | 31 (22.1%) | |
| 3 | 7 (5%) | |
| 4 | 5 (3.6%) | |
| 5 | 6 (4.3%) | |
| Nodule dimensions | ||
| Nodule diameter, mm | 30 (20; 40) | |
| Total tumor volume, cm3 | 14.13 (5.45-36.43) | |
| Milan criteria | ||
| Within | 99 (71.2%) | |
| Beyond | 40 (28.8%) | |
| Up-to-7 criteria | ||
| Within | 113 (81.3%) | |
| Beyond | 26 (18.7%) | |
| Treatment | ||
| Type | ||
| Surgical resection | 28 (20%) | |
| Local ablation | 49 (35%) | |
| TACE | 54 (38.6%) | |
| Sorafenib | 2 (1.4%) | |
| Support | 7 (5%) | |
| Number | ||
| < 2 | 63 (45%) | |
| ≥ 2 | 77 (55%) | |
| Response at 1 mo after treatment | ||
| Complete response | 72 (51.4%) | |
| Of whom treated with curative treatment | 56 (77.7%) | |
| Partial response | 40 (28.6%) | |
| Of whom treated with curative treatment | 17 (42.5%) | |
| Stable disease | 14 (10%) | |
| Of whom treated with curative treatment | 1 (7.1%) | |
| Disease progression | 10 (10%) | |
| Of whom treated with curative treatment | 1 (10%) | |
| Ultrasound surveillance every 6 mo | ||
| Adhesion to ultrasound surveillance | ||
| Under surveillance | 81 (57.9%) | |
| Not under surveillance | 59 (42.1%) | |
| Nodule diameter, mm | P < 0.001 | |
| Under surveillance | 25 (20; 35) | |
| Not under surveillance | 34 (25; 45) | |
| Number of nodules at diagnosis | P < 0.001 | |
| Under surveillance, < 2 nodules | 69 (85.2%) | |
| Not under surveillance, < 2 nodules | 22 (37.3%) | |
| Choice of curative treatment | P = 0.037 | |
| Under surveillance | 54 (66.6%) | |
| Not under surveillance | 29 (49.2%) | |
| Survival time, mo | ||
| Overall survival | 35 (17;67) | |
| Survival related to gender | NS | |
| Male | 34 (20; 80) | |
| Female | 35 (16; 64) | |
| Survival related to etiology | NS | |
| Viral | 32 (15; 65) | |
| Non-viral | 41 (19; 67) | |
| Survival related to treatment choice | P = 0.013 | |
| Curative (surgery/ablation) | 48 (18; 68) | |
| Non-curative (TACE/sorafenib/support) | 23 (14; 34) | |
| Survival related to ultrasound surveillance | P = 0.002 | |
| Under surveillance | 48 (20; 75) | |
| Not under surveillance | 30 (12; 49) | |
| Survival related to AFP | P < 0.001 | |
| AFP ≤ 200 ng/mL | 55 (34; 75) | |
| AFP > 200 ng/mL | 22 (12; 54) |
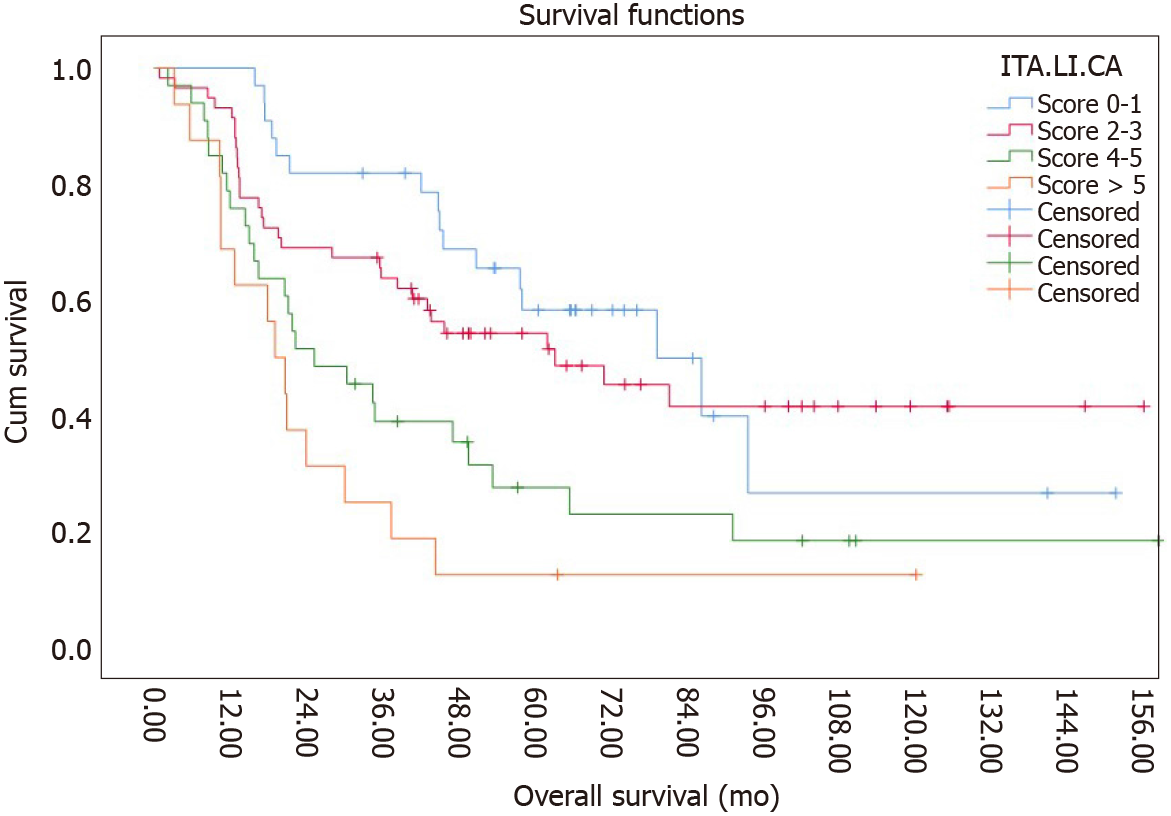
Using the ITA.LI.CA prognostic system, 28.6%, 40.7%, 22.1% and 8.6% of patients fell within stages 0-1, 2-3, 4-5 and > 5 respectively. The median survival was 57.9 mo for stages 0-1, 43 mo for stages 2-3, 21.7 mo for stages 4-5 and 10, 4 mo for stage > 5. 1-, 3-, and 5-year survival rates were 95%, 65% and 20% for stage 0-1, 94.7%, 43.9% and 26.3% for stage 2-3, 71%, 25.8% and 16.1% for stage 4-5 and 50%, 16.7% and 8.3% for stage >5. The Kaplan-Meier curves are shown in Figure 1.
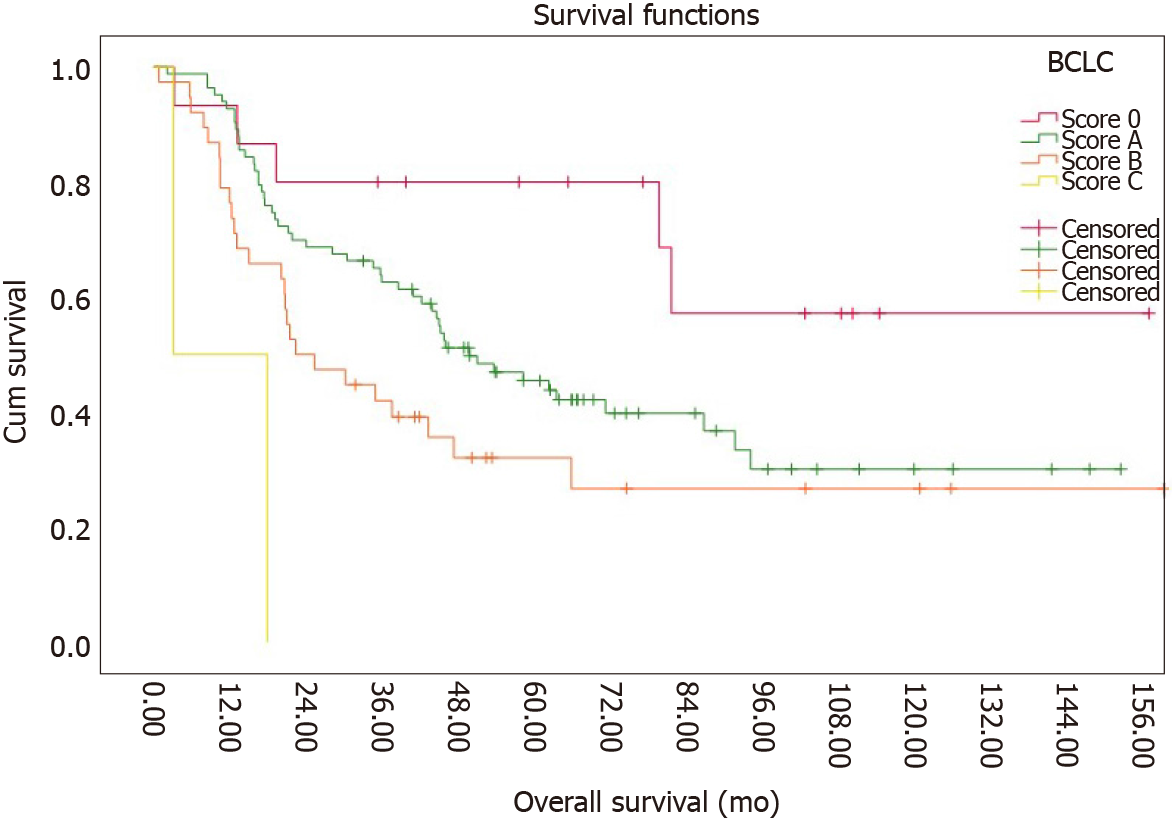
Using the BCLC staging system 10.7%, 59.3%, 27.1%, 1.4% and 0% of patients fell within stages 0, A, B, C and D respectively. The median survival was > 81, 1 mo for stage 0, 44, 9 mo for stage A, 21, 3 mo for stage B and 3, 1 mo for stage C. 1-, 3-, and 5-year survival rates were 86.7%, 60% and 46.7% for stage 0, 91.6%, 50.6% and 20,5% for stage A, 73.7%, 23.7% and 13.2% for stage B and 2%, 0% and 0% for stage C. The Kaplan-Meier curves are shown in Figure 2.
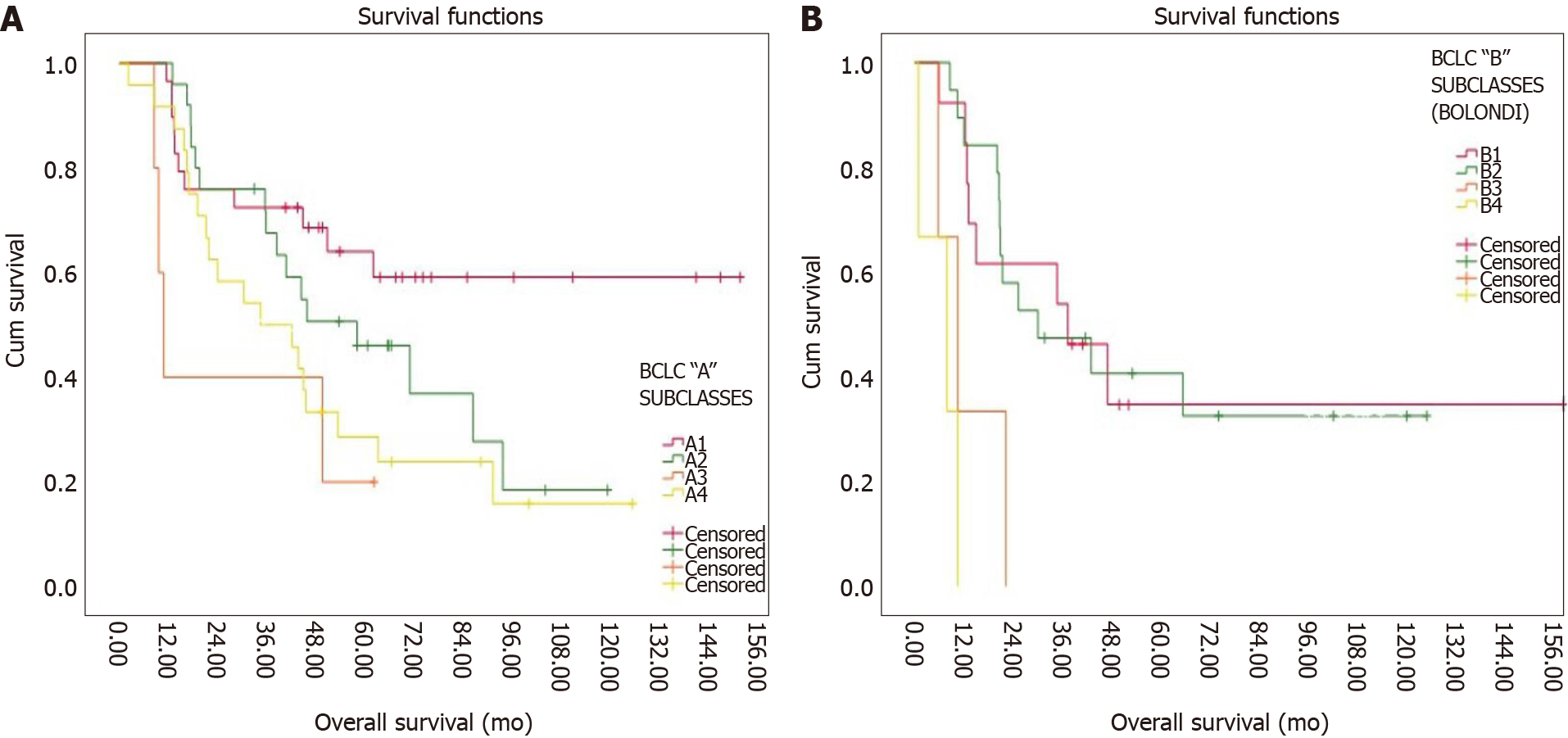
With BCLC stage A substaging 29 (35%), 25 (30.1%), 5 (6%) and 24 (28.9%) patients fell within stages A1, A2, A3, and A4 respectively. The median survival, 1-, 3-, and 5-year survival rates are shown in Table 3, while Kaplan-Meier curves are shown in Figure 3A. With Bolondi’s intermediate BCLC subclassification, 13 (34.2%), 19 (50%), 3 (7.9%), and 3 (7.9%) patients fell within stages B1, B2, B3, and B4 respectively. The median survival 1-, 3-, and 5-year survival rates are listed in Table 3, while the Kaplan-Meier curves are shown in Figure 3B.
| Score | Number of patients | Percentage | Median survival in mo | Statistical significance for prognostic stratification | Median survival in the original study in mo |
| ITA.LI.CA | P < 0.001 | ||||
| 0 | 7 | 5% | 93.5 | ||
| 1 | 33 | 23.6% | 57.9 | ||
| 2 | 19 | 13.6% | 63.1 | ||
| 3 | 38 | 27.1% | 40.6 | ||
| 4 | 20 | 14.3% | 25.2 | ||
| 5 | 11 | 7.8% | 21.1 | ||
| 6 | 5 | 3.6% | 20.8 | ||
| 7 | 4 | 2.9% | 10.3 | ||
| 8 | 3 | 2.1% | 4.3 | ||
| > 8 | 0 | 0% | |||
| ITA.LI.CA | |||||
| 0-1 | 40 | 28.6% | 57.9 | 57-61 | |
| 2-3 | 57 | 40.7% | 43 | 43-48 | |
| 4-5 | 31 | 22.1% | 21.7 | 23 | |
| > 5 | 12 | 8.6% | 10.4 | 9-8 | |
| BCLC | P = 0.001 | ||||
| 0 | 15 | 10.7% | > 81.1 | > 60 | |
| A | 83 | 59.3% | 44.9 | > 60 | |
| B | 38 | 27.1% | 21.3 | 20 | |
| C | 2 | 1.4% | 3.1 | 11 | |
| D | 0 | 0% | < 3 | ||
| BCLC A | P = 0.022 | ||||
| A1 | 29 | 20.7% | 61.9 | 43.4 | |
| A2 | 25 | 17.6% | 44.3 | 28.9 | |
| A3 | 5 | 3.5% | 10.7 | 25.4 | |
| A4 | 24 | 17.1% | 34.4 | 22.3 | |
| BCLC B (Bolondi) | P = 0.007 | ||||
| B1 | 13 | 9.3% | 34.7 | 31.9 | |
| B2 | 19 | 15.7% | 25.2 | 26.9 | |
| B3 | 3 | 0.7% | 10.4 | 13.5 | |
| B4 | 3 | 1.4% | 7.8 | 10.9 | |
| CLIP | P = 0.001 | 32 | |||
| 0 | 59 | 42.1% | 50.7 | 27 | |
| 1 | 47 | 33.6% | 53.3 | 15 | |
| 2 | 19 | 13.6% | 20.5 | 9 | |
| 3 | 12 | 8.6% | 17.8 | 7 | |
| 4 | 3 | 2.1% | 3.1 | 5 | |
| > 4 | 0 | 0% | 3 | ||
| JIS | P = 0.049 | ||||
| 0 | 27 | 19.3% | 70.8 | 22.6 | |
| 1 | 66 | 47.1% | 44.3 | 22 | |
| 2 | 40 | 28.6% | 42 | 20.6 | |
| 3 | 7 | 5% | 10.4 | 16.9 | |
| 4-5 | 0 | 0% | 12.1-5.9 | ||
| HKLC | P < 0.001 | ||||
| 1 | 93 | 67.4% | 47 | 79.7 | |
| 2a | 10 | 7.3% | 19 | 33.4 | |
| 2b | 18 | 13% | 34.7 | 32.7 | |
| 3a | 5 | 3.6% | 10.4 | 12.5 | |
| 3b | 10 | 7.3% | 20.8 | 5.5 | |
| 4a | 1 | 0.7% | 17.8 | 3.9 | |
| 4b | 1 | 0.7% | 3.1 | 1.9 | |
| 5 (a/b) | 0 | 0% | 32.5/1.6 | ||
| Tokyo | P = 0.002 | ||||
| 0 | 10 | 7.1% | 93.5 | ||
| 1 | 48 | 34.3% | 47 | ||
| 2 | 41 | 29.3% | 43.6 | ||
| 3 | 21 | 15% | 30.3 | ||
| 4 | 14 | 10% | 20.8 | ||
| 5 | 4 | 2.9% | 10.4 | ||
| 6 | 1 | 0.7% | 10.3 | ||
| 7 | 0 | 0% | |||
| 8 | 1 | 0.7% | 0.8 | ||
| Okuda | P = 0.026 | ||||
| 1 | 102 | 72.9% | 45.5 | 15.8 | |
| 2 | 36 | 25.7% | 20.5 | 3.6 | |
| 3 | 2 | 1.4% | 0.8 | 1.3 | |
| GRETCH | P < 0.001 | ||||
| A | 75 | 53.6 % | 57.6 | 29.3 | |
| B | 62 | 44.3 % | 30 | 7.4 | |
| C | 3 | 2.1 % | 7.8 | 2.1 | |
| NIACE | P = 0.001 | ||||
| 0 | 77 | 55 % | 45.7 | 44 | |
| 1 | 10 | 7.1 % | 43 | 22 | |
| 1.5 | 39 | 27.9 % | 21.7 | 20 | |
| 2.5 | 5 | 3.6 % | 10.4 | 14 | |
| 3 | 6 | 4.3 % | 16.5 | 9 | |
| 4 | 3 | 2.1 % | 3.1 | 7 | |
| > 4 | 0 | 0 % | 4 | ||
| MESH | P < 0.001 | ||||
| 0 | 40 | 28.6 % | 57.9 | 66 | |
| 1 | 46 | 32.9 % | 43 | 37 | |
| 2 | 30 | 21.4 % | 19.5 | 21 | |
| 3 | 19 | 13.6 % | 20.8 | 10 | |
| 4 | 5 | 3.5 % | 10.4 | 5 | |
| > 4 | 0 | 0 % | 4 | ||
| ALBI | P = 0.008 | ||||
| 1 | 43 | 31.9 % | 79.2 | 24.7 | |
| 2 | 87 | 64.4 % | 34.7 | 11.4 | |
| 3 | 5 | 3.7 % | 15.7 | 4.9 | |
| ALBI | P = 0.008 | ||||
| 2a | 53 | 39.2 % | 44.3 | 14.5 | |
| 2b | 34 | 25.2 % | 25.2 | 6.6 | |
| BCLC based on ALBI | P = 0.048 | ||||
| 0 | 15 | 10.9 % | > 81.1 | ||
| A | 75 | 54.3 % | 44.9 | ||
| B | 20 | 14.5 % | 22.2 | ||
| C | 1 | 0.7 % | 3.1 | ||
| D | 27 | 19.6 % | 21.7 | ||
| ALBI-T | P = 0.002 | ||||
| 0 | 12 | 9 % | 93.5 | 137.7 | |
| 1 | 42 | 31.6 % | 63.1 | 83.2 | |
| 2 | 49 | 36.8 % | 42 | 53.4 | |
| 3 | 28 | 21.1 % | 21.3 | 27.4 | |
| 4 | 2 | 1.5 % | 0.8 | 5 | |
| 5 | 0 | 0 % | 1.4 | ||
| HAP | P = 0.004 | ||||
| A | 31 | 22.2 % | 45.7 | 25.5 | |
| B | 51 | 36.4 % | 45.7 | 18.1 | |
| C | 41 | 29.3 % | 35.7 | 8.9 | |
| D | 17 | 12.1 % | 20.6 | 5.9 | |
| STATE | P = 0.322 | 20.5 (≥ 18 points) | |||
| > 37 | 8 | 5.7 % | 25.2 | ||
| 27-37 | 17 | 12.1 % | 40.6 | ||
| 18-27 | 16 | 11.4 % | 44.9 | ||
| < 18 | 13 | 9.3 % | 20 | ||
| Median STATE score | 29.1 (range: 2.4 – 45.6) | 6.1 | |||
| SNACOR | P = 0.09 | ||||
| 0-2 | 31 | 22.1 % | 25.2 | 31.5 | |
| 3-6 | 17 | 12.1 % | 19 | 19.9 | |
| 7-10 | 1 | 0.7 % | 10.3 | 9.2 | |
| NSP | P = 0.03 | ||||
| 0 | 63 | 45 % | 79.2 | ||
| 1 | 49 | 35 % | 42 | ||
| 2 | 11 | 7.9 % | 14.9 | ||
| 3 | 10 | 7.1 % | 20 | ||
| 4 | 4 | 2.9 % | 22.2 | ||
| 5 | 3 | 2.1 % | 5.6 | ||
| NSP | P = 0.002 | ||||
| 0-1 | 13 | 9.3 % | 47 | 51.5 | |
| > 1 | 25 | 17.9 % | 20.5 | 17.3 |
Median survivals within different stages and 1-, 3- or 5-year survivals for CLIP scoring system, JIS scoring system, HKLC scoring system, Okuda classification, GRETCH scoring system, NIACE scoring system, MESH scoring system, ALBI score, STATE scoring system, SNACOR staging system, NSP staging system are listed in Table 3. The best prognostic performance was achieved by the ITA.LI.CA score (P < 0.001), followed by HKLC, GRETCH, BCLC and CLIP (P = 0.001); the other showed less accuracy, with STATE and SNACOR staging systems showing no intergroup differences (P = 0.322 and P = 0.09 respectively). Also, the comparison between the median survival expected from the original studies and median survival in the study population according to the different scores is also shown in Table 3.
The main aim of this study was to assess the prognostic efficacy of different staging systems in the local patient population. Fifteen staging systems were analyzed and subsequently compared to data available from the current literature, showing considerably heterogeneous performances ranging from significant prognostic stratification and comparable median survivals to statistical insignificance and differences in overall survival. The most relevant differences were found for the BCLC, CLIP, JIS, HKLC, Okuda, and GRETCH staging systems and for the ALBI grade, as reported in Table 3.
Despite the unequivocal statistical significance in prognostic stratification of the CLIP and GRETCH staging systems in the study population, the original studies reported substantially shorter survival for almost every stage, although they were validated in European cohorts. However, the reason behind this difference might be related to the advances in treatment for HCC that took place over time since the 1992 and 1994, when the studies were censored. Despite being statistically significant in the study population, the original studies for the Okuda, JIS, and HKLC staging systems reported notably different median overall survival rates. In this case, although the JIS staging system was proven effective by some studies also for Western patients, the explanation is likely to be found in the patient population recruited for the analysis, since validation was performed using only Eastern cohorts along with other factors such as prevalent etiology and different treatment protocols. Moreover, the worse median survival from the original study for the Okuda staging system can be justified by the higher efficacy that therapeutic procedures have reached since 1984. The shorter median survival of patients from the ALBI original study can be explained by the European population employed as the reference, for all the patients had advanced HCC and were treated with sorafenib. Furthermore, if the study population’s median survivals are compared with those of the Japanese population of the study, that also included patients who underwent surgical resection, the differences appear much less significant. Despite the difference in survival, however, the ALBI grade showed statistical significance in the study population.
The median survival from the BCLC staging system clearly differs for stages A (and BCLC stage A subclassification) and C in the study population. The difference in survival for stage A might be explained with the heterogeneity in treatment that these patients received in the study population, while the reason for the difference in stage C is to be found in the low number of patients falling within this category in the study population. Nevertheless, BCLC stage B showed similar survivals, and so happened also for the BCLC intermediate subclassification according to Bolondi. The BCLC staging system, BCLC stage A subclassification and Bolondi’s BCLC B substaging all resulted statistically significant.
The NIACE staging systems presented median survivals similar to the validation study, and similarly, the MESH staging system presented median survivals comparable to those of the original study, except for stages with lower numbers of patients.
Among all of the staging systems, not only did the ITA.LI.CA show one of the highest statistical significance (P < 0.001) for prognostic stratification of the patients, but it also showed almost complete correspondence of median overall survivals for all different stages. Only patients in stage > 5 showed a median survival 2 mo longer than that of the original study (10.4 vs 8.9 mo), probably related to the relatively low numerosity of patients in this stage (12 patients, 8.6%). This study further supports the external validation process for the ITA.LI.CA prognostic system in Western patients affected by HCC[48].
The study also assessed the prognostic performance of scoring systems related to treatment. The median survivals of all three scoring systems (STATE, SNACOR, NSP) in the study population were similar to those of the original studies, but only the NSP system reached inter-group statistical significance.
As could be expected, the median overall survival of patients undergoing ultrasound surveillance every 6 mo was longer than those of patients who were not followed (48 vs 30 mo), attributable to an early detection of HCC nodules. In fact, as shown in Table 2, patients undergoing ultrasound surveillance had smaller nodule diameter (25 vs 34 mm, P < 0.001) and showed lower prevalence of 32 nodules at diagnosis. Also, patients with AFP > 200 ng/mL showed reduced survival if compared to patients with lower AFP levels (22 vs 55 mo, P < 0.001).
In terms of the treatment regimen, median overall survival was 48 (20; 75) mo for curative (surgery/ablation) treatment and 23 (14; 34) mo for non-curative (TACE/sorafenib/support) treatment. Further analyses were carried out assessing the difference in survival of patients who did and did not receive the treatment recommended for their stage by the BCLC staging system.For patients treated with surgical resection or TACE, there was no significant difference in survival between the two groups, proving that the BCLC score does not affect the overall survival for the same type of therapy. As could be expected, patients with BCLC stage A who underwent curative treatment (as recommended by the BCLC staging system) presented a significantly better survival compared to those who did not, but at the same time patients with BCLC stage B showed a benefit from curative treatment (not recommended by the BCLC staging system) compared to those who underwent TACE (as recommended), with a median survival of 34.7 mo instead of 22.2 mo. Therefore, the rigorous application of treatment recommendations for each BCLC stage, may shorten patients’ survival. In fact, treatment choices based on the sub-classification of the BCLC stage B can furtherly stratify patients and provide the most suitable treatment[49-53].
In conclusion, the study identified the ITA.LI.CA as the most effective staging system in the local population. In addition, the ITA.LI.CA does not propose a treatment algorithm, as opposed to other staging systems such as the BCLC, since numerous variables influence treatment choice, and the use of rigid and categorical flowcharts may not always guarantee the most suitable therapy, as partly shown also in this study. ITA.LI.CA seems a promising prognostic score system with a good applicability and reproducibility for patients with HCC.
Hepatocellular carcinoma represents the most common primitive liver malignancy.
Currently there is a widespread lack of agreement on staging systems, prognostic scores and treatment allocation algorithms.
Define the prognostic ability of fifteen different prognostic scores.
Retrospective study, 10-year enrollment of patients.
With the Italian Liver Cancer (ITA.LI.CA) prognostic system 28.6%, 40.7%, 22.1% and 8.6% of patients fell within stages 0-1, 2-3, 4-5 and > 5 respectively. The median survival was 57.9 mo for stages 0-1, 43 mo for stages 2-3, 21.7 mo for stages 4-5 and 10.4 mo for stage > 5. 1-, 3-, and 5-year survival rates were 95%, 65% and 20% for stages 0-1, 94.7%, 43.9% and 26.3% for stages 2-3, 71%, 25.8% and 16.1% for stages 4-5 and 50%, 16.7% and 8.3% for stage > 5.
The median overall survival of the cohort of patients was 35 (17; 67) mo, and it was statistically different in relation to treatment choice, ultrasound surveillance and serum AFP.
External validation to the ITA.LI.CA staging system.
Special Acknowledgments to Leonardo Da Rio, MD and Riccardo Patti, MD who significantly contributed to data collection and analysis.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhang Q S-Editor: Zhang H L-Editor: Filipodia P-Editor: Liu JH
| 1. | McGlynn KA, London WT. The global epidemiology of hepatocellular carcinoma: present and future. Clin Liver Dis. 2011;15:223-243, vii-vix. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 360] [Cited by in RCA: 375] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 2. | Global Burden of Disease Liver Cancer Collaboration, Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, Al-Raddadi R, Alvis-Guzman N, Amoako Y, Artaman A, Ayele TA, Barac A, Bensenor I, Berhane A, Bhutta Z, Castillo-Rivas J, Chitheer A, Choi JY, Cowie B, Dandona L, Dandona R, Dey S, Dicker D, Phuc H, Ekwueme DU, Zaki MS, Fischer F, Fürst T, Hancock J, Hay SI, Hotez P, Jee SH, Kasaeian A, Khader Y, Khang YH, Kumar A, Kutz M, Larson H, Lopez A, Lunevicius R, Malekzadeh R, McAlinden C, Meier T, Mendoza W, Mokdad A, Moradi-Lakeh M, Nagel G, Nguyen Q, Nguyen G, Ogbo F, Patton G, Pereira DM, Pourmalek F, Qorbani M, Radfar A, Roshandel G, Salomon JA, Sanabria J, Sartorius B, Satpathy M, Sawhney M, Sepanlou S, Shackelford K, Shore H, Sun J, Mengistu DT, Topór-Mądry R, Tran B, Ukwaja KN, Vlassov V, Vollset SE, Vos T, Wakayo T, Weiderpass E, Werdecker A, Yonemoto N, Younis M, Yu C, Zaidi Z, Zhu L, Murray CJL, Naghavi M, Fitzmaurice C. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3:1683-1691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1459] [Cited by in RCA: 1492] [Article Influence: 186.5] [Reference Citation Analysis (0)] |
| 3. | Bosetti C, Levi F, Boffetta P, Lucchini F, Negri E, La Vecchia C. Trends in mortality from hepatocellular carcinoma in Europe, 1980-2004. Hepatology. 2008;48:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 181] [Article Influence: 10.6] [Reference Citation Analysis (1)] |
| 4. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12135] [Cited by in RCA: 12985] [Article Influence: 1442.8] [Reference Citation Analysis (2)] |
| 5. | Colombo M, de Franchis R, Del Ninno E, Sangiovanni A, De Fazio C, Tommasini M, Donato MF, Piva A, Di Carlo V, Dioguardi N. Hepatocellular carcinoma in Italian patients with cirrhosis. N Engl J Med. 1991;325:675-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 536] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 6. | Mittal S, El-Serag HB, Sada YH, Kanwal F, Duan Z, Temple S, May SB, Kramer JR, Richardson PA, Davila JA. Hepatocellular Carcinoma in the Absence of Cirrhosis in United States Veterans is Associated With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol 2016; 14: 124-31. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 490] [Article Influence: 54.4] [Reference Citation Analysis (1)] |
| 7. | Giuffrè M, Campigotto M, Campisciano G, Comar M, Crocè LS. A story of liver and gut microbes: how does the intestinal flora affect liver disease? A review of the literature. Am J Physiol Gastrointest Liver Physiol 2020; 5: G889-G906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 8. | Wong GL, Wong VW, Tan GM, Ip KI, Lai WK, Li YW, Mak MS, Lai PB, Sung JJ, Chan HL. Surveillance programme for hepatocellular carcinoma improves the survival of patients with chronic viral hepatitis. Liver Int. 2008;28:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6569] [Article Influence: 469.2] [Reference Citation Analysis (1)] |
| 10. | European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4517] [Article Influence: 347.5] [Reference Citation Analysis (2)] |
| 11. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3015] [Article Influence: 430.7] [Reference Citation Analysis (3)] |
| 12. | Hennedige T, Venkatesh SK. Imaging of hepatocellular carcinoma: diagnosis, staging and treatment monitoring. Cancer Imaging. 2013;12:530-547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 128] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 13. | Parikh ND, Singal AG. The ITA.LI.CA Staging System: A Novel Staging System for Hepatocellular Carcinoma. PLoS Med. 2016;13:e1002005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Kamarajah SK, Frankel TL, Sonnenday C, Cho CS, Nathan H. Critical evaluation of the American Joint Commission on Cancer (AJCC) 8th edition staging system for patients with Hepatocellular Carcinoma (HCC): A Surveillance, Epidemiology, End Results (SEER) analysis. J Surg Oncol. 2018;117:644-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 108] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 15. | Chan AC, Fan ST, Poon RT, Cheung TT, Chok KS, Chan SC, Lo CM. Evaluation of the seventh edition of the American Joint Committee on Cancer tumour-node-metastasis (TNM) staging system for patients undergoing curative resection of hepatocellular carcinoma: implications for the development of a refined staging system. HPB (Oxford). 2013;15:439-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Poon RT, Fan ST, Lo CM, Liu CL, Ng IO, Wong J. Long-term prognosis after resection of hepatocellular carcinoma associated with hepatitis B-related cirrhosis. J Clin Oncol. 2000;18:1094-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 149] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Subramaniam S, Kelley RK, Venook AP. A review of hepatocellular carcinoma (HCC) staging systems. Chin Clin Oncol. 2013;2:33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 18. | Maida M, Orlando E, Cammà C, Cabibbo G. Staging systems of hepatocellular carcinoma: a review of literature. World J Gastroenterol. 2014;20:4141-4150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 95] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 19. | A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 977] [Cited by in RCA: 963] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 20. | Cammà C, Di Marco V, Cabibbo G, Latteri F, Sandonato L, Parisi P, Enea M, Attanasio M, Galia M, Alessi N, Licata A, Latteri MA, Craxì A. Survival of patients with hepatocellular carcinoma in cirrhosis: a comparison of BCLC, CLIP and GRETCH staging systems. Aliment Pharmacol Ther. 2008;28:62-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J Gastroenterol. 2003;38:207-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 537] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 22. | Sherman M. Staging for hepatocellular carcinoma: complex and confusing. Gastroenterology. 2014;146:1599-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Adhoute X, Penaranda G, Bronowicki JP, Raoul JL. Usefulness of the HKLC vs. the BCLC staging system in a European HCC cohort. J Hepatol. 2015;62:492-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Kinoshita A, Onoda H, Fushiya N, Koike K, Nishino H, Tajiri H. Staging systems for hepatocellular carcinoma: Current status and future perspectives. World J Hepatol. 2015;7:406-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (2)] |
| 25. | Choi WM, Yu SJ, Ahn H, Cho H, Cho YY, Lee M, Yoo JJ, Cho Y, Lee DH, Cho EJ, Lee JH, Kim YJ, Yoon JH. A model to estimate survival in ambulatory patients with hepatocellular carcinoma: Can it predict the natural course of hepatocellular carcinoma? Dig Liver Dis. 2017;49:1273-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Ogasawara S, Chiba T, Ooka Y, Suzuki E, Kanogawa N, Saito T, Motoyama T, Tawada A, Kanai F, Yokosuka O. Liver function assessment according to the Albumin-Bilirubin (ALBI) grade in sorafenib-treated patients with advanced hepatocellular carcinoma. Invest New Drugs. 2015;33:1257-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 27. | Hiraoka A, Kumada T, Michitaka K, Toyoda H, Tada T, Ueki H, Kaneto M, Aibiki T, Okudaira T, Kawakami T, Kawamura T, Yamago H, Suga Y, Miyamoto Y, Tomida H, Azemoto N, Mori K, Miyata H, Ninomiya T, Kawasaki H. Usefulness of albumin-bilirubin grade for evaluation of prognosis of 2584 Japanese patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 2016;31:1031-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 198] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 28. | Liu PH, Hsu CY, Hsia CY, Lee YH, Huang YH, Su CW, Lee FY, Lin HC, Huo TI. Proposal and validation of a new model to estimate survival for hepatocellular carcinoma patients. Eur J Cancer. 2016;63:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Adhoute X, Pénaranda G, Raoul JL, Blanc JF, Edeline J, Conroy G, Perrier H, Pol B, Bayle O, Monnet O, Beaurain P, Muller C, Castellani P, Bronowicki JP, Bourlière M. Prognosis of advanced hepatocellular carcinoma: a new stratification of Barcelona Clinic Liver Cancer stage C: results from a French multicenter study. Eur J Gastroenterol Hepatol. 2016;28:433-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Zhang YF, Zhou J, Wei W, Zou RH, Chen MS, Lau WY, Shi M, Guo RP. Intermediate-stage hepatocellular carcinoma treated with hepatic resection: the NSP score as an aid to decision-making. Br J Cancer. 2016;115:1039-1047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Kadalayil L, Benini R, Pallan L, O'Beirne J, Marelli L, Yu D, Hackshaw A, Fox R, Johnson P, Burroughs AK, Palmer DH, Meyer T. A simple prognostic scoring system for patients receiving transarterial embolisation for hepatocellular cancer. Ann Oncol. 2013;24:2565-2570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 278] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 32. | Hucke F, Pinter M, Graziadei I, Bota S, Vogel W, Müller C, Heinzl H, Waneck F, Trauner M, Peck-Radosavljevic M, Sieghart W. How to STATE suitability and START transarterial chemoembolization in patients with intermediate stage hepatocellular carcinoma. J Hepatol. 2014;61:1287-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 33. | Mähringer-Kunz A, Weinmann A, Schmidtmann I, Koch S, Schotten S, Pinto Dos Santos D, Pitton MB, Dueber C, Galle PR, Kloeckner R. Validation of the SNACOR clinical scoring system after transarterial chemoembolisation in patients with hepatocellular carcinoma. BMC Cancer. 2018;18:489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2871] [Article Influence: 110.4] [Reference Citation Analysis (1)] |
| 35. | Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL, Adam R, Neuhaus P, Salizzoni M, Bruix J, Forner A, De Carlis L, Cillo U, Burroughs AK, Troisi R, Rossi M, Gerunda GE, Lerut J, Belghiti J, Boin I, Gugenheim J, Rochling F, Van Hoek B, Majno P; Metroticket Investigator Study Group. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1570] [Article Influence: 92.4] [Reference Citation Analysis (1)] |
| 36. | Weinmann A, Koch S, Sprinzl M, Kloeckner R, Schulze-Bergkamen H, Düber C, Lang H, Otto G, Wörns MA, Galle PR. Survival analysis of proposed BCLC-B subgroups in hepatocellular carcinoma patients. Liver Int. 2015;35:591-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 37. | Barman PM, Su GL. Limitations of the barcelona clinic liver cancer staging system with a focus on transarterial chemoembolization as a key modality for treatment of hepatocellular carcinoma. Clin Liver Dis (Hoboken). 2016;7:32-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | Yopp AC, Parikh ND, Singal AG. Is the Hong Kong Liver Cancer Staging System Ready to Replace the Barcelona Clinic Liver Cancer System? Clin Gastroenterol Hepatol. 2017;15:756-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 39. | Bolondi L, Burroughs A, Dufour JF, Galle PR, Mazzaferro V, Piscaglia F, Raoul JL, Sangro B. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012;32:348-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 302] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 40. | Ha Y, Shim JH, Kim SO, Kim KM, Lim YS, Lee HC. Clinical appraisal of the recently proposed Barcelona Clinic Liver Cancer stage B subclassification by survival analysis. J Gastroenterol Hepatol. 2014;29:787-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 41. | Yamakado K, Miyayama S, Hirota S, Mizunuma K, Nakamura K, Inaba Y, Maeda H, Matsuo K, Nishida N, Aramaki T, Anai H, Koura S, Oikawa S, Watanabe K, Yasumoto T, Furuichi K, Yamaguchi M. Subgrouping of intermediate-stage (BCLC stage B) hepatocellular carcinoma based on tumor number and size and Child-Pugh grade correlated with prognosis after transarterial chemoembolization. Jpn J Radiol. 2014;32:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 42. | Yamakado K, Hirota S. Sub-classification of intermediate-stage (Barcelona Clinic Liver Cancer stage-B) hepatocellular carcinomas. World J Gastroenterol. 2015;21:10604-10608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 43. | Arizumi T, Ueshima K, Iwanishi M, Minami T, Chishina H, Kono M, Takita M, Kitai S, Inoue T, Yada N, Hagiwara S, Minami Y, Ida H, Sakurai T, Kitano M, Nishida N, Kudo M. Validation of Kinki Criteria, a Modified Substaging System, in Patients with Intermediate Stage Hepatocellular Carcinoma. Dig Dis. 2016;34:671-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Wang JH, Kee KM, Lin CY, Hung CH, Chen CH, Lee CM, Lu SN. Validation and modification of a proposed substaging system for patients with intermediate hepatocellular carcinoma. J Gastroenterol Hepatol. 2015;30:358-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 45. | Chan AW, Kumada T, Toyoda H, Tada T, Chong CC, Mo FK, Yeo W, Johnson PJ, Lai PB, Chan AT, To KF, Chan SL. Integration of albumin-bilirubin (ALBI) score into Barcelona Clinic Liver Cancer (BCLC) system for hepatocellular carcinoma. J Gastroenterol Hepatol. 2016;31:1300-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 46. | Farinati F, Vitale A, Spolverato G, Pawlik TM, Huo TL, Lee YH, Frigo AC, Giacomin A, Giannini EG, Ciccarese F, Piscaglia F, Rapaccini GL, Di Marco M, Caturelli E, Zoli M, Borzio F, Cabibbo G, Felder M, Sacco R, Morisco F, Biasini E, Foschi FG, Gasbarrini A, Svegliati Baroni G, Virdone R, Masotto A, Trevisani F, Cillo U; ITA. LI.CA study group. Development and Validation of a New Prognostic System for Patients with Hepatocellular Carcinoma. PLoS Med. 2016;13:e1002006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 47. | Yoo JJ, Lee JH, Lee SH, Lee M, Lee DH, Cho Y, Lee YB, Yu SJ, Kim HC, Kim YJ, Yoon JH, Kim CY, Lee HS. Comparison of the effects of transarterial chemoembolization for advanced hepatocellular carcinoma between patients with and without extrahepatic metastases. PLoS One. 2014;9:e113926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 48. | Borzio M, Dionigi E, Rossini A, Marignani M, Sacco R, De Sio I, Bertolini E, Francica G, Giacomin A, Parisi G, Vicari S, Toldi A, Salmi A, Boccia S, Mitra M, Fornari F. External validation of the ITA.LI.CA prognostic system for patients with hepatocellular carcinoma: A multicenter cohort study. Hepatology. 2018;67:2215-2225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 49. | Chevret S, Trinchet JC, Mathieu D, Rached AA, Beaugrand M, Chastang C. A new prognostic classification for predicting survival in patients with hepatocellular carcinoma. Groupe d'Etude et de Traitement du Carcinome Hépatocellulaire. J Hepatol. 1999;31:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 360] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 50. | Okuda K, Obata H, Nakajima Y, Ohtsuki T, Okazaki N, Ohnishi K. Prognosis of primary hepatocellular carcinoma. Hepatology. 1984;4:3S-6S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 159] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 51. | Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology 2014; 146: 1691-700. e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 543] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 52. | Kim JY, Sinn DH, Gwak GY, Choi GS, Saleh AM, Joh JW, Cho SK, Shin SW, Carriere KC, Ahn JH, Paik YH, Choi MS, Lee JH, Koh KC, Paik SW. Transarterial chemoembolization versus resection for intermediate-stage (BCLC B) hepatocellular carcinoma. Clin Mol Hepatol. 2016;22:250-258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 53. | Ciria R, López-Cillero P, Gallardo AB, Cabrera J, Pleguezuelo M, Ayllón MD, Luque A, Zurera L, Espejo JJ, Rodríguez-Perálvarez M, Montero JL, de la Mata M, Briceño J. Optimizing the management of patients with BCLC stage-B hepatocellular carcinoma: Modern surgical resection as a feasible alternative to transarterial chemoemolization. Eur J Surg Oncol. 2015;41:1153-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 54. | Tateishi R, Yoshida H, Shiina S, Imamura H, Hasegawa K, Teratani T, Obi S, Sato S, Koike Y, Fujishima T, Makuuchi M, Omata M. Proposal of a new prognostic model for hepatocellular carcinoma: an analysis of 403 patients. Gut. 2005;54:419-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 203] [Article Influence: 10.2] [Reference Citation Analysis (0)] |