Published online Nov 27, 2020. doi: 10.4254/wjh.v12.i11.919
Peer-review started: August 6, 2020
First decision: September 17, 2020
Revised: September 19, 2020
Accepted: September 27, 2020
Article in press: September 27, 2020
Published online: November 27, 2020
Processing time: 109 Days and 16.6 Hours
An adequate balance between electrolytes and clear water is of paramount importance to maintaining physiologic homeostasis. Natremia imbalance and, in particular, hyponatremia is the most frequent electrolyte abnormality observed in hospitalized subjects, involving approximately one-fourth of them. Pathological changes occurring during liver cirrhosis predispose patients to an increased risk of sodium imbalance, and hypervolemic hyponatremia has been reported in nearly 50% of subjects with severe liver disease and ascites. Splanchnic vasodilatation, portal-systemic collaterals’ opening and increased excretion of vasoactive modulators are all factors impairing clear water handling during liver cirrhosis. Of concern, sodium imbalance has been consistently reported to be associated with increased risk of complications and reduced survival in liver disease patients. In the last decades clinical interest in sodium levels has been also extended in the field of liver transplantation. Evidence that [Na+] in blood is an independent risk factor for in-list mortality led to the incorporation of sodium value in prognostic scores employed for transplant priority, such as model for end-stage liver disease-Na and UKELD. On the other hand, severe hyponatremic cirrhotic patients are frequently delisted by transplant centers due to the elevated risk of mortality after grafting. In this review, we describe in detail the relationship between sodium imbalance and liver cirrhosis, focusing on its impact on peritransplant phases. The possible therapeutic approaches, in order to improve transplant outcome, are also discussed.
Core Tip: Sodium imbalance represents an important issue in cirrhotic patients. In the last decades, the impact of altered sodium levels in the peritransplant phases has also gained a relevant clinical interest. In this review, we examined: (1) The determinants of an impaired sodium balance in the course of severe liver diseases; (2) The consequences of sodium imbalance on liver transplant; and (3) The possible corrective measures for this condition.
- Citation: Lenci I, Milana M, Grassi G, Signorello A, Aglitti A, Baiocchi L. Natremia and liver transplantation: The right amount of salt for a good recipe. World J Hepatol 2020; 12(11): 919-930
- URL: https://www.wjgnet.com/1948-5182/full/v12/i11/919.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i11.919
We are all well aware that the human body is composed of a high percentage of water (approximately 60% by weight), with significant changes among different tissues and according to sex and age[1-3]. However, when we examine the human physio-pathological mechanisms, it would be better to keep in mind that this large fluid mass is actually composed of “salt” water[4]. The accurate proportion/relationship between electrolytes and water is of paramount importance to preserve cellular and tissue homeostasis. Moreover, specific transporters, such as the sodium pump or aquaporin channels, exist to keep definite gradients of electrolytes and water between the inside and outside of the cell[5]. Failure to maintain adequate solute concentration (osmolality) between the intra- and extracellular compartments may affect tonicity, determining cellular damage for shrinkage or swelling. The accumulation in the cell of sodium (Na+), the most concentrated cation in extracellular fluid, is prevented by the sodium pump. For this reason, Na+ is not only the main determinant of plasma osmolality but it also plays an important role in maintaining isotonicity between the intra- and extracellular environment[6].
Central regulation of the sodium-water balance and tonicity are obtained by brain/kidney crosstalk[7,8]. In brief, during physiologic conditions, neurohypophysis detects hypertonic signals recorded by osmoreceptors and activates vasopressin [i.e. antidiuretic hormone; antidiuretic hormone (ADH)] release by the pituitary gland. ADH, in turn, stimulates kidney reabsorption of water, thus reducing hyperosmolarity. Thirst is also stimulated by osmoreceptors as long as homeostasis is not reacquired. In hypotonic conditions, the mechanism is the reverse, with decreased ADH secretion and inhibition of thirst. Despite this fine-tuned regulation of osmolality, altered sodium serum levels are frequently encountered in clinical practice; in this case, our attention is usually recalled by the Na+ reported value; however, changes in plasmatic sodium are more frequently the expression of an impaired water balance rather than electrolyte increase or loss.
In this review, we examined natremia changes in pathological conditions focusing on the aspects observed during liver cirrhosis. The consequences of altered sodium levels on the outcome of patients undergoing liver transplantation (LT) and the possible corrective measures will also be discussed.
While natremia imbalance should be considered a symptom rather than a disease, evidence in clinical practice of a plasma sodium concentration lower than 135 or higher than 145 mEq/L, respectively, defines hypo or hypernatremia. Hyponatremia is by far the more frequently encountered electrolyte abnormality in hospitalized patients[9-11]. In a large retrospective study, prevalence of Na+ < 135 mEq/L was 22.1% and 14.7% in hospitalized and ambulatory patients, respectively[12]. Despite the finding of hyponatremia, a hypo, hyper or isotonic plasma/cell condition should not be ruled out since other solutes not able to cross plasma membrane (such as glucose) may impact tonicity[13,14]. However, hypotonic or dilutional hyponatremia caused by excess water intake or impaired kidney water disposal is the most frequent form[14].
Hyponatremia should also be discriminated in the clinic according to the volume of extracellular fluid in hypo, normo, or hypervolemic forms[15]. In chronic diseases such as cardiac insufficiency, nephrotic syndrome, or liver cirrhosis, a hypervolemic hyponatremia is usually present, characterized by edema. Concern exists in hospitalized patients because of the strict relationship between morbidity/mortality and hyponatremia[16]. Moreover, an increased risk of mortality, in sodium-deficient hospitalized patients, seems to be present also for mild reduction of blood [Na+] (130-134 mEq/L) and persists also in the 5-year follow-up after discharge[17,18].
With regard to mortality, the most dramatic complications of acute hyponatremia (also when a too rapid correction of sodium depletion occurs) are considered the neurologic ones[19,20]. Symptoms of onset are represented by lethargy, vomiting, headache and confusion, among others, reflecting impairment of the central nervous system as a consequence of a rapid change of extracellular tonicity exiting in brain edema and possible demyelination[14]. This condition is characterized by a relevant morbidity in more than one-third of subjects[21]. However, (1) since this important complication requires rapid modification of plasma sodium concentration and (2) considering that an adaptive response to osmotic changes exists in the brain (constituted mainly by the shift of fluid in the subarachnoid space[22] and loss of solutes by brain cells[23]), increased overall mortality observed in patients with hyponatremia does not seem completely explained by neurological involvement. In this perspective, besides patients in which sodium depletion is the main cause of death, there are others in whom hyponatremia is only the innocent expression of a chronic severe disease, thus not eliciting a direct impact (or with just a partial impact) on mortality[24].
Hypernatremia, recognized by a plasma [Na+] > 145 mEq/L (as also stated before), is a condition with a lower prevalence in comparison with hyponatremia, but it is associated, like the latter, with significant mortality. While hyponatremia may be present in different conditions of extracellular fluid tonicity, the hypernatremia is constantly characterized by a hypertonic plasma[14]. In the pathogenesis of the increased plasma sodium concentration, an imbalance between clear water and Na+ loss/intake occurs, with concomitant failure of the compensatory systems, including the brain-kidney axis and the thirst triggering mechanism[25]. Given the important defense operated by thirst stimulation against hypernatremia, increased plasma sodium levels usually occur when the sensitivity to central stimuli is absent or greatly reduced. Therefore, in clinical practice, patients with reduced consciousness, such as those in an intensive care unit are at major risk for hypernatremia[26-28]. In outpatient settings increased sodium levels are rarely observed and the main causes are represented by diarrhea (in elderly and small children), excessive use of diuretic drugs, diabetes insipidus, and others[25,26].
From all the above, it is clear that complex diagnostic flow charts are sometimes needed to achieve a correct diagnosis in patients with sodium imbalance. In other clinical situations, such as in liver cirrhosis, changes in sodium blood levels are instead characteristic of the disease.
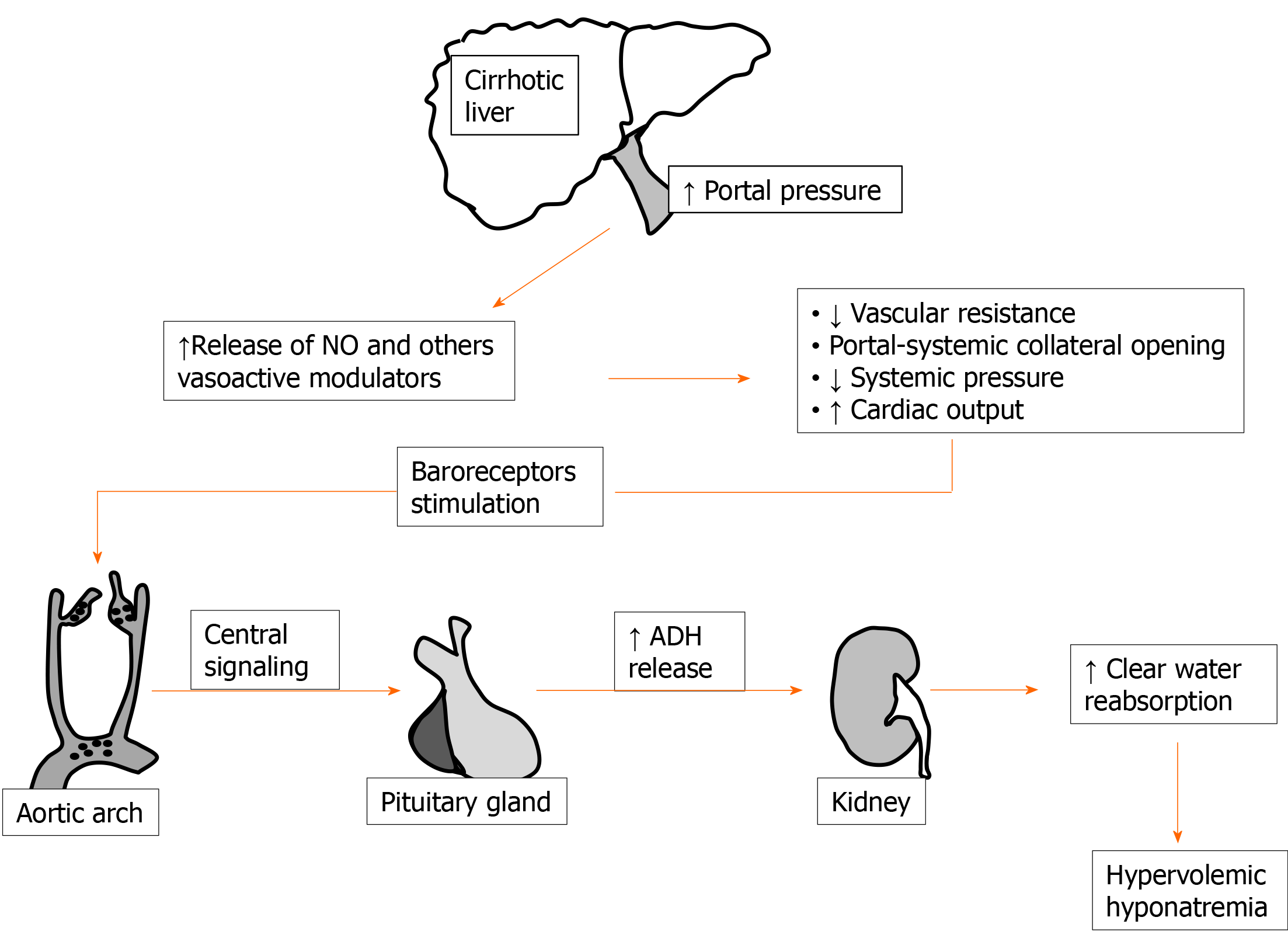
Impaired water handling has long been demonstrated in liver cirrhosis[29]; this is well documented by the frequent finding of ascites and edema in patients with relevant liver impairment[30]. Ascites and dilutional hyponatremia are, in liver cirrhosis, the ending evolution of the same pathological sequence of events. In fact, as an important step, a hyperdynamic circulation with increased cardiac output and decreased blood pressure for reduced peripheral vascular resistance develops[31]. Fall of peripheral resistance, which is not justified by increased requirement of oxygen by tissues, is clearly evident also by the subject examination usually exhibiting warm extremities, palmar erythema, spider naevi, and other typical signs. Rearrangement of the systemic circulation is thought to occur as a response to portal hypertension, stimulating splanchnic vasodilatation and portal-systemic collaterals’ opening[32]. Vasodilation in this setting seems to be maintained by the excretion of several modulators[33]. Among these, nitric oxide has long been identified as of major importance[32,34-36].
Finally, arterial underfilling activates baroreceptors stimulating chronic ADH release. Increased reabsorption of clear water is then the determinant of fluid accumulation and hypervolemic hyponatremia in decompensated cirrhosis[37]. A simplified scheme of events leading to dilutional hyponatremia during cirrhosis is reported in Figure 1. Considering (1) the constant occurrence of altered clear water handling in the majority of patients with advanced liver disease and (2) in order to have a more tailored indication on pathological sodium levels in these patients, the beginning of the threshold limit for hyponatremia in cirrhotic patients was reduced to [Na+] < 130 mEq/L[38,39].
Detection of persistent hyponatremia in cirrhotic patients represents a worrisome event because of its relationship with complications and mortality[40,41]. In 2006, a prospective survey on 997 cirrhotic patients with ascites, coming from 28 worldwide centers, was performed in order to assess the impact of natremia on the onset of complications[42]. This study demonstrated a prevalence of hyponatremia (Na+ < 135 mEq/L) of nearly 50%, while 21.6% exhibited a Na+ < 130 mEq/L. Moreover, low sodium levels were associated with a more severe grade of liver disease (Child-Pugh C) and an increased risk of major complications, such as hepatic encephalopathy, hepatorenal syndrome (HRS), and spontaneous bacterial peritonitis, excluding only gastrointestinal bleeding. Of particular concern is the association between natremia and HRS. The latter, that is currently diagnosed on the basis of acute kidney injury criteria (baseline increase of serum creatinine ≥ 0.3 mg/DL and/or ≥ 50% within 48 h), has, in fact, a recorded mortality higher than 50%[43,44]. HRS is still in search of adequate medical treatment, and the complex interplay between hyponatremia and HRS are discussed in detail in a 2015 review by Mohanty et al[45].
Finally, hyponatremia has been demonstrated to impact the health-related quality of life (HRQL) of cirrhotic patients. This is also observed in the absence of hepatic encephalopathy[46]. In a study, sodium correction in 21 patients with cirrhosis determined a statistical improvement in HRQL and cognitive performance in comparison with the corresponding baseline values[47]. This finding, as also suggested in an editorial of the same journal, underscored the fact that correction of hyponatremia in cirrhosis did not correspond to just “treating a number” but to a cure for several clinical complications, including a reduced HRQL[48].
Hypernatremia is more seldom observed and less studied in cirrhotic patients, accounting for 2%-5% of cases only[42,49]. It has been described in severely ill patients after the lactulose-induced loss of fluid or in concomitance with decompensated diabetes; however, a definite picture of hypernatremia prevalence, morbidity, and mortality in cirrhosis is not available at present[50,51].
Given the importance of sodium imbalance in liver diseases, with regard to its relationship with complications and mortality, the attention to sodium was also translated in the field of liver transplantation in the last decades. Natremia levels were, in fact, examined for their possible role as a prognostic factor in the waiting list, as a predictor of outcome after grafting, and as possible effectors of complications in the peritransplant phases.
Starting from the new millennium, it was evident in the United States that liver transplantation should be offered on a “sickest first basis” rather than on-list waiting time[52]. In this perspective, prognostic scores to forecast a short term decrease in cirrhotic patients acquired paramount importance in order to select subjects more in need of a transplant with a consequent reduction of in-list mortality. The model for end-stage liver disease (MELD) score (calculated on the base of bilirubin, creatinine, and international normalized ratio values)[53], originally proposed to evaluate 3-mo liver-related mortality in cirrhotic patients undergoing trans-jugular-portal-systemic shunt, seemed at that time adequate also for the selection of best liver transplant (LT) candidates[54,55]. However, assessment of the MELD score in the real LT world suggested that dynamic evaluation of these parameters or adoption of adjunctive parameters should determine a better selection of patients for LT[56].
A study on 507 patients evaluated for LT in the United States aimed to find additional parameters (not included in MELD) indicating short-term mortality[41]. This research evidenced that persistent ascites and sodium serum levels < 135 mEq/L represented important predictors of mortality that improved the performance of low (< 21) MELD score. Since the impact on survival of water imbalance was not captured by the MELD score and in need to find a possible objective parameter paralleling the severity of edema, a study was conducted to evaluate the predictive value of MELD and sodium serum levels in the waiting list for LT[57]. In 554 cirrhotic patients in a single United States center: (1) A natremia < 126 mEq/L at listing was associated with an increased (nearly 8 times) risk of death; (2) The estimation of risk based on sodium levels was independent by MELD score; and (3) The inclusion of Na with MELD seemed to improve performance in estimating mortality at 3 mo and 6 mo. The role of sodium levels as an independent factor predicting in-list mortality was then definitively demonstrated in a very large study with data retrieved from the Organ Procurement and Transplantation Network database[58]. This research demonstrated a better graft allocation employing the MELD score and Na+ value together (more evident for low MELD score), with a possible 7% reduction of mortality in the list when this scheme was applied to retrospective results.
Further evidence-based studies led to the incorporation of natremia in the MELD score, giving origin to MELD-Na in the United States and UKELD in the United Kingdom, respectively[59,60]. Both scores were demonstrated to perform better than the original MELD at 3 mo and 6 mo[61], even if their superiority was not demonstrated when evaluating patients with acute liver failure[62]. At present, the importance of sodium serum level inclusion in the MELD score has also been confirmed in an updated model undergoing optimization of coefficient bounds[63]. However, some authors advanced concern in including natremia for LT allocation since this parameter could be artificially altered in the clinical setting, and its contribution in the MELD model seems to be limited as well as restricted to low score[64].
Interest in sodium serum levels and LT then rose also with regard to surgical outcome. In an early retrospective European study on 241 cirrhotic patients undergoing LT, a prevalence of hyponatremia (< 130 mEq/L) of 8% was found[65]. Hyponatremic patients had ascites in 100% of cases and more severe liver disease before LT. In the first month after LT, the incidence of neurologic, infectious, or kidney complications was statistically more frequent in patients transplanted with a [Na+] < 130 mEq/L in comparison with others. This translated into a significant reduction of 3 mo survival (84% vs 95%; P < 0.05). A subsequent United Kingdom multicenter study reassessed this issue on 5152 patients undergoing LT and in whom pre-transplant sodium data were available[66]. Patients were stratified according to blood [Na+] in severely hyponatremic (< 130 mEq/L), hyponatremic (130-134 mEq/L), normal (135-145 mEq/L), and hypernatremic > 145 mEq/L. The 3-mo mortality was increased in patients with sodium < 130 mEq/L, accounting for approximately 15% of cases, while the impact on mortality of hypernatremia was even more evident, accounting for 25% of cases. However, the finding of increased sodium levels was 20-times less frequent than hyponatremia in the study. Finally, patients with sodium serum levels falling between 130-134 mEq/L did not exhibit a difference in mortality in comparison with eunatremic subjects.
Despite the fact that the main cause of death in all groups in the study was represented by infections, thus evolving in multi-organ failure, the authors suggested that the occurrence of central nervous system complications was the first trigger increasing mortality in groups with natremia imbalance. In fact, a previous study demonstrated that rapid corrective osmotic changes occurring during transplant and in early postoperative phases might be responsible in patients with deranged sodium serum levels of pontine and extrapontine myelinolysis[67,68]. Unfortunately, the occurrence of this complication was not assessed in the study. Prevalence of central pontine myelinolysis after LT and according to pretransplant natremia levels was then evaluated in a large United States study[69]. Central pontine myelinolysis was evidenced in 0.5% of the entire cohort (2175 patients) and was associated with the presence of hyponatremia. Interestingly in this American study, differently from previous European data, even if Na+ levels were associated with longer intensive care unit and in-hospital stay, an increased 90 d mortality after LT was not found.
The possible role of hyponatremia on LT short-term survival was again challenged by a following United States large study[70]. In this cohort of nearly 20.000 patients, there was no difference in short-term (90 d) survival after LT between hyponatremic and normonatremic patients. On the other hand, an important (statistically significant) reduced survival was observed in hypernatremic (Na+ > 145 mEq/L) subjects. The interesting discrepancy between the European and American studies does not have a clear explanation so far. However, it is possible that in European studies: (1) Hyponatremia was the expression of more severe liver disease; (2) The use of marginal graft was more largely applied; and (3) Different etiologies of liver diseases (with worse outcomes) were more represented[70]. More recently, a monocentric study with a limited number of patients (n = 306) reassessed the issue of natremia and short-term neurological complications[71]. In this research, while either hypo (< 130 mEq/L) or hypernatremia (< 145 mEq/L) did not have an effect on short-term survival after LT, a relationship between the magnitude of sodium levels correction (> 10 mEq/L), neurological complication, and reduced outcome was observed.
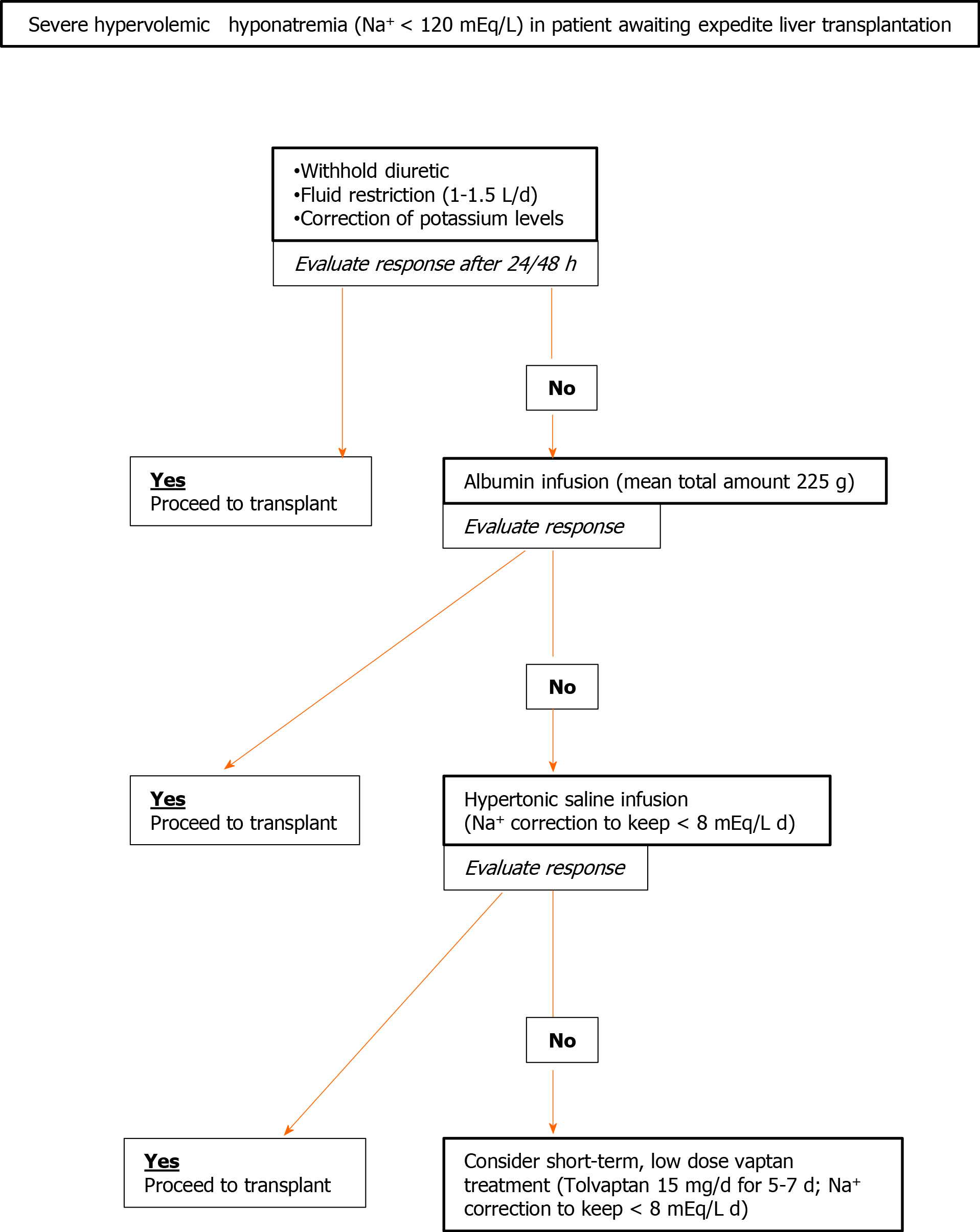
As reported above, the general issue of sodium imbalance in cirrhosis acquires particular importance with regard to patients proceeding toward LT. The management of hyponatremia in liver disease patients (the most frequent electrolyte alterations observed) changes widely according to the clinical picture. Acute hypovolemic hyponatremia (observed for extended diuretic therapy or fluid loss) may be managed with success by employing sodium and fluid replacement therapy[37]. On the other hand, treatment of chronic hypervolemic (dilutional) hyponatremia, that represents the expression of a more general impairment of clear water handling, is complex and overall results remain unsatisfactory. The heterogeneous management of this condition by different transplant centers, in the lack of a shared guideline, reflects the complexity of this pathological alteration and the physicians’ concern. The United States data show that the majority of LT centers delist patients with Na+ < 120 mEq/L and that a specific protocol to manage low natremic levels before surgery is adopted in less than one-third of transplant centers[72]. These findings suggest that the establishment of an optimal therapy of dilutional hyponatremia in cirrhotic patients may increment their access to transplants, also reducing in-list mortality and possible peritransplant complications.
In clinical practice and in this setting, treatment for decreased natremia is a multistep route to be tailored according to therapy response and target[73]. Low-level hyponatremia (Na+ < 135; ≥ 130 mEq/L) is not deemed worthy of treating since it is considered a frequent and harmless feature of severe liver disease. Sodium values lower than 130 mEq/L may be considered for corrective measures, even if symptoms are seldom observed above 125 mEq/L. Less aggressive, first-line therapy is represented by the withholding of diuretic drugs and fluid restriction. An adequate reduction of fluid intake (1-1.5 L/d) is frequently difficult to obtain because of compliance issues. When this measure is effective, blood sodium level rises within 24-48 h[72,74]. Correction of hypokalemia and administration of albumin may also improve sodium levels[72-74]. Potassium, when it is administered to restore the normal serum range, migrates in the cell, shifting sodium in the extracellular space to equilibrate the net charge concentration. The use of albumin, for sodium correction in cirrhotic patients, despite the efficacy, remains more controversial due to the cost and its transient effect[75]; however, in hyponatremic patients awaiting LT, it seems a reasonable second-line treatment to reduce possible perisurgical complications after the failure of fluid restriction.
In a large United States study on cirrhotic hyponatremic (< 130 mEq/L) patients, albumin administration was statistically associated with normalization of sodium levels; this, in turn, had a positive effect on 30-d survival[76]. The effect of albumin on natremia may be related to both its oncotic and non-oncotic (inhibition of vasodilators release) properties, as underlined in a commentary in the same journal[77].
The possibility to administer hypertonic saline (generally contraindicated in cirrhotic patients since it increases ascites and edema) may be considered as a short–term treatment, in the few days preceding LT, in severely hyponatremic patients. However, drastic changes in sodium serum levels (> 8 mEq/L daily) should be carefully avoided for the possible onset of significant adverse events in these fragile patients[73]. In this perspective, it seems reasonable to postpone hypertonic saline infusion after attempting to correct sodium levels with fluid restriction or albumin infusion. In fact, the latter strategies are not flawed by significant complications.
Finally, a more complex therapeutic approach includes the use of vaptans; the latter are specific inhibitors of vasopressin (V)-receptors[78]. Among different V receptors (V1a, V1b, and V2), the one involved in kidney clear water reabsorption is the V2. Great interest was raised on V2-vaptans inhibitors (lixivaptan, stavaptan, and tolvaptam) for their possible beneficial effects in cirrhosis since they seemed to target the specific physio-pathological mechanisms leading to dilutional hyponatremia and edema during end-stage liver diseases. Three meta-analyses, focusing on cirrhotic patients, during the last decade consistently demonstrated correction of natremia and reduction of ascites and related complications, such as spontaneous bacterial peritonitis. However, a clear effect on survival was never observed also after a long-term (> 26 wk) follow-up[79-81]. Moreover, a safety warning was released by the Food and Drug Administration in 2013 to avoid tolvaptan in patients with underlying liver disease, since increased liver enzymes were observed in 4.4% of patients (significant alteration in 1%) in a trial on autosomal dominant polycystic kidney disease[82]. On the other hand, despite the fact that early data on autosomal dominant polycystic kidney disease evidenced changes in liver biochemistry during vaptans (tolvaptan) therapy[83], a significant increase in adverse events in cirrhotic patients was never observed. A more worrisome complication related to V2-specific vaptans use might be considered the too rapid (> 8 mEq/L day) correction of sodium levels, with possible neurologic deleterious effect. For this reason, their administration should be decided, managed, and monitored by expert centers. However, the utility of long-term routine use of tolvaptan (the only V2-specific oral vaptan available) for chronic hyponatremia in cirrhotic patients, remains undefined and uncertain since the quick reversal of the therapeutic effect when treatment is withdrawn and the possible adverse events. For these reasons, hyponatremia in cirrhosis remains an “off label” indication for this drug in the majority of countries.
The clinical setting of LT is nevertheless different from the general management of liver cirrhosis. Sometimes, a patient needing an accelerated LT because of a poor prognosis may present with severe hyponatremia. This condition exposes the candidate to a well-recognized risk of neurological complications in the perisurgical phase and maybe to an “a priori” exclusion from transplant. In this complex and specific clinical situation, in which possible drug-induced liver toxicity appears negligible as LT is already required, it seems correct to consider vaptans treatment[72,84]. In this perspective, tolvaptan was experimented for the first time by our group in two LT candidates presenting with severely reduced sodium levels[85]. These subjects were in need of an expedited LT (MELD > 30), both with a natremia approaching 120 mEq/L. After the failure of fluid restriction and hypertonic saline administration, they underwent a short-term (5 d) low-dose (15 mg daily) administration of tolvaptan, with a rise of sodium levels > 130 mEq/L. The drug administration (after acquisition of an informed consent due to the risk of increased liver damage) was carefully followed–up, with frequent testing of liver function parameters and sodium levels, as the patients were hospitalized in our liver sub-intensive unit. We did not observe any major changes in patient biochemical tests. One patient was transplanted and the outcome was uneventful, while the other died of multi-organ failure since an appropriate graft was not retrievable despite the urgency. Our data demonstrated that this short-term low-dose administration was effective, safe, and feasible also in the presence of relevant cholestasis and coagulopathy (both patients had bilirubin > 10 mg/dL and international normalized ratio > 2.5). Our experience was also replicated in a case report of an LT candidate with a less severe liver impairment (MELD17)[86].
Finally, a study was undertaken with tolvaptan in LT candidates in order to improve refractory ascites[87]. Ten patients were treated with a very low dose of tolvaptan (starting from 3.75 mg/d) together with standard diuretic therapy. Six of them had a reduction > 1.5 Kg of body weight within 1 wk of treatment. No major changes were observed with regard to liver function tests or natremic levels.
Also in the lack of unequivocal data and clear guidelines, a possible multi-step approach to severe hypervolemic hyponatremia, for candidates to expedite LT, is proposed in Figure 2.
Sodium imbalance, and in particular dilutional hyponatremia, is frequently encountered in patients with severe liver disease. Low sodium levels in cirrhosis should be considered as the expression of a more complex impairment of water handling rather than a mere electrolyte deficiency. In this perspective, the scarce effects of standard maneuvers for natremia correction are not surprising since the ideal treatment should be targeted to reverse the mechanisms at the base of hyperdynamic circulation or the increased excretion of nitric oxide and ADH. While waiting for appropriate medical treatment, sodium imbalance continues to affect survival and to increase complications in cirrhotic patients. It also sometimes prevents their transplantability, increasing their list mortality and the complexity of post-transplant outcomes. A definitive solution to these aspects would be an important achievement in the future. For the time being, we have to consider that LT represents the most effective therapy for chronic natremia unbalance in cirrhosis and that hyponatremic patients are those experiencing the major survival benefit from this procedure[88].
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dabbous H, Singh SA S-Editor: Zhang L L-Editor: Filipodia P-Editor: Wang LL
| 1. | Watson PE, Watson ID, Batt RD. Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am J Clin Nutr. 1980;33:27-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1012] [Cited by in RCA: 969] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 2. | Friis-Hansen BJ, Holiday M, Stapleton T, Wallace WM. Total body water in children. Pediatrics. 1951;7:321-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 3. | Forbes RM, Cooper AR, Mitchell HH. The composition of the adult human body as determined by chemical analysis. J Biol Chem. 1953;203:359-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 161] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Sterns RH. Disorders of plasma sodium--causes, consequences, and correction. N Engl J Med. 2015;372:55-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 329] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 5. | Illarionova NB, Gunnarson E, Li Y, Brismar H, Bondar A, Zelenin S, Aperia A. Functional and molecular interactions between aquaporins and Na,K-ATPase. Neuroscience. 2010;168:915-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Seay NW, Lehrich RW, Greenberg A. Diagnosis and Management of Disorders of Body Tonicity-Hyponatremia and Hypernatremia: Core Curriculum 2020. Am J Kidney Dis. 2020;75:272-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 7. | Davenport A. The brain and the kidney--organ cross talk and interactions. Blood Purif. 2008;26:526-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Knepper MA, Kwon TH, Nielsen S. Molecular physiology of water balance. N Engl J Med. 2015;372:1349-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 178] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 9. | Anderson RJ, Chung HM, Kluge R, Schrier RW. Hyponatremia: a prospective analysis of its epidemiology and the pathogenetic role of vasopressin. Ann Intern Med. 1985;102:164-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 395] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 10. | Upadhyay A, Jaber BL, Madias NE. Incidence and prevalence of hyponatremia. Am J Med. 2006;119:S30-S35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 494] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 11. | Mannesse CK, Vondeling AM, van Marum RJ, van Solinge WW, Egberts TC, Jansen PA. Prevalence of hyponatremia on geriatric wards compared to other settings over four decades: a systematic review. Ageing Res Rev. 2013;12:165-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Hawkins RC. Age and gender as risk factors for hyponatremia and hypernatremia. Clin Chim Acta. 2003;337:169-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 257] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 13. | Gennari FJ. Current concepts. Serum osmolality. Uses and limitations. N Engl J Med. 1984;310:102-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 169] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Adrogué HJ, Madias NE. Hyponatremia. N Engl J Med. 2000;342:1581-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1255] [Cited by in RCA: 1104] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 15. | Hoorn EJ, Zietse R. Diagnosis and Treatment of Hyponatremia: Compilation of the Guidelines. J Am Soc Nephrol. 2017;28:1340-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 197] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 16. | Zilberberg MD, Exuzides A, Spalding J, Foreman A, Jones AG, Colby C, Shorr AF. Epidemiology, clinical and economic outcomes of admission hyponatremia among hospitalized patients. Curr Med Res Opin. 2008;24:1601-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 131] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 17. | Waikar SS, Mount DB, Curhan GC. Mortality after hospitalization with mild, moderate, and severe hyponatremia. Am J Med. 2009;122:857-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 499] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 18. | Mohan S, Gu S, Parikh A, Radhakrishnan J. Prevalence of hyponatremia and association with mortality: results from NHANES. Am J Med 2013; 126: 1127-37. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 183] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 19. | Arieff AI. Hyponatremia, convulsions, respiratory arrest, and permanent brain damage after elective surgery in healthy women. N Engl J Med. 1986;314:1529-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 353] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 20. | Alkali NH, Jibrin YB, Dunga JA, Abdu A. Osmotic demyelination syndrome following acute kidney injury with hypernatremia. Niger J Clin Pract. 2019;22:1166-1168. [PubMed] |
| 21. | Ayus JC, Achinger SG, Arieff A. Brain cell volume regulation in hyponatremia: role of sex, age, vasopressin, and hypoxia. Am J Physiol Renal Physiol. 2008;295:F619-F624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 153] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 22. | Fisher SK, Heacock AM, Keep RF, Foster DJ. Receptor regulation of osmolyte homeostasis in neural cells. J Physiol. 2010;588:3355-3364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Verbalis JG. Brain volume regulation in response to changes in osmolality. Neuroscience. 2010;168:862-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 24. | Hoorn EJ, Zietse R. Hyponatremia and mortality: moving beyond associations. Am J Kidney Dis. 2013;62:139-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 149] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 25. | Muhsin SA, Mount DB. Diagnosis and treatment of hypernatremia. Best Pract Res Clin Endocrinol Metab. 2016;30:189-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 26. | Lindner G, Funk GC. Hypernatremia in critically ill patients. J Crit Care 2013; 28: 216.e11-216. e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Funk GC, Lindner G, Druml W, Metnitz B, Schwarz C, Bauer P, Metnitz PG. Incidence and prognosis of dysnatremias present on ICU admission. Intensive Care Med. 2010;36:304-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 260] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 28. | Waite MD, Fuhrman SA, Badawi O, Zuckerman IH, Franey CS. Intensive care unit-acquired hypernatremia is an independent predictor of increased mortality and length of stay. J Crit Care. 2013;28:405-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Epstein M. Derangements of renal water handling in liver disease. Gastroenterology. 1985;89:1415-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 44] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Moore KP, Aithal GP. Guidelines on the management of ascites in cirrhosis. Gut. 2006;55 Suppl 6:vi1-v12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 207] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 31. | Abelmann WH. Hyperdynamic circulation in cirrhosis: a historical perspective. Hepatology. 1994;20:1356-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Bolognesi M, Di Pascoli M, Verardo A, Gatta A. Splanchnic vasodilation and hyperdynamic circulatory syndrome in cirrhosis. World J Gastroenterol. 2014;20:2555-2563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 133] [Cited by in RCA: 149] [Article Influence: 13.5] [Reference Citation Analysis (3)] |
| 33. | Martell M, Coll M, Ezkurdia N, Raurell I, Genescà J. Physiopathology of splanchnic vasodilation in portal hypertension. World J Hepatol. 2010;2:208-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 34. | Vallance P, Moncada S. Hyperdynamic circulation in cirrhosis: a role for nitric oxide? Lancet. 1991;337:776-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 460] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 35. | Battista S, Bar F, Mengozzi G, Zanon E, Grosso M, Molino G. Hyperdynamic circulation in patients with cirrhosis: direct measurement of nitric oxide levels in hepatic and portal veins. J Hepatol. 1997;26:75-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 76] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 36. | Sogni P, Moreau R, Gadano A, Lebrec D. The role of nitric oxide in the hyperdynamic circulatory syndrome associated with portal hypertension. J Hepatol. 1995;23:218-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Ginès P, Guevara M. Hyponatremia in cirrhosis: pathogenesis, clinical significance, and management. Hepatology. 2008;48:1002-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 194] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 38. | Arroyo V, Rodés J, Gutiérrez-Lizárraga MA, Revert L. Prognostic value of spontaneous hyponatremia in cirrhosis with ascites. Am J Dig Dis. 1976;21:249-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 122] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 39. | Ginés P, Berl T, Bernardi M, Bichet DG, Hamon G, Jiménez W, Liard JF, Martin PY, Schrier RW. Hyponatremia in cirrhosis: from pathogenesis to treatment. Hepatology. 1998;28:851-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 176] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 40. | Fernández-Esparrach G, Sánchez-Fueyo A, Ginès P, Uriz J, Quintó L, Ventura PJ, Cárdenas A, Guevara M, Sort P, Jiménez W, Bataller R, Arroyo V, Rodés J. A prognostic model for predicting survival in cirrhosis with ascites. J Hepatol. 2001;34:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 166] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 41. | Heuman DM, Abou-Assi SG, Habib A, Williams LM, Stravitz RT, Sanyal AJ, Fisher RA, Mihas AA. Persistent ascites and low serum sodium identify patients with cirrhosis and low MELD scores who are at high risk for early death. Hepatology. 2004;40:802-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 114] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 42. | Angeli P, Wong F, Watson H, Ginès P; CAPPS Investigators. Hyponatremia in cirrhosis: Results of a patient population survey. Hepatology. 2006;44:1535-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 273] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 43. | Acevedo JG, Cramp ME. Hepatorenal syndrome: Update on diagnosis and therapy. World J Hepatol. 2017;9:293-299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (3)] |
| 44. | Allegretti AS, Ortiz G, Wenger J, Deferio JJ, Wibecan J, Kalim S, Tamez H, Chung RT, Karumanchi SA, Thadhani RI. Prognosis of Acute Kidney Injury and Hepatorenal Syndrome in Patients with Cirrhosis: A Prospective Cohort Study. Int J Nephrol. 2015;2015:108139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 45. | Mohanty A, Garcia-Tsao G. Hyponatremia and Hepatorenal Syndrome. Gastroenterol Hepatol (N Y). 2015;11:220-229. [PubMed] |
| 46. | Ahluwalia V, Wade JB, Thacker L, Kraft KA, Sterling RK, Stravitz RT, Fuchs M, Bouneva I, Puri P, Luketic V, Sanyal AJ, Gilles H, Heuman DM, Bajaj JS. Differential impact of hyponatremia and hepatic encephalopathy on health-related quality of life and brain metabolite abnormalities in cirrhosis. J Hepatol. 2013;59:467-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 47. | Ahluwalia V, Heuman DM, Feldman G, Wade JB, Thacker LR, Gavis E, Gilles H, Unser A, White MB, Bajaj JS. Correction of hyponatraemia improves cognition, quality of life, and brain oedema in cirrhosis. J Hepatol. 2015;62:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 48. | Cárdenas A, Riggio O. Correction of hyponatraemia in cirrhosis: treating more than a number! J Hepatol. 2015;62:13-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 49. | Bernardi M, Zaccherini G. Approach and management of dysnatremias in cirrhosis. Hepatol Int. 2018;12:487-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 50. | Warren SE, Mitas JA 2nd, Swerdlin AH. Hypernatremia in hepatic failure. JAMA. 1980;243:1257-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 51. | Nelson DC, McGrew WR Jr, Hoyumpa AM Jr. Hypernatremia and lactulose therapy. JAMA. 1983;249:1295-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 52. | Schaffer RL 3rd, Kulkarni S, Harper A, Millis JM, Cronin DC 2nd. The sickest first? Liver Transpl. 2003;9:1211-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 53. | Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2063] [Article Influence: 82.5] [Reference Citation Analysis (0)] |
| 54. | Brown RS Jr, Kumar KS, Russo MW, Kinkhabwala M, Rudow DL, Harren P, Lobritto S, Emond JC. Model for end-stage liver disease and Child-Turcotte-Pugh score as predictors of pretransplantation disease severity, posttransplantation outcome, and resource utilization in United Network for Organ Sharing status 2A patients. Liver Transpl. 2002;8:278-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 174] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 55. | Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, Kremers W, Lake J, Howard T, Merion RM, Wolfe RA, Krom R; United Network for Organ Sharing Liver Disease Severity Score Committee. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1825] [Cited by in RCA: 1863] [Article Influence: 84.7] [Reference Citation Analysis (0)] |
| 56. | Merion RM, Wolfe RA, Dykstra DM, Leichtman AB, Gillespie B, Held PJ. Longitudinal assessment of mortality risk among candidates for liver transplantation. Liver Transpl. 2003;9:12-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 165] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 57. | Biggins SW, Rodriguez HJ, Bacchetti P, Bass NM, Roberts JP, Terrault NA. Serum sodium predicts mortality in patients listed for liver transplantation. Hepatology. 2005;41:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 292] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 58. | Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, Edwards E, Therneau TM. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 935] [Cited by in RCA: 1046] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 59. | Biggins SW, Kim WR, Terrault NA, Saab S, Balan V, Schiano T, Benson J, Therneau T, Kremers W, Wiesner R, Kamath P, Klintmalm G. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology. 2006;130:1652-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 545] [Article Influence: 28.7] [Reference Citation Analysis (1)] |
| 60. | Neuberger J, Gimson A, Davies M, Akyol M, O'Grady J, Burroughs A, Hudson M; Liver Advisory Group; UK Blood and Transplant. Selection of patients for liver transplantation and allocation of donated livers in the UK. Gut. 2008;57:252-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 156] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 61. | Biselli M, Gitto S, Gramenzi A, Di Donato R, Brodosi L, Ravaioli M, Grazi GL, Pinna AD, Andreone P, Bernardi M. Six score systems to evaluate candidates with advanced cirrhosis for orthotopic liver transplant: Which is the winner? Liver Transpl. 2010;16:964-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 62. | Manka P, Bechmann LP, Tacke F, Sowa JP, Schlattjan M, Kälsch J, Jochum C, Paul A, Saner FH, Trautwein C, Gerken G, Canbay A. Serum sodium based modification of the MELD does not improve prediction of outcome in acute liver failure. BMC Gastroenterol. 2013;13:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 63. | Leise MD, Kim WR, Kremers WK, Larson JJ, Benson JT, Therneau TM. A revised model for end-stage liver disease optimizes prediction of mortality among patients awaiting liver transplantation. Gastroenterology. 2011;140:1952-1960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 121] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 64. | O'Leary JG, Trotter JF. Is MELD fit enough? Gastroenterology. 2011;140:1871-1874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 65. | Londoño MC, Guevara M, Rimola A, Navasa M, Taurà P, Mas A, García-Valdecasas JC, Arroyo V, Ginès P. Hyponatremia impairs early posttransplantation outcome in patients with cirrhosis undergoing liver transplantation. Gastroenterology. 2006;130:1135-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 135] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 66. | Dawwas MF, Lewsey JD, Neuberger JM, Gimson AE. The impact of serum sodium concentration on mortality after liver transplantation: a cohort multicenter study. Liver Transpl. 2007;13:1115-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 96] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 67. | Wszolek ZK, McComb RD, Pfeiffer RF, Steg RE, Wood RP, Shaw BW Jr, Markin RS. Pontine and extrapontine myelinolysis following liver transplantation. Relationship to serum sodium. Transplantation. 1989;48:1006-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 68. | Abbasoglu O, Goldstein RM, Vodapally MS, Jennings LW, Levy MF, Husberg BS, Klintmalm GB. Liver transplantation in hyponatremic patients with emphasis on central pontine myelinolysis. Clin Transplant. 1998;12:263-269. [PubMed] |
| 69. | Yun BC, Kim WR, Benson JT, Biggins SW, Therneau TM, Kremers WK, Rosen CB, Klintmalm GB. Impact of pretransplant hyponatremia on outcome following liver transplantation. Hepatology. 2009;49:1610-1615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 70. | Leise MD, Yun BC, Larson JJ, Benson JT, Yang JD, Therneau TM, Rosen CB, Heimbach JK, Biggins SW, Kim WR. Effect of the pretransplant serum sodium concentration on outcomes following liver transplantation. Liver Transpl. 2014;20:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 71. | Mihaylov P, Nagai S, Ekser B, Mangus R, Fridell J, Kubal C. Prognostic Impact of Peritransplant Serum Sodium Concentrations in Liver Transplantation. Ann Transplant. 2019;24:418-425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 72. | Leise M, Cárdenas A. Hyponatremia in Cirrhosis: Implications for Liver Transplantation. Liver Transpl. 2018;24:1612-1621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 73. | Attar B. Approach to Hyponatremia in Cirrhosis. Clin Liver Dis (Hoboken). 2019;13:98-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 74. | John S, Thuluvath PJ. Hyponatremia in cirrhosis: pathophysiology and management. World J Gastroenterol. 2015;21:3197-3205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 108] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (6)] |
| 75. | McCormick PA, Mistry P, Kaye G, Burroughs AK, McIntyre N. Intravenous albumin infusion is an effective therapy for hyponatraemia in cirrhotic patients with ascites. Gut. 1990;31:204-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 76. | Bajaj JS, Tandon P, O’Leary JG, Biggins SW, Wong F, Kamath PS, Garcia-Tsao G, Maliakkal B, Lai JC, Fallon M, Thuluvath P, Vargas HE, Subramanian RM, Thacker LR, Reddy RK. The Impact of Albumin Use on Resolution of Hyponatremia in Hospitalized Patients With Cirrhosis. Am J Gastroenterol. 2018;113:1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 77. | Paine CH, Pichler RH. Treatment of Hyponatremia in End-Stage Liver Disease: New Tools in the Shed. Am J Gastroenterol. 2018;113:1728-1729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 78. | Decaux G, Soupart A, Vassart G. Non-peptide arginine-vasopressin antagonists: the vaptans. Lancet. 2008;371:1624-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 248] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 79. | Dahl E, Gluud LL, Kimer N, Krag A. Meta-analysis: the safety and efficacy of vaptans (tolvaptan, satavaptan and lixivaptan) in cirrhosis with ascites or hyponatraemia. Aliment Pharmacol Ther. 2012;36:619-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 80. | Yan L, Xie F, Lu J, Ni Q, Shi C, Tang C, Yang J. The treatment of vasopressin V2-receptor antagonists in cirrhosis patients with ascites: a meta-analysis of randomized controlled trials. BMC Gastroenterol. 2015;15:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 81. | Li M, Bi Z, Huang Z. Impact of Vaptans on Clinical Outcomes in Cirrhosis Patients: A Meta-Analysis of Randomized Controlled Trials. Front Pharmacol. 2019;10:1365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 82. | Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS; TEMPO 3:4 Trial Investigators. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367:2407-2418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1179] [Cited by in RCA: 1144] [Article Influence: 88.0] [Reference Citation Analysis (0)] |
| 83. | Watkins PB, Lewis JH, Kaplowitz N, Alpers DH, Blais JD, Smotzer DM, Krasa H, Ouyang J, Torres VE, Czerwiec FS, Zimmer CA. Clinical Pattern of Tolvaptan-Associated Liver Injury in Subjects with Autosomal Dominant Polycystic Kidney Disease: Analysis of Clinical Trials Database. Drug Saf. 2015;38:1103-1113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 152] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 84. | Verbalis JG, Goldsmith SR, Greenberg A, Korzelius C, Schrier RW, Sterns RH, Thompson CJ. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. 2013;126:S1-S42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 627] [Cited by in RCA: 651] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 85. | Lenci I, Milana M, Angelico M, Baiocchi L. Short-Term, Low-Dose Use of Tolvaptan as a Bridge Therapy to Expedite Liver Transplant for Severe Hyponatremic, Cirrhotic Patients With High Model for End-Stage Liver Disease Scores. Exp Clin Transplant. 2017;15:689-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 86. | Parekh A, Rajaram P, Patel G, Subramanian RM. Utility of Tolvaptan in the Perioperative Management of Severe Hyponatremia During Liver Transplantation: A Case Report. Transplant Proc. 2017;49:2399-2401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 87. | Kiritani S, Kaneko J, Miyata Y, Matsumura M, Akamatsu N, Ishizawa T, Arita J, Tamura S, Kokudo N, Hasegawa K. A selective oral vasopressin V2-receptor antagonist for patients with end-stage liver disease awaiting liver transplantation: a preliminary study. Biosci Trends. 2019;13:189-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 88. | Sharma P, Schaubel DE, Goodrich NP, Merion RM. Serum sodium and survival benefit of liver transplantation. Liver Transpl. 2015;21:308-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |