Published online Dec 27, 2020. doi: 10.4254/wjh.v12.i12.1211
Peer-review started: June 30, 2020
First decision: September 14, 2020
Revised: September 30, 2020
Accepted: October 29, 2020
Article in press: October 29, 2020
Published online: December 27, 2020
Processing time: 170 Days and 15.6 Hours
Anti-programmed death therapy has thrust immunotherapy into the spotlight. However, such therapy has a modest response in hepatocellular carcinoma (HCC). Epigenetic immunomodulation is a suggestive combinatorial therapy with immune checkpoint blockade. Non-coding ribonucleic acid (ncRNA) driven regulation is a major mechanism of epigenetic modulation. Given the wide range of ncRNAs that co-opt in programmed cell-death protein 1 (PD-1)/programmed death ligand 1 (PD-L1) regulation, and based on the literature, we hypothesized that miR-155-5p, miR-194-5p and long non-coding RNAs (lncRNAs) X-inactive specific transcript (XIST) and MALAT-1 are involved in a regulatory upstream pathway for PD-1/PD-L1. Recently, nutraceutical therapeutics in cancers have received increasing attention. Thus, it is interesting to study the impact of oleuropein on the respective study key players.
To explore potential upstream regulatory ncRNAs for the immune checkpoint PD-1/PD-L1.
Bioinformatics tools including microrna.org and lnCeDB software were adopted to detect targeting of miR-155-5p, miR-194-5p and lncRNAs XIST and MALAT-1 to PD-L1 mRNA, respectively. In addition, Diana tool was used to predict targeting of both aforementioned miRNAs to lncRNAs XIST and MALAT-1. HCC and normal tissue samples were collected for scanning of PD-L1, XIST and MALAT-1 expression. To study the interaction among miR-155-5p, miR-194-5p, lncRNAs XIST and MALAT-1, as well as PD-L1 mRNA, a series of transfections of the Huh-7 cell line was carried out.
Bioinformatics software predicted that miR-155-5p and miR-194-5p can target PD-L1, MALAT-1 and XIST. MALAT-1 and XIST were predicted to target PD-L1 mRNA. PD-L1 and XIST were significantly upregulated in 23 HCC biopsies compared to healthy controls; however, MALAT-1 was barely detected. MiR-194 induced expression elevated the expression of PD-L1, XIST and MALAT-1. However, overexpression of miR-155-5p induced the upregulation of PD-L1 and XIST, while it had a negative impact on MALAT-1 expression. Knockdown of XIST did have an impact on PD-L1 expression; however, following knockdown of the negative regulator of X-inactive specific transcript (TSIX), PD-L1 expression was elevated, and abolished MALAT-1 activity. Upon co-transfection of miR-194-5p with siMALAT-1, PD-L1 expression was elevated. Co-transfection of miR-194-5p with siXIST did not have an impact on PD-L1 expression. Upon co-transfection of miR-194 with siTSIX, PD-L1 expression was upregulated. Interestingly, the same PD-L1 expression pattern was observed following miR-155-5p co-transfections. Oleuropein treatment of Huh-7 cells reduced the expression profile of PD-L1, XIST, and miR-155-5p, upregulated the expression of miR-194-5p and had no significant impact on the MALAT-1 expression profile.
This study reported a novel finding revealing that opposing acting miRNAs in HCC, have the same impact on PD-1/PD-L1 immune checkpoint by sharing a common signaling pathway.
Core Tip: Due to the immune rich milieu of hepatocellular carcinoma (HCC), it is a good candidate for immune-based therapies. In this study, our aim was to identify potential upstream epigenetic regulators of immune checkpoint programmed cell-death protein 1/programmed death ligand 1 in HCC which could be regarded as therapeutic targets. The findings of this study revealed the re-questioning of the role of certain non-coding ribonucleic acids in HCC. Here we deduced a novel shared upstream regulatory signaling pathway for programmed cell-death protein 1/programmed death ligand 1 immune checkpoint between paradoxically acting tumor suppressor miR-194-5p and onco-miR-155-5p, in HCC through X-inactive specific transcript expression modulation.
- Citation: Atwa SM, Handoussa H, Hosny KM, Odenthal M, El Tayebi HM. Pivotal role of long non-coding ribonucleic acid-X-inactive specific transcript in regulating immune checkpoint programmed death ligand 1 through a shared pathway between miR-194-5p and miR-155-5p in hepatocellular carcinoma. World J Hepatol 2020; 12(12): 1211-1227
- URL: https://www.wjgnet.com/1948-5182/full/v12/i12/1211.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i12.1211
Hepatocellular carcinoma (HCC) constitutes a global burden and is one of the leading causes of cancer mortality[1]. A myriad of therapeutic modalities is available for HCC including tumor resection or ablation, transarterial chemoembolization, liver transplantation and treatment with tyrosine kinase inhibitors[2]. Nevertheless, HCC is a highly therapy resistant disease and is frequently diagnosed at an advanced stage; thus, the identification of a novel therapeutic modality is essential[3].
Recently, tumour immunotherapy has been thrust into the spotlight to inhibit tumour progression, relapse and metastasis. Immunotherapeutic techniques comprise both activation of tumour specific immune responses as well as enhancement of cellular or humoral immunity thus causing disruption of immune tolerance[4]. HCC immunotherapy has greatly changed due to extensive ongoing immunological studies which have incorporated immunotherapy into the HCC treatment armamentarium[5]. The rationale behind such a revolutionary therapeutic technique is the fact that HCC develops in an inflammatory milieu brimming with tumour infiltrating lymphocytes boosting HCC immunogenicity[6].
Immune checkpoint inhibitors have been featured as a sensational paradigm shift in cancer immunotherapy[7]. Physiologically, immune checkpoints are co-inhibitory molecules that act as “brakes” in the immune system to avoid an exaggerated response and restore its activity to a normal level[8,9]. Programmed cell-death protein 1 (PD-1) is one of the highly expressed immune checkpoints on T-cells in most solid tumours[10]. PD-1 was originally described by Ishida et al[11] in 1992 as a cell death inducer, a discovery that paved the way for Noble prize winning immune checkpoint inhibitor studies in 2018. Tumour immune surveillance evasion can then occur upon engagement of PD-1 with its ligand, Programmed death ligand 1 (PD-L1), expressed on tumour cells leading to effector T-cell exhaustion and dysfunction[12,13]. PD-1/PD-L1 immune checkpoint blockade has shown considerable survival benefits in patients with different metastatic tumours[14-17]. In 2017, the Food and Drug Administration approved Nivolumab, a human immunoglobulin G monoclonal antibody against PD-1, for patients with advanced HCC, due to durable responses observed in these patients[18].
Accumulating evidence has shown that PD-1/PD-L1 immune checkpoint is epigenetically regulated through immunomodulatory non-coding ribonucleic acids (ncRNAs) as microRNAs (miRNAs) and long non-coding RNAs (lncRNAs) in several cancers including colorectal cancer[19], lung cancer[20] and pancreatic cancer[21]. Furthermore, our research group demonstrated the epigenetic regulation of PD-1/PD-L1 in breast cancer[22]. Nevertheless, such immunomodulatory loops orchestrating PD-1/PD-L1 expression and activity are still under investigation in HCC.
Due to the breakthrough established in next generation sequencing which enabled the profiling of the whole transcriptomic expression at the molecular level, our understanding of biological systems has improved[23]. Such studies have revealed the expression deregulation of a multitude of ncRNAs[24].
Based on bioinformatics analysis, the miRNAs, oncomiR and miR-155-5p, and tumor suppressor miR-194-5p were predicted to target PD-L1 transcriptome as well as the candidate lncRNAs, X-inactive specific transcript (XIST) and MALAT-1. Moreover, lncRNAs XIST and MALAT-1 were predicted to target PD-L1 transcript where both lncRNAs have demonstrated their role in HCC pathogenesis in several studies.
Therefore, it is interesting to study the expression profile of PD-L1 in Huh-7 cells relative to the expression manipulation of candidate ncRNAs in order to explore novel potential upstream regulatory ncRNAs for PD-L1 in HCC and the capacity of these ncRNAs as therapeutic targets. In addition, it is of value to determine the clinical relevance of the proposed regulatory signaling pathways for PD-L1 in HCC patients by assessing the expression pattern of PD-L1 as well as the lncRNAs XIST and MALAT-1 in HCC tissues.
The trend towards integrating phytochemicals in cancer therapy is being augmented worldwide, especially with increased tolerance and resistance to traditional cancer therapeutic modalities. The olive tree (Olea europaea L.) which belongs to the Oleacaea family is native to tropical and warm temperate regions[25]. Several studies have postulated that the olive plant has anti-inflammatory[26] and anti-cancer activities[27]. Such activities are mainly attributed to the unique polyphenolic content of the olive plant.
Oleuropein is one of the highly abundant phenolic compounds in olive leaves[28]. It is reported to have a plethora of beneficial health benefits that are attributed to a compilation of pharmacological action including anti-oxidant[29], anti-inflammatory[30], and anti-angiogenic[31] activities which pave the way for its interesting anticancer activity[32]. Oleuropein has been demonstrated to have an anti-inflammatory and immunomodulatory effect via down-regulation of MAPKs and NF-κB signaling pathways as well as controlling the production of inflammatory mediators such as IL-6 and TNF-α cytokines, MMP-1 and MMP-3 levels[33]. Interestingly, Ruzzolini et al[34] revealed the promising potential of oleuropein as an adjuvant therapy against BRAF melanoma, by manipulating the pAKT/pS6 pathway. Moreover, a recent study demonstrated the potential indirect modulatory impact of oleuropein on PD-L1 in esophageal cancer, by manipulating the expression of hypoxia-inducible factor-1[35]. Nevertheless, to the best of our knowledge, the immunomodulatory impact of oleuropein on HCC has not been extensively studied. Hence, the impact of this promising compound on our study key players was determined.
To detect possible microRNAs targeting 3’UTR of PD-L1 mRNA, microrna.org (www.microrna.org) bioinformatics target prediction software was used. Based on the binding scores and number of hits, miRNAs with good scores were chosen. Diana tools software (http://carolina.imis.athena-innovation.gr) was used to analyze potential binding of miR-194 and miR-155 to the 3’UTR region of lncRNAs XIST and MALAT1. The lnCeDB (Database of Human Long Noncoding RNA Acting as Competing Endogenous RNA) prediction software algorithm (http://gyanxet-beta.com/lncedb/) was used to analyze potential binding of lncRNA XIST and MALAT-1 to PD-L1.
The present study included 23 patients with HCC, who underwent liver transplant surgery in the Kasr El Einy Hospital (Cairo University, Cairo, Egypt). Four samples of cirrhotic tissues were taken from a subset of these patients with focal HCC lesions. As per the pathology report of these patients, summarized in Table 1, almost 70% of patients had > 1 focal lesion. Ten liver biopsies were obtained from healthy donors. Ethical approval for this study was issued by the Institutional Review Board of Cairo University. In addition, all participants provided written informed consent. The institutional ethics committees approving this research comply with the principles set forth in the international reports and guidelines of the Helsinki Declaration and the International Ethical Guidelines for Biomedical Research Involving Human Subjects, issued by the Council for International Organizations of Medical Sciences.
| Parameter | Value |
| Age (yr) | 49 ± 13.5 |
| Aspartate aminotransferase (U/L) | 100.5 ± 65.8 |
| Alanine aminotransferase (U/L) | 85.6 ± 95.6 |
| Alkaline phosphatase (U/L) | 110.2 ± 60.7 |
| Serum albumin (g/dL) | 4.6 ± 1.5 |
| Serum α fetoprotein (ng/mL) | 155.7 ± 22.3 |
Huh-7 cells were purchased from Vacsera Egypt. They were maintained in Dulbecco's modified Eagle's medium (DMEM, Lonza, Germany, cat. no. 12-604F), supplemented with 4.5 g/L glucose, 4 mmol/L L-glutamine, 10% fetal bovine serum (Applied Biosystems; Thermo Fisher Scientific Inc., cat. no. 10270098) and Mycozap (1:500; Lonza, cat. no. LT07-818) at 37°C in a 5% carbon dioxide atmosphere.
Twenty-four hours prior to transfection, 1-5 × 104 or 2-8 × 104 Huh-7 cells (40%-80% confluency) per well were seeded in a 96-well plate or 24-well plate, respectively. The cells were incubated under normal growth conditions (37°C and 5% carbon dioxide). The Huh-7 cell line was transfected with miScript™ miRNA mimics/inhibitors of miR-155-5p (Syn-hsa-miR-155-5p miScript miRNA Mimic, Qiagen, cat. no. MSY0000646 and Anti-hsa-miR-155-5p miScript miRNA Inhibitor, Qiagen, cat. no. MIN0000646) and miR-194 (Syn-hsa-miR-194-5p miScript miRNA Mimic, Qiagen, cat. no. MSY0000460 and Anti-hsa-miR-194-5p miScript miRNA Inhibitor, Qiagen, cat. no. MIN0000460). Transfections with siRNAs for each of XIST (Hs_XIST_3 FlexiTube siRNA, Qiagen Germany, cat. no. SI03654483), the negative regulator of X-inactive specific transcript (TSIX), (Hs_TSIX_7 FlexiTube siRNA, Qiagen Germany, cat. no. SI04708795) and MALAT-1 (Hs_MALAT1_1 FlexiTube siRNA, Qiagen Germany, cat. no. SI03670541) were also carried out. Co-transfections of each of the miR-155 and miR194 mimics were carried out with the siRNAs of each of the three lncRNAs MALAT-1, XIST and TSIX, respectively. All transfection experiments were performed in triplicate using HiPerfect Transfection Reagent (Qiagen Germany, cat. no. 301705) according to the manufacturer's instructions, and experiments were repeated three times. Cells that were exposed only to the transfection reagent were designated mock cells; cells transfected with miR-155 or miR-194 mimics were designated miR-155 cells and miR-194 cells, respectively; cells transfected with the miR-155 or miR-194 inhibitors were designated as anti-miR-155 cells and anti-miR-194 cells, respectively; cells transfected with XIST siRNAs were designated as XIST siRNA cells; cells transfected with MALAT-1 siRNAs were designated as MALAT-1 siRNA cells; cells transfected with TSIX siRNAs were designated as TSIX siRNA cells; cells co-transfected with miR-155 and XIST siRNA were designated as miR-155/siXIST; cells co-transfected with miR-155 and MALAT-1siRNA were designated as miR-155/siMALAT-1; cells co-transfected with miR-155 and TSIX siRNA were designated as miR-155/siTSIX; cells co-transfected with miR-194 and XIST siRNA were designated as miR-194/siXIST; cells co-transfected with miR-194 and MALAT-1 siRNA were designated as miR-194/siMALAT-1; cells co-transfected with miR-194 and TSIX siRNA were designated as miR-194/siTSIX; Cells were lysed 48 h post-transfection and total RNA was extracted for further analysis.
Olive leaves were collected from northern Sinai, Egypt and authenticated by Mrs. Therasa Labib, Taxonomist, Orman Botanical Garden, Egypt. Voucher specimen number (00396) was deposited at the Herbarium of the Pharmaceutical Biology Department, Faculty of Pharmacy and Biotechnology, German University in Cairo. Exhaustive extraction of olive leaves was carried out using 70% aqueous-ethanol, followed by re-suspension of the residue in H2O and fractionation against petroleum ether, chloroform and ethyl acetate to yield 17 g, 6.5 g and 4.5, g respectively. The ethyl acetate polar fraction was applied over an open column (64 cm L × 5.5 cm ID) packed with silica (250 g) as stationary phase. A CHCl3:CH3OH:H2O gradient was used for the elution process to ensure purification of the sub-fractions.
The sub-fraction of interest (30 mg) was obtained using CHCl3:CH3OH:H2O in a ratio of 3:4:3, then injected into a preparative high performance liquid chromatograph (Waters 600 E multisolvent delivery system, Waters 600 E pump and Waters 2998 PDA) which was employed using Lichrospher 100 RP-18 (250 mm × 10 mm i.d.; 10 µm) (Merck KGaA, Darmstadt, Germany). The mobile phase used was composed of 0.2% H3PO4 (v/v), methanol and acetonitrile in a ratio of 96:2:2. NMR spectra were obtained using a Bruker Avance 500 spectrometer (Bremen, Germany) 5 mm-Zgrad probe, operating at 500.13 MHz for 1H and 125.77 MHz for 13C. The purity of oleuropein was confirmed using analytical HPLC (Agilent Technologies, Waldbronn, Germany), equipped with a PDA detector G 1314 C (SL). Chromatographic separation was carried out on a Superspher 100 RP-18 (75 mm × 4 mm i.d.; 4 μm) column (Merck, Darmstadt, Germany) using mobile phases: (A) 2% acetic acid (pH 2.6) and (B) 80% methanol. A gradient starting from 5% B to 50% B was employed for the elution process with 100 μL/min flow rate at 30°C and compared vs standard material (Sigma Aldrich) using HPLC. Confirmation of oleuropein identity was carried out by comparing its spectral data to the obtained literature[36].
A stock solution of oleuropein 100 mmol/L was prepared by dissolving 0.108 g in 2 mL of free DMEM. A solution of 80 µmol/L concentration that was previously reported as LC50 on Huh-7 cells[37] was prepared using this stock.
RNA was isolated from Huh-7 cells and liver biopsies using the TRIzol™ LS Reagent (Applied Biosystems; Thermo Fisher Scientific Inc., cat. no. 10296010) extraction protocol.
Total RNA extracted was reverse-transcribed into single-stranded complementary DNA (cDNA) using the high-capacity cDNA reverse transcription kit (Applied Biosystems; Thermo Fisher Scientific Inc., cat. no. 4368814). The relative expression of miR-155 as well as miR-194 to that of RNU6B (housekeeping gene), in addition to PD-L1 mRNA, XIST and MALAT-1 lncRNAs to that of β-2-microglobulin (β2M; a housekeeping gene) were quantified with TaqMan RT-quantitative polymerase chain reaction [quantified real-time polymerase chain reaction (qRT-PCR); Applied Biosystems Assay IDs: 002287, 000493, 0001093, Hs01079824_m1, Hs00273907_ml and Hs00984230_m1 and Hs01060665_g1, respectively] using StepOne™ Systems (Applied Biosystems Life Technologies). The PCR for miR quantification included 1 µL TaqMan Small RNA Assay (20 X) specific for each of miR-155 or miR-194 or RNU6B and 1.33 µL cDNA from each miR-155 or miR-194 or RNU6B RT reactions, respectively. Taqman target gene assay expression assay (1 µL) specific for each of PD-L1, XIST and MALAT-1 as well as 4 µL of the respective cDNA were used for quantification. The RT-qPCR run was performed in the standard mode, consisting of two stages: A first 10 min stage at 95°C where the Taq-polymerase enzyme was activated, followed by a second stage of 40 amplification cycles (15 s at 95°C and 60 s at 60°C). Relative expression was calculated using the 2−ΔΔCq method. All PCR reactions, including controls, were run in triplicate.
All data were expressed in relative quantitation. For the purpose of comparison between two different studied groups, the Student's unpaired t-test was used. Data were expressed as mean ± SD error of the mean. A P value less than 0.05 was considered statistically significant. dP < 0.0001, cP < 0.001, bP < 0.01, aP < 0.05. Analysis was performed using GraphPad Prism 7.02.
According to miRANDA software and the miRDB database, a total of 146 miRNAs were predicted to target PD-L1 mRNA. Both miR-155 and miR-194 were predicted to bind to the 3’UTR region of PD-L1 mRNA using miRANDA software and Targetscan software, while binding of miR-194 and miR-155 to the 3’UTR region of lncRNAs XIST and MALAT1 was predicted using Diana tools software. MALAT1 and XIST were predicted to target PD-L1 mRNA according to LnCeDB software algorithms.
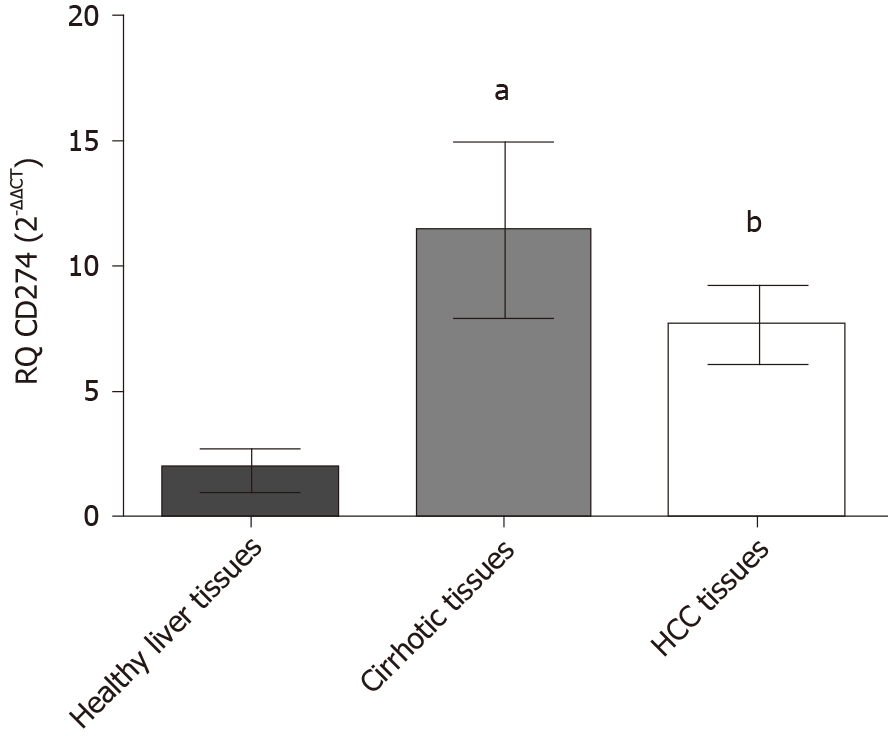
The expression profile of PD-L1 was assessed in HCC patients, and adjacent cirrhotic biopsies in a subset of patients together with 10 donor healthy controls, using qRT-PCR. PD-L1 was significantly elevated in both HCC biopsies (P = 0.0065) and cirrhotic biopsies (P = 0.0251) in comparison to healthy controls (Figure 1).
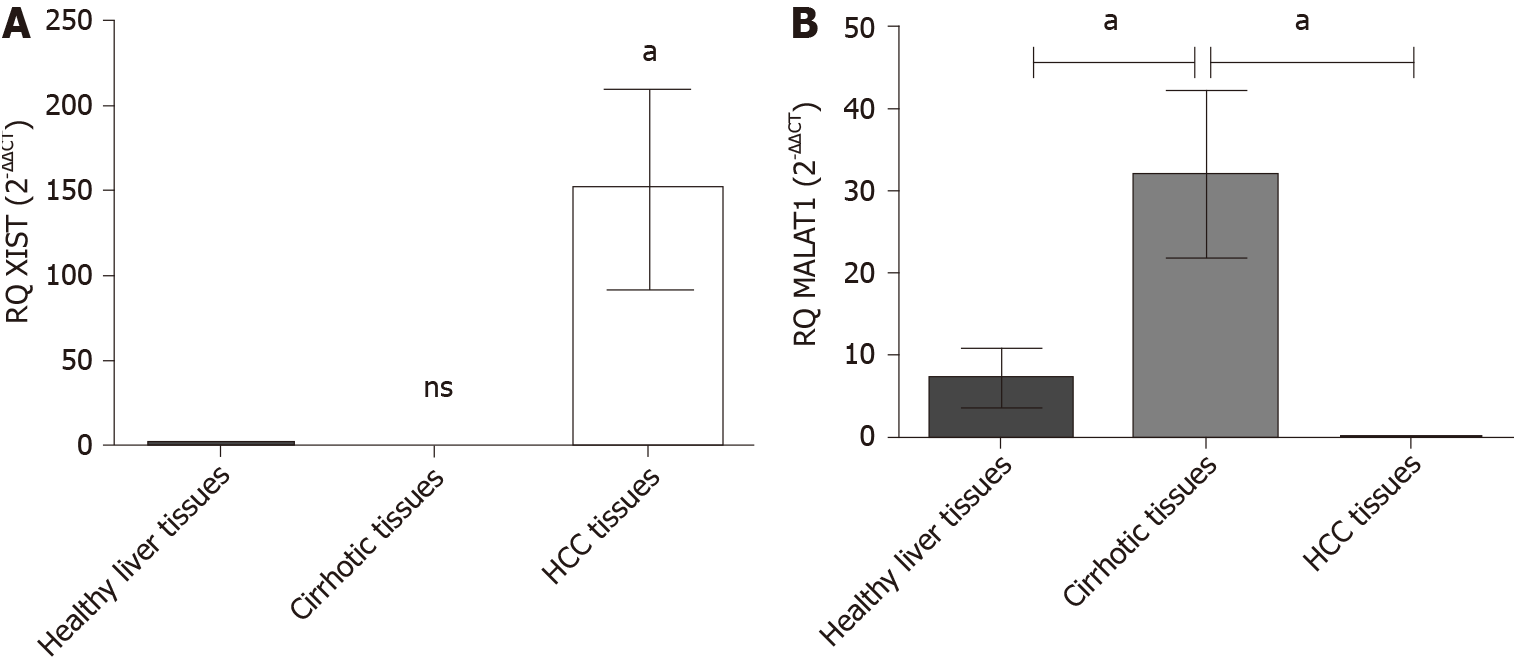
The expression profile of the endogenous lncRNAs XIST and MALAT-1 was examined in HCC patients and adjacent cirrhotic biopsies in a subset of patients together with 10 healthy donors using qRT-PCR. HCC patients showed a significant upregulation of XIST expression (P = 0.048) compared to healthy controls. MALAT-1 expression in HCC patients was barely detected (P = 0.043) and a significant upregulation was found in the cirrhotic tissues (P = 0.0136) (Figure 2).
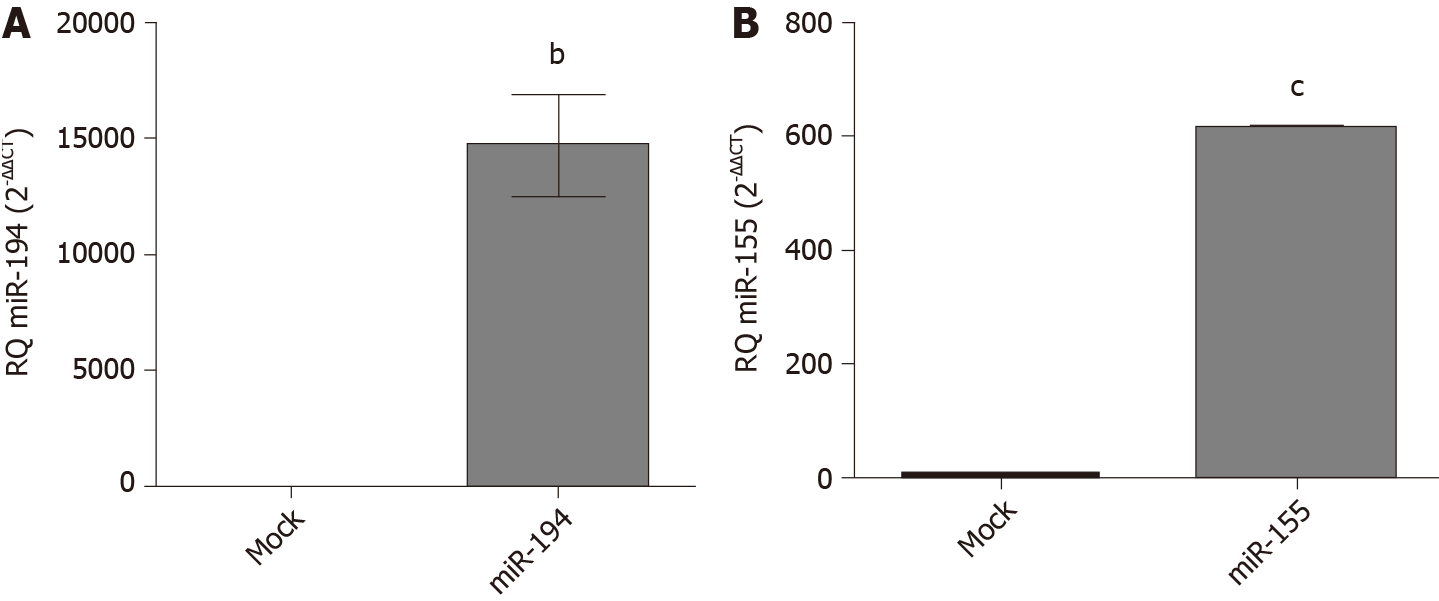
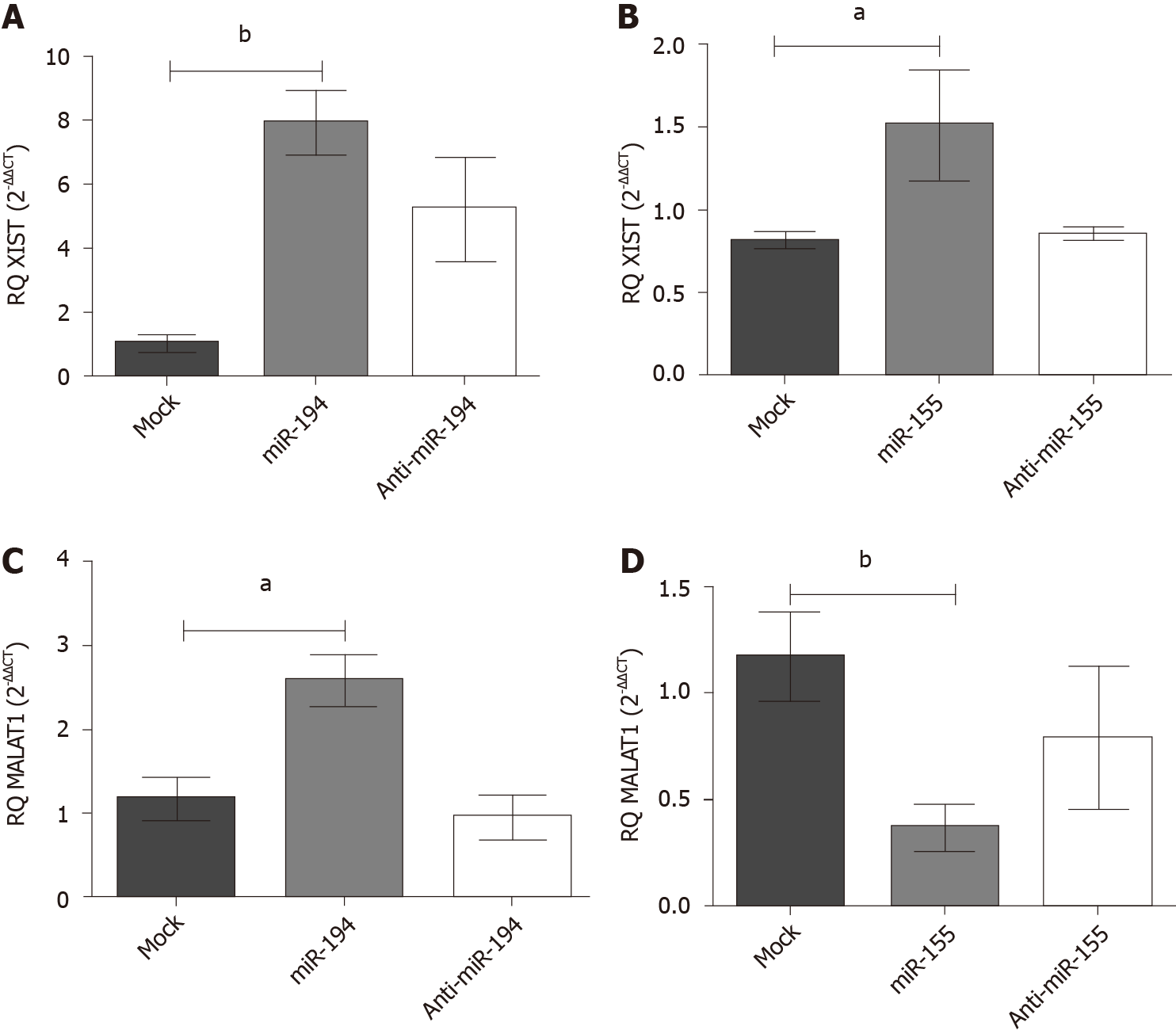
Transfection efficiency of miR-194-5p and miR-155-5p oligonucleotides: In order to manipulate the expression of miR-194-5p and miR-155-5p in Huh-7 cells, the cells were transfected with each of the respective miRNA mimics and antagomirs, respectively. Efficient transfection was assessed 48 h post-transfection using qRT-PCR, and both miR-194-5p (Figure 3A) and miR-155-5p (Figure 3B) were markedly increased in mimicked cells compared to mock cells, (P = 0.0026) and (P < 0.0001), respectively.
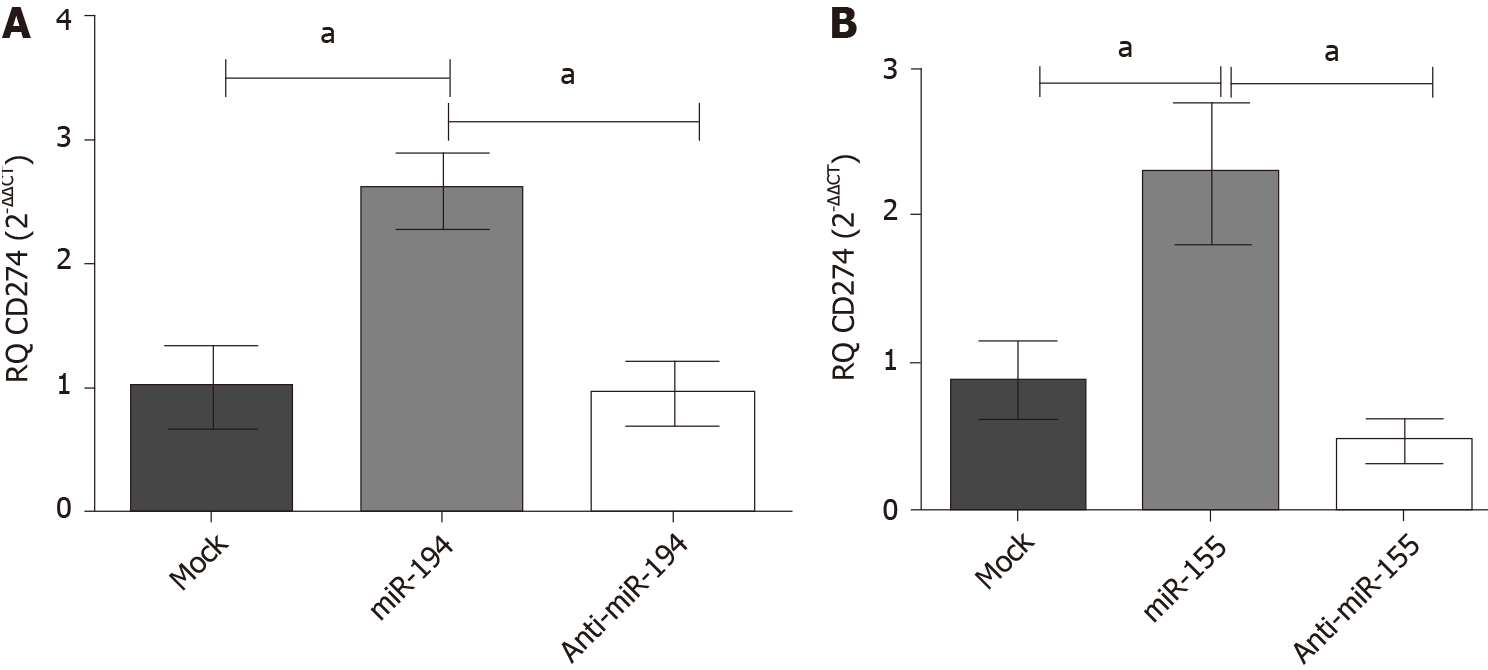
Impact of miR-194-5p and miR-155-5p on PD-L1 transcript expression in Huh-7 cells: Mimicking of both miRNAs miR-155 and miR-194 in Huh-7 cells showed an up-regulation of PD-L1 expression (P = 0.0219) (P = 0.0209), respectively, compared to the mock untransfected cells (Figure 4). However, antagonizing both miRNAs resulted in a significant downregulation of PD-L1 transcript expression compared to mock untransfected cells.
Impact of miR-155-5p and miR-194-5p on lncRNAs XIST and MALAT-1 expression in Huh7 cells: Following ectopic expression manipulation of each of the respective miRNAs in Huh-7 cells, the lncRNAs XIST and MALAT-1 expression profiles were assessed using qRT-PCR and normalized to B2M an endogenous housekeeping gene. (A) Mimicking of miR-194-5p and miR-155-5p resulted in an upregulated expression profile of XIST compared to the mock untransfected cells, (P = 0.0026, P = 0.0477), respectively, as shown in Figure 5A; (B) Meanwhile, as shown in Figure 5B, mimicking of miR-194-5p and miR-155-5p had a paradoxical impact on the MALAT-1 expression profile. Mimicking of miR-194-5p induced the expression of MALAT-1 (P = 0.0135) compared to mock untransfected cells. On the other hand, mimicking of miR-155-5p induced the downregulation of MALAT-1 expression compared to mock untransfected cells (P = 0.0053).
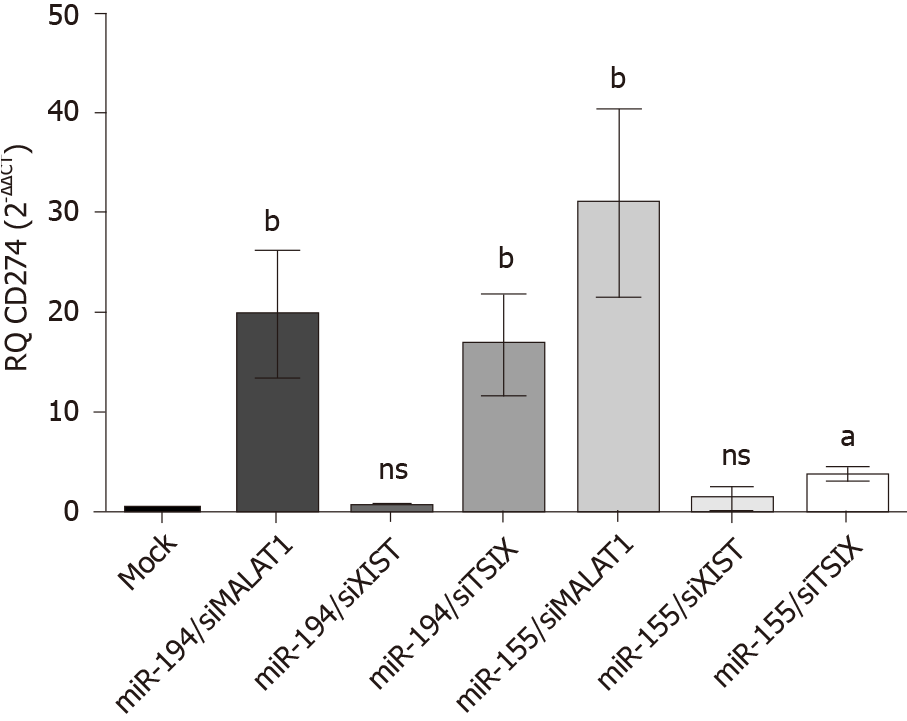
Knockdown of MALAT-1 significantly down regulated PD-L1 expression (P = 0.001) compared to mock cells. On the other hand, transfection with siRNAs of TSIX induced the upregulation of PD-L1 expression (P = 0.0358) compared to mock cells. Knockdown of XIST resulted in an insignificant change in the PD-L1 expression profile compared to untransfected mock cells (Figure 6).
The expression profile of PD-L1 transcript was studied following co-transfection of Huh-7 cells with different combinations of each miRNA; miR-194-5p and miR-155-5p, respectively, with each of the siRNAs of lncRNAs; MALAT-1, XIST and TSIX. Values were normalized to the endogenous housekeeping gene B2M and compared to mock untransfected cells. Following transfection of miR-194-5p with siRNA of MALAT-1, PD-L1 expression was significantly induced (P = 0.0074). However, following knockdown of XIST, miR-194-5p did not have a significant impact on PD-L1 expression compared to mock cells. However, co-transfection of miR-194-5p with siRNA TSIX, did have a positive impact on the PD-L1 expression profile compared to mock cells (P = 0.0067). Co-transfection of miR-155-5p siRNA MALAT-1 showed a significant upregulation of the PD-L1 transcript expression (P = 0.0060). However, miR-155-5P was unable to elevate PD-L1 expression following knockdown of XIST as there was no significant change in PD-L1 expression compared to mock cells. Knockdown of TSIX and co-transfection with miR-155-5P significantly induced the expression of PD-L1 (P = 0.0188) (Figure 7).
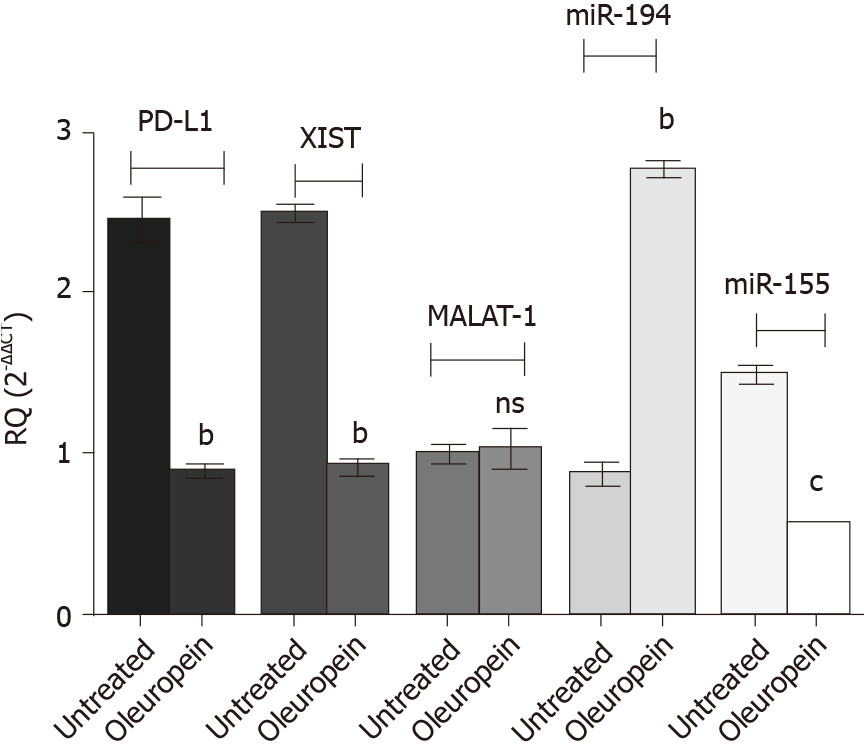
Treatment of Huh-7 cells with pure isolated oleuropein showed surprising results (Figure 8). Oleuropein treatment significantly downregulated the expression of PD-L1 (P = 0.0011), XIST (P = 0.0020) and miR-155 (P = 0.0001); however, MALAT-1 expression profile was not affected following oleuropein treatment. The miR-194-5p expression pattern was upregulated following oleuropein treatment (P = 0.0022).
The high expression pattern of immune checkpoints is a major cause of inefficient anti-tumor immunity. In this framework, immune checkpoint blockade has been revitalized to unleash the potential of anti-tumor immunity[38]. Nevertheless, immunotherapeutic approaches have modest responses in HCC. Thus, combinatorial therapeutic strategies including epigenetic modulation through ncRNAs and immunomodulation techniques are implemented to circumvent the limitation of immunotherapeutic techniques[39]. Recently, a novel interaction circuit has been demonstrated in the competing endogenous RNA (ceRNAs) network, composed of three RNAs “lncRNA-miRNA-mRNA”. Here, we showed that PD-L1 in HCC is a member of a ceRNA network orchestrated by miR-155, miR-194 and lncRNA XIST.
Based on in-silico analysis, the oncogenic miR-155-5p and tumour suppressor miR-194-5p were predicted to target PD-L1 mRNA. It has been postulated that miR-155 promotes tumorigenic properties in HCC-derived cell lines and hence is an oncogenic miRNA in HCC pathogenesis[40-42]. On the other hand, miR-194 has tumour suppressor activity in HCC as it was downregulated in HCC biopsies[43-45]. Interestingly, a paradoxical function of the tumour suppressor miR-194-5p in HCC was revealed in this study, and was able to elevate the abundance of the oncogenic mediator, PD-L1. Similarly, another study demonstrated the contradictory role of the oncomiR miR-125b in hematological malignancies, in which its oncogenic activity could be overcome in some instances in chronic lymphocytic leukemia to act as a tumour suppressor[46].
Inspired by the ceRNA regulatory network, we investigated the impact of the key miRNAs players on the proposed lncRNAs. Bioinformatics analysis was adopted to predict the potential lncRNAs targeted by miR-194-5p and miR-155-5p. Based on the literature, two lncRNAs were selected, XIST and MALAT-1. LncRNA XIST is reported to be an oncogenic RNA as it is associated with worsening of survival in HCC patients, in which its oncogenic activity is mediated by AKt signaling pathway activation through the miR-139-5p/PDK1 axis[47]. Nevertheless, overexpression of miR-194-5p and miR-155-5p induced an elevation in XIST. This finding also confirms the potential paradoxical role of miR-194-5p in HCC pathogenesis.
Several studies have shown upregulation of MALAT-1 in HCC biopsies[48,49]. However, one study reported that following MALAT-1 knockdown in a hepatoma cell line, no variations in the proliferation pattern, cell cycle progression or nuclear architecture were observed[50]. Surprisingly, overexpression of miR-194-5p induced the elevation of MALAT-1. In contrast, induced expression of miR-155-5p resulted in downregulation of MALAT-1. Taken together, these findings demonstrate the paradoxical functions of miRNAs in tumours, in which miR-194-5p expression induction elevated the expression of oncogenic members in the Huh-7 cell line. A plausible explanation for this anomaly is the fact that a single miRNA can target tens to hundreds of mRNAs, some of which are tumour suppressors and others are oncogenes. According to the balance in expression of the targeted mRNAs, a net effect of oncogenic or tumour suppressor activity can emerge[46].
Our study showed that knockdown of MALAT-1 using siMALAT-1 resulted in downregulation of PD-L1 transcript. On the other hand, following knockdown of XIST negative regulator, TSIX, PD-L1 transcript was significantly elevated. These findings are considered to be helpful in clarifying the interesting role of tumour-suppressor miR-194-5p in elevating PD-L1, an activity that could be mediated through XIST and MALAT-1. However, the role of MALAT-1 in PD-L1 transcript elevation in HCC is still questionable, as despite the downregulation of MALAT-1 upon miR-155-5p overexpression, PD-L1 transcript was found to be highly abundant.
In order to have a full understanding of the ceRNA network involved in PD-L1 transcript level modulation in HCC, the combined effect of the respective miRNAs and lncRNAs on PD-L1 transcript abundance was studied. MiR-194-5p elevated PD-L1 transcript abundance even in the absence of MALAT-1. However, when XIST was knocked down, miR-194-5p was unable solely to affect PD-L1 abundance level. Nevertheless, upon XIST upregulation together with mimicking of miR-194-5P, PD-L1 transcript level was restored. These findings provide solid evidence of the pivotal role of XIST in increased PD-L1 transcript abundance. Surprisingly, similar findings were observed following co-transfection of miR-155-5p mimics with each of the siRNAs of the respective lncRNAs, comparable to their co-transfection with miR-194-5p. These findings provide extra proof of the insignificant role of MALAT-1 in the PD-L1 expression pattern in comparison with XIST and both respective miRNAs.
The in-vitro results of our study also demonstrated the dual activity of miR-194-5p. Based on the literature, miR-194-5p has tumour suppressor activity in HCC by exerting a negative impact on cell viability and proliferation[43]. However, our results indicated that overexpression of miR-194-5p increased the abundance of the two oncogenic HCC members PD-L1 and XIST similar to the impact of oncogenic miR-155-5p. Hence, our next aim was to determine the results ex-vivo by screening HCC biopsies for PD-L1, XIST and MALAT-1 expression. An elevated expression of XIST in HCC biopsies was noted which was in accordance with several other studies that have reported the oncogenic role of XIST in HCC[47,51]. Also, PD-L1 was found to be significantly overexpressed in HCC biopsies compared to normal donor biopsies. This result is similar to that in other studies which reported the elevated expression of PD-L1 in HCC and its mechanistic role in immune evasion[52]. To our surprise, MALAT-1 was barely detected in HCC biopsies, in contrast to other studies that have reported the oncogenic role of MALAT-1 in HCC[53]. The interesting finding of downregulated MALAT-1 in HCC biopsies is in accordance with the in-vitro finding of the insignificant role of MALAT-1 in PD-L1 expression in HCC cells.
This study highlights the potential therapeutic targets in HCC including the members of the aforementioned upstream regulatory pathways of PD-L1. Nevertheless, the clinical application of ncRNAs as therapeutics is still limited and understudied. Thus, a trend towards using nutraceuticals in cancer therapy has developed due to the feasibility of their clinical application[54]. Phytochemicals did not only demonstrate epigenetic immunomodulation by targeting lncRNAs and miRNAs, but have also revealed their role in immune checkpoint modulation[55].
Due to the favorable role of polyphenolic nutraceuticals in epigenetic modulation, the nutraceutical oleuropein was selected for this study in order to determine its impact on the study key players, based on its aforementioned anti-inflammatory and immunomodulatory effects[33].
At 80 µmol/L[37], oleuropein significantly reduced the abundance of PD-L1 in Huh-7 cells. When the abundance of potential upstream regulatory ncRNAs was measured, it was found that XIST expression was significantly down regulated. However, oleuropein did not have a significant impact on MALAT-1 expression. Measurement of the impact of oleuropein on miR-194-5p and miR-155-5p revealed that miR-194-5p expression was markedly upregulated. In contrast, miR-155-5p was significantly downregulated. This finding is in accordance with another study that reported the negative impact of oleuropein on miR-155 in a breast cancer cell line, which manifested anti-proliferative, apoptotic, and anti-metastatic effects in the breast cancer cell line[56]. Finally, the potential of oleuropein as a therapeutic agent in HCC requires further investigation in order to support these promising findings.
Some limitations must be acknowledged in this study. First, the limited number of patients and subsequently, number of tissue biopsies; however, statistically significant results were obtained. Further studies using a larger number of tissue biopsies should be performed to validate the proposed pathway in a larger cohort of patients. Second, a further robust study design is necessary to analyze the study key players in peripheral blood samples of advanced HCC patients and to investigate the impact of mimicking the miRNAs, miR-155-5p and miR-194-5p, on PD-L1 protein levels in HCC cell lines.
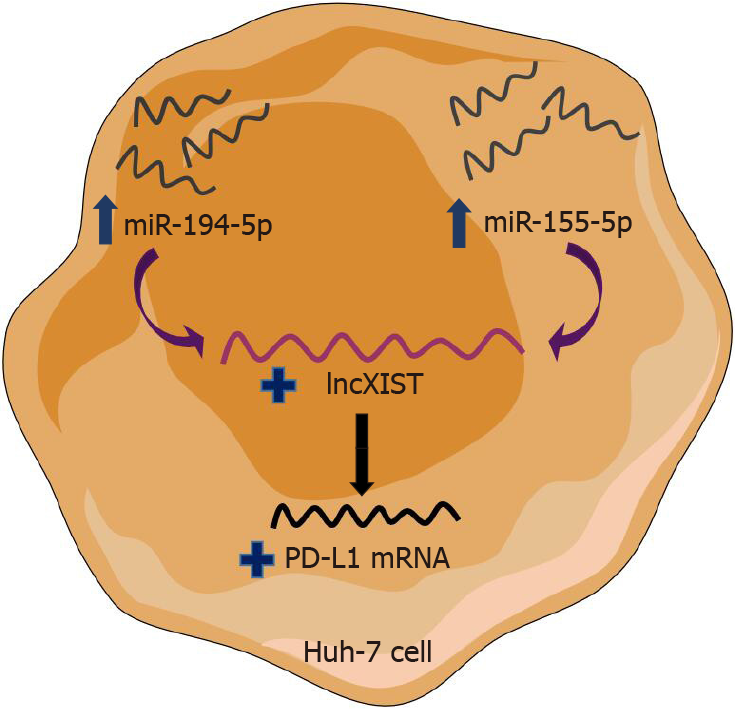
In conclusion, this study reported the controversial role of miR-194-5p in HCC as it has the paradoxical function of being both a tumour suppressor and oncogenic activity in HCC, and had the same impact on upregulation of PD-L1 and XIST. Transfection of each of the siRNAs of the respective lncRNAs, showed that XIST and MALAT-1 can have a positive impact on PD-L1 transcript abundance. However, following a series of co-transfections, it was demonstrated that XIST is a cornerstone in PD-L1 expression, while MALAT-1 has no significant impact compared to the respective miRNAs and XIST. Thus, a novel shared upstream regulatory signaling pathway for PD-1/PD-L1 immune checkpoint paradoxically acting on miR-194-5p and miR-155-5p occurs, through XIST expression modulation (Figure 9). Thus, the key regulators of the ceRNA circuit could be employed as therapeutic targets in HCC.
Hepatocellular carcinoma (HCC) develops in an inflammatory milieu containing tumor infiltrating lymphocytes, thus boosting tumor immunogenicity and provides an aspect for developing immunotherapies against HCC. However, immunotherapies have a modest response in HCC, accordingly combinatorial therapies with epigenetic immunomodulation may be a promising modality. Growing scientific evidence has suggested a modulatory role for miRNAs and long non-coding ribonucleic acids (lncRNAs) on programmed cell-death protein 1 (PD-1)/programmed death ligand 1 (PD-L1) immune checkpoint in HCC.
HCC is considered a therapy-resistant disease, and is frequently diagnosed at an advanced stage. Thus, the development of a novel therapeutic modality is essential. It is noteworthy that immune checkpoint blockade therapy in HCC is gaining attention. Additionally, given the wide range of non-coding RNAs (ncRNAs) that orchestrate PD-1/PD-L1 immune checkpoint, we investigated how selected ncRNAs regulate PD-1/PD-L1 immune checkpoint. Hence, the therapeutic potential of combining epigenetic immunomodulation through ncRNAs with immune checkpoint blockade was studied.
This study aimed at exploring potential upstream regulatory ncRNAs of immune checkpoint PD-1/PD-L1. Hence, the potential of combining immune checkpoint blockade with epigenetic immunomodulation was investigated.
Based on bioinformatics software and the literature, ncRNAs including miR-155-5p and miR-194-5p as well as lncRNAs X-inactive specific transcript (XIST) and MALAT-1 were selected. 23 HCC tissue biopsies and 10 healthy donor tissue biopsies were used to screen the expression of PD-L1 as well as lncRNAs XIST and MALAT-1. To study the interaction between miR-155-5p, miR-194-5p, lncRNAs XIST and MALAT-1, as well as PD-L1 mRNA, a series of transfections and co-transfections of the Huh-7 cell line was carried out. Quantified real-time polymerase chain reaction was then utilized to study the abundance of selected ncRNAs as well as PD-L1 transcripts in Huh-7 cells in the transfections experiments.
Based on bioinformatics software and the literature, we hypothesized that a potential upstream regulatory pathway to immune checkpoint PD-L1 is present in HCC, composed of both miRNAs, tumor suppressor miR-194-5p and oncomiR-155-5p, as well as both lncRNAs XIST and MALAT-1. Following the screening of 23 HCC biopsies, PD-L1 and XIST were found to be significantly upregulated compared to healthy controls; however, MALAT-1 was barely detected. Induced expression of miR-194-5p and miR-155-5p in the Huh-7 cell line showed the same pattern of upregulation of both PD-L1 transcript and XIST. However, ectopic expression of the respective miRNAs had a paradoxical impact on MALAT-1 abundance, i.e. miR-194-5p induced the upregulation of MALAT-1 while miR-155-5p downregulated the abundance of MALAT-1. Knockdown of XIST had no impact on PD-L1 expression; however, following knockdown of the negative regulator of X-inactive specific transcript (TSIX), PD-L1 expression was elevated, and MALAT-1 activity was abolished. Upon co-transfection of miR-194-5p with siMALAT-1, PD-L1 expression was elevated. On the other hand, co-transfection of miR-194-5p with siXIST did not have an impact on PD-L1 expression. Following co-transfection of miR-194 with siTSIX, PD-L1 expression was upregulated. Interestingly, the same PD-L1 expression pattern was revealed following the oncomiR-155-5p co-transfection series.
In conclusion, this study reported the controversial role of miR-194-5p in HCC and despite its paradoxical function of a tumour suppressor and having oncogenic activity in HCC, both had the same impact on upregulation of XIST. LncXIST is thought to be an intermediate player whose upregulation increased PD-L1 transcript abundance.
Although further investigations are needed, this study proposes a novel competing endogenous RNA circuit made up of both miR-155-5p and miR-194-5p as well as lncXIST and PD-L1 mRNA. This circuit could be regarded as a potential therapeutic target in HCC.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Aoki T, Hu B S-Editor: Zhang L L-Editor: Webster JR P-Editor: Liu JH
| 1. | Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2184] [Cited by in RCA: 2889] [Article Influence: 481.5] [Reference Citation Analysis (17)] |
| 2. | Llovet JM, Villanueva A, Lachenmayer A, Finn RS. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat Rev Clin Oncol. 2015;12:408-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 390] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 3. | Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018;15:599-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1458] [Cited by in RCA: 1378] [Article Influence: 196.9] [Reference Citation Analysis (0)] |
| 4. | Xie Y, Xiang Y, Sheng J, Zhang D, Yao X, Yang Y, Zhang X. Immunotherapy for Hepatocellular Carcinoma: Current Advances and Future Expectations. J Immunol Res. 2018;2018:8740976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 5. | Ji J, Yin Y, Ju H, Xu X, Liu W, Fu Q, Hu J, Zhang X, Sun B. Long non-coding RNA Lnc-Tim3 exacerbates CD8 T cell exhaustion via binding to Tim-3 and inducing nuclear translocation of Bat3 in HCC. Cell Death Dis. 2018;9:478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 126] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 6. | Flecken T, Schmidt N, Hild S, Gostick E, Drognitz O, Zeiser R, Schemmer P, Bruns H, Eiermann T, Price DA, Blum HE, Neumann-Haefelin C, Thimme R. Immunodominance and functional alterations of tumor-associated antigen-specific CD8+ T-cell responses in hepatocellular carcinoma. Hepatology. 2014;59:1415-1426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 288] [Cited by in RCA: 298] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 7. | Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2348] [Cited by in RCA: 2903] [Article Influence: 207.4] [Reference Citation Analysis (0)] |
| 8. | Kudo M. Immune Checkpoint Inhibition in Hepatocellular Carcinoma: Basics and Ongoing Clinical Trials. Oncology. 2017;92 Suppl 1:50-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 140] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 9. | Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 2006;27:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 557] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 10. | Kamphorst AO, Ahmed R. Manipulating the PD-1 pathway to improve immunity. Curr Opin Immunol. 2013;25:381-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887-3895. [PubMed] |
| 12. | Wei SC, Duffy CR, Allison JP. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018;8:1069-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1430] [Cited by in RCA: 2188] [Article Influence: 312.6] [Reference Citation Analysis (0)] |
| 13. | Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci. 2002;99:12293-12297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2103] [Cited by in RCA: 2451] [Article Influence: 106.6] [Reference Citation Analysis (0)] |
| 14. | Whiteside TL, Demaria S, Rodriguez-Ruiz ME, Zarour HM, Melero I. Emerging Opportunities and Challenges in Cancer Immunotherapy. Clin Cancer Res. 2016;22:1845-1855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 248] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 15. | Weber JS, D'Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH Jr, Lao CD, Linette GP, Thomas L, Lorigan P, Grossmann KF, Hassel JC, Maio M, Sznol M, Ascierto PA, Mohr P, Chmielowski B, Bryce A, Svane IM, Grob JJ, Krackhardt AM, Horak C, Lambert A, Yang AS, Larkin J. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1853] [Cited by in RCA: 2105] [Article Influence: 210.5] [Reference Citation Analysis (0)] |
| 16. | Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, Castellano D, Choueiri TK, Gurney H, Donskov F, Bono P, Wagstaff J, Gauler TC, Ueda T, Tomita Y, Schutz FA, Kollmannsberger C, Larkin J, Ravaud A, Simon JS, Xu LA, Waxman IM, Sharma P; CheckMate 025 Investigators. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373:1803-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4375] [Cited by in RCA: 4597] [Article Influence: 459.7] [Reference Citation Analysis (0)] |
| 17. | Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2870] [Cited by in RCA: 3570] [Article Influence: 357.0] [Reference Citation Analysis (0)] |
| 18. | El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3278] [Cited by in RCA: 3301] [Article Influence: 412.6] [Reference Citation Analysis (1)] |
| 19. | Zhao L, Yu H, Yi S, Peng X, Su P, Xiao Z, Liu R, Tang A, Li X, Liu F, Shen S. The tumor suppressor miR-138-5p targets PD-L1 in colorectal cancer. Oncotarget. 2016;7:45370-45384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 177] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 20. | Cortez MA, Ivan C, Valdecanas D, Wang X, Peltier HJ, Ye Y, Araujo L, Carbone DP, Shilo K, Giri DK, Kelnar K, Martin D, Komaki R, Gomez DR, Krishnan S, Calin GA, Bader AG, Welsh JW. PDL1 Regulation by p53 via miR-34. J Natl Cancer Inst. 2016;108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 313] [Cited by in RCA: 502] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 21. | Yao K, Wang Q, Jia J, Zhao H. A competing endogenous RNA network identifies novel mRNA, miRNA and lncRNA markers for the prognosis of diabetic pancreatic cancer. Tumour Biol. 2017;39:1010428317707882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 22. | Salama EA, Adbeltawab RE, El Tayebi HM. XIST and TSIX: Novel Cancer Immune Biomarkers in PD-L1-Overexpressing Breast Cancer Patients. Front Oncol. 2019;9:1459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 23. | Koduru SV, Tiwari AK, Leberfinger A, Hazard SW, Kawasawa YI, Mahajan M, Ravnic DJ. A Comprehensive NGS Data Analysis of Differentially Regulated miRNAs, piRNAs, lncRNAs and sn/snoRNAs in Triple Negative Breast Cancer. J Cancer. 2017;8:578-596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 24. | Klingenberg M, Matsuda A, Diederichs S, Patel T. Non-coding RNA in hepatocellular carcinoma: Mechanisms, biomarkers and therapeutic targets. J Hepatol. 2017;67:603-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 279] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 25. | Ghanbari R, Anwar F, Alkharfy KM, Gilani AH, Saari N. Valuable nutrients and functional bioactives in different parts of olive (Olea europaea L.)-a review. Int J Mol Sci. 2012;13:3291-3340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 377] [Cited by in RCA: 304] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 26. | Lockyer S, Corona G, Yaqoob P, Spencer JP, Rowland I. Secoiridoids delivered as olive leaf extract induce acute improvements in human vascular function and reduction of an inflammatory cytokine: a randomised, double-blind, placebo-controlled, cross-over trial. Br J Nutr. 2015;114:75-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 27. | Boss A, Bishop KS, Marlow G, Barnett MP, Ferguson LR. Evidence to Support the Anti-Cancer Effect of Olive Leaf Extract and Future Directions. Nutrients. 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 28. | Goldsmith CD, Bond DR, Jankowski H, Weidenhofer J, Stathopoulos CE, Roach PD, Scarlett CJ. The Olive Biophenols Oleuropein and Hydroxytyrosol Selectively Reduce Proliferation, Influence the Cell Cycle, and Induce Apoptosis in Pancreatic Cancer Cells. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 29. | Rizzo M, Ventrice D, Giannetto F, Cirinnà S, Santagati NA, Procopio A, Mollace V, Muscoli C. Antioxidant activity of oleuropein and semisynthetic acetyl-derivatives determined by measuring malondialdehyde in rat brain. J Pharm Pharmacol. 2017;69:1502-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Qabaha K, Al-Rimawi F, Qasem A, Naser SA. Oleuropein Is Responsible for the Major Anti-Inflammatory Effects of Olive Leaf Extract. J Med Food. 2018;21:302-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 31. | Song H, Lim DY, Jung JI, Cho HJ, Park SY, Kwon GT, Kang YH, Lee KW, Choi MS, Park JHY. Dietary oleuropein inhibits tumor angiogenesis and lymphangiogenesis in the B16F10 melanoma allograft model: a mechanism for the suppression of high-fat diet-induced solid tumor growth and lymph node metastasis. Oncotarget. 2017;8:32027-32042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 32. | Shamshoum H, Vlavcheski F, Tsiani E. Anticancer effects of oleuropein. Biofactors. 2017;43:517-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 33. | Castejón ML, Rosillo MÁ, Montoya T, González-Benjumea A, Fernández-Bolaños JG, Alarcón-de-la-Lastra C. Oleuropein down-regulated IL-1β-induced inflammation and oxidative stress in human synovial fibroblast cell line SW982. Food Funct. 2017;8:1890-1898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 34. | Ruzzolini J, Peppicelli S, Andreucci E, Bianchini F, Scardigli A, Romani A, la Marca G, Nediani C, Calorini L. Oleuropein, the Main Polyphenol of Olea europaea Leaf Extract, Has an Anti-Cancer Effect on Human BRAF Melanoma Cells and Potentiates the Cytotoxicity of Current Chemotherapies. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 35. | Zhang F, Zhang M. Oleuropein inhibits esophageal cancer through hypoxic suppression of BTG3 mRNA. Food Funct. 2019;10:978-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Olmo-Cunillera A, López-Yerena A, Lozano-Castellón J, Tresserra-Rimbau A, Vallverdú-Queralt A, Pérez M. NMR spectroscopy: a powerful tool for the analysis of polyphenols in extra virgin olive oil. J Sci Food Agric. 2020;100:1842-1851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 37. | Yan CM, Chai EQ, Cai HY, Miao GY, Ma W. Oleuropein induces apoptosis via activation of caspases and suppression of phosphatidylinositol 3-kinase/protein kinase B pathway in HepG2 human hepatoma cell line. Mol Med Rep. 2015;11:4617-4624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 38. | Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9936] [Cited by in RCA: 10332] [Article Influence: 794.8] [Reference Citation Analysis (34)] |
| 39. | Hong YK, Li Y, Pandit H, Li S, Pulliam Z, Zheng Q, Yu Y, Martin RCG. Epigenetic modulation enhances immunotherapy for hepatocellular carcinoma. Cell Immunol. 2019;336:66-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 40. | Yan XL, Jia YL, Chen L, Zeng Q, Zhou JN, Fu CJ, Chen HX, Yuan HF, Li ZW, Shi L, Xu YC, Wang JX, Zhang XM, He LJ, Zhai C, Yue W, Pei XT. Hepatocellular carcinoma-associated mesenchymal stem cells promote hepatocarcinoma progression: role of the S100A4-miR155-SOCS1-MMP9 axis. Hepatology. 2013;57:2274-2286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 41. | Xie Q, Chen X, Lu F, Zhang T, Hao M, Wang Y, Zhao J, McCrae MA, Zhuang H. Aberrant expression of microRNA 155 may accelerate cell proliferation by targeting sex-determining region Y box 6 in hepatocellular carcinoma. Cancer. 2012;118:2431-2442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 42. | El Tayebi HM, Waly AA, Assal RA, Hosny KA, Esmat G, Abdelaziz AI. Transcriptional activation of the IGF-II/IGF-1R axis and inhibition of IGFBP-3 by miR-155 in hepatocellular carcinoma. Oncol Lett. 2015;10:3206-3212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 43. | Zhao Y, Li F, Zhang X, Liu A, Qi J, Cui H, Zhao P. MicroRNA-194 acts as a prognostic marker and inhibits proliferation in hepatocellular carcinoma by targeting MAP4K4. Int J Clin Exp Pathol. 2015;8:12446-12454. [PubMed] |
| 44. | Bao C, Li Y, Huan L, Zhang Y, Zhao F, Wang Q, Liang L, Ding J, Liu L, Chen T, Li J, Yao M, Huang S, He X. NF-κB signaling relieves negative regulation by miR-194 in hepatocellular carcinoma by suppressing the transcription factor HNF-1α. Sci Signal. 2015;8:ra75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 45. | Jung HS, Seo YR, Yang YM, Koo JH, An J, Lee SJ, Kim KM, Kim SG. Gα12gep oncogene inhibits FOXO1 in hepatocellular carcinoma as a consequence of miR-135b and miR-194 dysregulation. Cell Signal. 2014;26:1456-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 46. | Svoronos AA, Engelman DM, Slack FJ. OncomiR or Tumor Suppressor? Cancer Res. 2016;76:3666-3670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 580] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 47. | Mo Y, Lu Y, Wang P, Huang S, He L, Li D, Li F, Huang J, Lin X, Li X, Che S, Chen Q. Long non-coding RNA XIST promotes cell growth by regulating miR-139-5p/PDK1/AKT axis in hepatocellular carcinoma. Tumour Biol. 2017;39:1010428317690999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 48. | Guerrieri F. Long non-coding RNAs era in liver cancer. World J Hepatol. 2015;7:1971-1973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 49. | Guo S, Chen W, Luo Y, Ren F, Zhong T, Rong M, Dang Y, Feng Z, Chen G. Clinical implication of long non-coding RNA NEAT1 expression in hepatocellular carcinoma patients. Int J Clin Exp Pathol. 2015;8:5395-5402. [PubMed] |
| 50. | Eißmann M, Gutschner T, Hämmerle M, Günther S, Caudron-Herger M, Groß M, Schirmacher P, Rippe K, Braun T, Zörnig M, Diederichs S. Loss of the abundant nuclear non-coding RNA MALAT1 is compatible with life and development. RNA Biol. 2012;9:1076-1087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 327] [Cited by in RCA: 333] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 51. | Kong Q, Zhang S, Liang C, Zhang Y, Kong Q, Chen S, Qin J, Jin Y. LncRNA XIST functions as a molecular sponge of miR-194-5p to regulate MAPK1 expression in hepatocellular carcinoma cell. J Cell Biochem. 2018;119:4458-4468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 52. | Ohaegbulam KC, Assal A, Lazar-Molnar E, Yao Y, Zang X. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol Med. 2015;21:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 616] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 53. | Liu D, Zhu Y, Pang J, Weng X, Feng X, Guo Y. Knockdown of long non-coding RNA MALAT1 inhibits growth and motility of human hepatoma cells via modulation of miR-195. J Cell Biochem. 2018;119:1368-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 54. | Zhu K, Wang W. Green tea polyphenol EGCG suppresses osteosarcoma cell growth through upregulating miR-1. Tumour Biol. 2016;37:4373-4382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 55. | Coombs MR, Harrison ME, Hoskin DW. Apigenin inhibits the inducible expression of programmed death ligand 1 by human and mouse mammary carcinoma cells. Cancer Lett. 2016;380:424-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 56. | Abtin M, Alivand MR, Khaniani MS, Bastami M, Zaeifizadeh M, Derakhshan SM. Simultaneous downregulation of miR-21 and miR-155 through oleuropein for breast cancer prevention and therapy. J Cell Biochem. 2018;119:7151-7165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |