Published online Nov 27, 2020. doi: 10.4254/wjh.v12.i11.1098
Peer-review started: July 21, 2020
First decision: September 14, 2020
Revised: September 26, 2020
Accepted: October 12, 2020
Article in press: October 12, 2020
Published online: November 27, 2020
Processing time: 126 Days and 2.3 Hours
Hepatectomy with inflow occlusion results in ischemia-reperfusion injury; however, pharmacological preconditioning can prevent such injury and optimize the postoperative recovery of hepatectomized patients. The normal inflammatory response after a hepatectomy involves increased expression of metalloproteinases, which may signal pathologic hepatic tissue reformation.
To investigate the effect of desflurane preconditioning on these inflammatory indices in patients with inflow occlusion undergoing hepatectomy.
This is a single-center, prospective, randomized controlled trial conducted at the 4th Department of Surgery of the Medical School of Aristotle University of Thessaloniki, between August 2016 and December 2017. Forty-six patients were randomized to either the desflurane treatment group for pharmacological preconditioning (by replacement of propofol with desflurane, administered 30 min before induction of ischemia) or the control group for standard intravenous propofol. The primary endpoint of expression levels of matrix metalloproteinases and their inhibitors was determined preoperatively and at 30 min posthepatic reperfusion. The secondary endpoints of neutrophil infiltration, coagulation profile, activity of antithrombin III (AT III), protein C (PC), protein S and biochemical markers of liver function were determined for 5 d postoperatively and compared between the groups.
The desflurane treatment group showed significantly increased levels of tissue inhibitor of metalloproteinases 1 and 2, significantly decreased levels of matrix metalloproteinases 2 and 9, decreased neutrophil infiltration, and less profound changes in the coagulation profile. During the 5-d postoperative period, all patients showed significantly decreased activity of AT III, PC and protein S (vs baseline values, P < 0.05). The activity of AT III and PC differed significantly between the two groups from postoperative day 1 to postoperative day 5 (P < 0.05), showing a moderate drop in activity of AT III and PC in the desflurane treatment group and a dramatic drop in the control group. Compared to the control group, the desflurane treatment group also had significantly lower international normalized ratio values on all postoperative days (P < 0.005) and lower serum glutamic oxaloacetic transaminase and serum glutamic pyruvic transaminase values on postoperative days 2 and 3 (P < 0.05). Total length of stay was significantly less in the desflurane group (P = 0.009).
Desflurane preconditioning can lessen the inflammatory response related to ischemia-reperfusion injury and may shorten length of hospitalization.
Core Tip: Ischemia-reperfusion injury remains a leading cause of morbidity and mortality in hepatectomies. In our study, 46 patients were randomly and equally allocated to receive pharmacological preconditioning with desflurane (intervention group) or not (control group) to compare inflammatory indices between the two groups. We found significantly reduced levels of matrix metalloproteinases 2 and 9, increased levels of tissue inhibitor matrix metalloproteinases 1 and 2, and decreased neutrophil infiltration in the intervention group. Thus, hepatoprotective strategies may ameliorate the pathophysiologic effects of ischemia-reperfusion during liver surgery, with our study suggesting a novel promising strategy that may benefit patients postoperatively.
- Citation: Koraki E, Mantzoros I, Chatzakis C, Gkiouliava A, Cheva A, Lavrentieva A, Sifaki F, Argiriadou H, Kesisoglou I, Galanos-Demiris K, Bitsianis S, Tsalis K. Metalloproteinase expression after desflurane preconditioning in hepatectomies: A randomized clinical trial. World J Hepatol 2020; 12(11): 1098-1114
- URL: https://www.wjgnet.com/1948-5182/full/v12/i11/1098.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i11.1098
Hepatectomy is one of the most frequently performed strategies to treat benign and malignant liver diseases, and the number of patients undergoing hepatectomy is increasing[1]. Unfortunately, the ischemia-reperfusion (IR) technique in the hepatectomy procedure can cause liver damage and represents one of the most important reasons for postoperative liver failure[2]. When prolonged ischemia is applied to a tissue, the cellular metabolism inevitably becomes anaerobic, leading to loss of cellular function and ultimately cell death[3,4]. The extent of the resection along with IR injury (IRI) results in tissue trauma and triggers the acute phase response. It consequently impacts the physiology of the liver and affects the mechanisms underlying production of clotting factors, including that of inflammatory cytokines, chemokines and complement products, and the recruitment of neutrophils to the site of injury[5,6]. Indeed, previous studies have demonstrated increased neutrophil infiltration in the livers of animals which have suffered from IRI and that exposure to pharmacological agents attenuating neutrophil activities leads to milder hepatic IRI[7-9].
The inflammatory response complicating IRI leads to induction of matrix metalloproteinases (MMPs), which are produced by hepatic stellate cells, Kupffer cells and hepatocytes[10]. While the MMPs in general play a major role in tissue remodeling and molecular signaling, and correlate with the inflammation process, MMP2 and MMP9 are expressed under pathological conditions and are responsible for the extracellular matrix disruption linked to IRI[11,12]. Parallel to this, the balance between MMPs and their endogenous inhibitors (known as the tissue inhibitors of metalloproteinases, or TIMPs) regulate their activity in pathologic conditions[13].
Pharmacological preconditioning may limit the anticipated rise in the concentration of MMPs following hepatic IRI. Although this procedure has been studied extensively in cardiac surgery[14-16], its efficacy in hepatic surgery remains unknown. Desflurane, a volatile anesthetic in routine clinical use, protects hepatic blood flow. It is also known to cause less toxicity to the liver than other volatile anesthetics, to have minor biological degradation, with a 0.02% calculated metabolism ratio, and to be less soluble in plasma and tissues[17]. Moreover, it was found to be superior to total intravenous anesthesia, regarding patient outcomes following liver surgery[18,19].
Taking the role of MMPs and pharmacological preconditioning in IRI into consideration, we aimed to investigate the effect of preconditioning with desflurane on MMP induction.
This single-center, prospective, randomized controlled trial was conducted in patients undergoing liver resection at the 4th Department of Surgery of the Medical School of Aristotle University of Thessaloniki, between August 2016 and December 2017. Patients were randomized, in a 1:1 ratio, into the hepatectomy with pharmacological preconditioning with desflurane group (intervention group) or the hepatectomy without preconditioning group (control group). The study was carried out following review and approval by the Institutional Review Board of the General Hospital “Georgios Papanikolaou” where the 4th Surgical Department of Aristotle University in Thessaloniki is located. Written informed consent was obtained from all participants. The study was also conducted in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans and is registered at clinicaltrials.gov (NCT03848780).
Patients eligible for study inclusion were older than 18 years of age and undergoing an elective extensive hepatic resection that included more than two segments of the liver. Exclusion criteria were infection with hepatitis B or C or human immunodeficiency virus, liver cirrhosis, autoimmune disease, inflammatory bowel disease, or pregnancy. Patients who received additional ablation therapies (cryosurgery or radiofrequency) or liver resections without inflow occlusion were also not eligible for study inclusion. During the study, there were no changes in the eligibility criteria.
Ultimately, 46 consecutive patients undergoing elective major hepatectomy were included in this prospective study. The patients were randomized equally into either the intervention group (receiving preconditioning with desflurane) or the control group. The randomization sequence was generated by a computer and sealed in envelopes. Each patient was operated by the same experienced hepatobiliary surgical team, who were all blinded to the intervention assignment.
The preoperative physical status of the patients was categorized into classes, according to the criteria of the American Society of Anesthesiologists (commonly known as ASA). Standard monitoring, along with invasive blood pressure, cardiac output/stroke volume variation, a central venous line and depth of anesthesia (measured with the bispectral index), were conducted routinely. General anesthesia was accomplished with 3 μg/kg fentanyl, 2-2.5 mg/kg propofol, 1 mg/kg lidocaine, and 0.2 mg/kg cis-atracurium. Anesthesia was maintained by the continuous infusion of 0.05-0.1 mg/kg/min propofol, 5 μg/kg fentanyl, 2-4 mg boluses cis-atracurium (according to clinical need) and 0.3-0.6 μg/kg/min remifentanil. Both the control and desflurane groups underwent the same protocol for administration of fentanyl and remifentanil.
In patients with preconditioning, propofol anesthesia was replaced by desflurane according to previous practice for pharmacological preconditioning in IR[20]. Specifically, at 30 min before the induction of ischemia, propofol administration was stopped and replaced by desflurane, set to achieve a minimal alveolar concentration of 1 (induction time of 5 min). The pharmacological preconditioning was performed for 20 min, after which the following 5 min were used to cease desflurane administration and reinitiate propofol (with washout of 5 min). Hepatic ischemia was then begun. The hemodynamic tolerance to clamping was the same in both groups.
Surgical procedures were performed in a standardized manner, by the same experienced hepatobiliary surgeon. At 30 min before clamping of the portal triad, the anesthesiologist was informed as to whether a pharmacological preconditioning with desflurane was to be performed or not, according to the randomized assignment. The surgeon remained blinded to the assignment for the entire operation. During resection, we aimed for a low central venous pressure (0-5 mmHg). Liver transection was performed by parenchyma crushing, using a 3-mm tip Kelly clamp. Vessels ≤ 2 mm were coagulated at 120W with the irrigated bipolar forceps. Clipping or ligation was applied for all other elements. A stapler device was used only for the transection of hepatic veins. The Pringle maneuver was applied intermittently and the cumulative duration was at least 30 min. Specifically, every interval of 10 min of inflow occlusion was followed by 5 min reperfusion time[21]. Postoperatively, all patients in both groups received thromboprophylaxis with tinzaparin.
Primary endpoints: Primary outcomes of the study were the serum levels of MMP2, MMP9, TIMP1 and TIMP2 in both groups. Blood samples were taken preoperatively and at 30 min after hepatic reperfusion had been permanently established. The samples were tested to determine the relative gene expression using real-time polymerase chain reaction (PCR). The comparative log fold-change method (also referred to as the 2-ΔΔCT method) was used to calculate the fold-change and then convert it to a percentage.
Secondary endpoints: A sample of hepatic tissue was taken immediately before ischemia induction and at 30 min after liver reperfusion, for histological analysis. Hematoxylin-eosin staining was used to assess the degree of steatosis, while Gomori and Masson staining was used to determine the level of fibrosis. Steatosis was characterized (100 × magnification) as mild (10%-30%), moderate (30%-60%) or severe (> 60%), according to the presence of fat droplets in the hepatic cells. Fibrosis was graded based on the METAVIR score, with absence graded as F0, portal fibrosis without septa as F1, with rare septa as F2 or numerous septa as F3, and cirrhosis as F4.
Coagulation markers and the activity of anticoagulation factors were monitored perioperatively and followed for the first 5 d after liver resection. Blood samples were collected preoperatively and on postoperative days (PODs) 1-5. Standard coagulation tests, international normalized ratio (INR), prothrombin time (PT), activated partial thromboplastin time (aPΤT), the fibrin degradation product D-dimer (D-d), platelet count, and natural anticoagulant activity levels [antithrombin III (AT III), protein C (PC), and protein S (PS)] were measured and compared between the two groups using chromogenic assays for plasma AT III and PC and electro-immunodiffusion assay for free PS (all from Dade-Behring Inc., Deerfield, IL, United States). The AT III assay had normal values at 80%-120%, an intra-assay coefficient of 7.7% and inter-assay coefficient of 8.2%. The PC assay had normal values at 70%-130%, an intra-assay coefficient of 8.1% and inter-assay coefficient of 8.6%. The PS assay had normal values of 70%-130%, an intra-assay coefficient of 8.2% and inter-assay coefficient of 8.7%. To assess activation of the fibrinolytic system, fibrinogen and D-d were measured, with the latter measured by latex semiquantitative assay (Diagnostica Stago, Asnières-sur-Seine, France) wherein a negative result was indicated by concentrations of < 0.5 μg/mL.
The lengths of intensive care unit and hospital stays were also recorded. In addition, biochemical markers of liver function, including aspartate aminotransferase, alanine transaminase, total bilirubin, gamma-glutamyl transferase and alkaline phosphatase, were evaluated in all patients preoperatively and up to POD 5. No changes were made to the trial outcomes after the trial commenced.
In order to determine the appropriate sample size of the study, power analysis was carried out in G*Power (version 3.1.7; Universität Kiel, Germany). Data from a previous study[20] were used as a proxy of the anticipated findings in the present study, with 80% power and 5% significance level. Power calculation resulted in 20 patients per group.
The independent samples t-test and independent samples Mann-Whitney test were implemented for group (intervention vs control) comparisons. Furthermore, Fisher’s exact test and the χ2 test were used for comparisons of groups relative to qualitative variables. Data on biological markers were analyzed within the frame of general linear models with the ANOVA method, according to the model which involves one factor between patients (factor “group” with two levels) and one factor within patients (factor “time” with five levels, with repeated measures). Comparisons of means were carried out with the least significant difference criterion. The significance level was preset at P ≤ 0.05 for all hypotheses testing procedures. The SPSS v.22.0 software (IBM Corp., Armonk, NY, United States) was utilized for all statistical analyses. All data were subjected to the Kolmogorov-Smirnov test and found to be normally distributed; as such, they are presented as mean ± standard deviation in the tables. The statistical methods used in this study were reviewed by Anna Bettina Haidich, Assistant Professor in Hygiene-Medical Statistics Department of the Aristotle University of Thessaloniki.
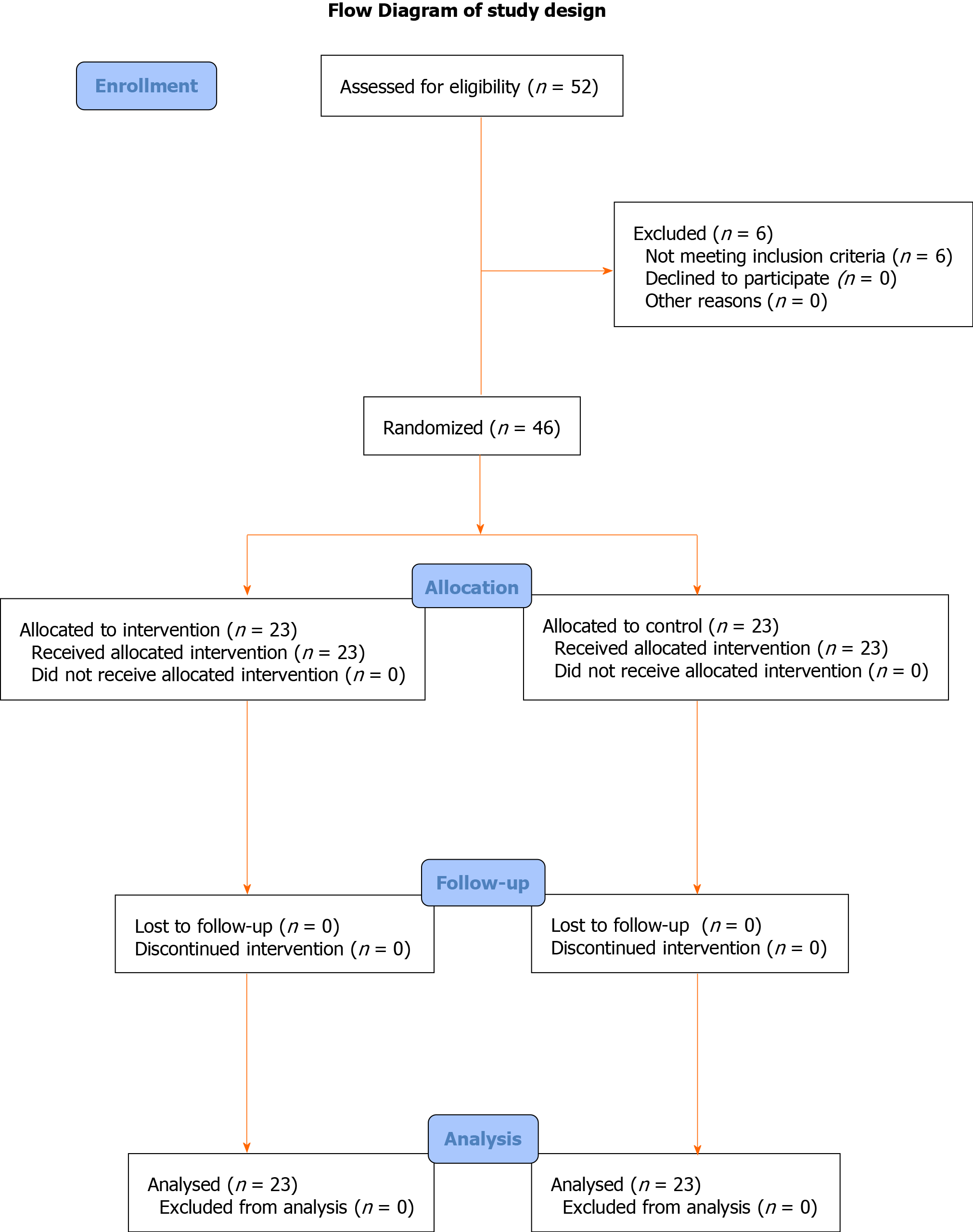
The selection process is displayed in Figure 1, as a flow diagram. Patients’ characteristics are presented in Table 1. The leading reason for liver resection among the study population was hepatic metastasis (n = 20). There was no significant difference between the two groups in terms of sex (P = 0.719), age (P = 0.612), body mass index (BMI) or ASA class (P = 0.963).
| Characteristics | Desflurane group, n = 23 | Control group, n = 23 | P value |
| Age, yr | 64.5 ± 10.6 | 61.5 ± 11,4 | 0.612 |
| Males | 12 (52.1) | 14 (60.8) | 0.719 |
| ΒΜΙ, kg/m2 | 25.6 ± 5.1 | 26.2 ± 4.8 | 0.953 |
| Cause of liver resection | 0.914 | ||
| Hepatic cancer | 10 (44) | 9 (39) | |
| Liver metastasis | 9 (39) | 11 (48) | |
| Hemangioma | 1 (4.3) | 1 (4.3) | |
| Klatskin tumor | 2 (8.6) | 1 (4.3) | |
| Focal nodular hyperplasia | 1 (4.4) | 1 (4.3) | |
| No of tumors | 0.772 | ||
| Solitary | 14 (60.9) | 12 (52.2) | |
| 2 | 5 (21.7) | 7 (30.4) | |
| 3 | 3 (13) | 4 (17.4) | |
| 4 | 1 (4.3) | 0 | |
| Size of largest tumor, cm | 12.5 ± 8.7 | 11.9 ± 7.5 | 0.711 |
| No of segments | 1 | ||
| 1 | 10 (43.5) | 11 (47.8) | |
| 2-3 | 8 (34.6) | 7 (30.4) | |
| > 3 | 5 (21.7) | 5 (21.7) | |
| Type of hepatectomy | 0.853 | ||
| Right extended | 3 (13) | 2 (8.6) | |
| Right | 8 (34.7) | 10 (43.5) | |
| Left | 10 (43.5) | 8 (34.7) | |
| Left extended | 2 (8.6) | 3 (13) | |
| Previous treatment status | 0.552 | ||
| No preoperative treatment | 14 (60.9) | 12 (52.2) | |
| Neo-adjuvant chemotherapy | 9 (39.1) | 11 (47.8) | |
| Intraoperative blood loss, mL | 630 ± 550 | 690 ± 580 | 0.847 |
| Intraoperative blood transfusion, units of red blood cells | 0.875 | ||
| 0 | 5 (21.7) | 5 (21.7) | |
| 1 | 6 (26) | 7 (30.4) | |
| 2 | 8 (34.7) | 7 (30.4) | |
| 3 | 3 (13) | 2 (8.6) | |
| 4 | 1 (4.3) | 2 (8.6) | |
The mean duration of ischemia during the hepatectomies was 61 ± 30 min in the intervention group and 55 ± 26 min in the control group (P = 0.654). Median duration of operation was 300 min (range: 241-380 min) in the intervention group and 270 min (range: 210-340 min) in the control group (P = 0.364). There was no statistically significant difference between the two groups in the units of blood administered intraoperatively (Table 1). The median length of intensive care unit stay after liver resection was also similar between the two groups [1 (1-2) vs 1 (1-2) d; P = 0.373]; however, a shorter hospital stay was observed for patients undergoing liver resection with desflurane preconditioning [9.5 (8-11) d vs 12 (11-20.3) d; P = 0.009]. Postoperative complications are presented in Table 2.
| Postoperative complications | Desflurane group, n = 23 | Control group, n = 23 | P value |
| Postoperative blood transfusion (red blood cells) | 0.555 | ||
| 0 U | 13 (56.5) | 11 (47.8) | |
| 1 U | 10 (43.5) | 10 (43.5) | |
| 2 U | 0 | 2 (8.7) | |
| Bile leak | 2 (8.7) | 2 (8.7) | 1.00 |
| Deep vein thrombosis | 2 (8.7) | 1 (4.3) | 0.555 |
| Pneumonia | 4 (17.4) | 5 (21.7) | 0.71 |
MMPs: After hepatic IR, the control group showed significantly higher levels of both MMP2 and MMP9 (vs the intervention group; Table 3).
TIMPs: After hepatic IR, the intervention group showed significantly higher levels of both TIMP1 and TIMP2 (vs the control group; Table 3).
Neutrophil infiltration: After hepatic IR, the control group showed a significantly higher infiltration of neutrophils (vs the intervention group; Table 4).
| Score | Desflurane group, n = 23 | Control group, n = 23 | P value |
| Neutrophil infiltration | |||
| 0 | 9 (39) | NA | b |
| 1 | 9 (39) | 5 (23) | |
| 2 | 5 (23) | 9 (39) | |
| 3 | NA | 9 (39) | |
| Fibrosis | |||
| 0 | 11 (48) | 7 (30) | 0.767 |
| 1 | 7 (30) | 9 (39) | |
| 2 | 3 (13) | 4 (18) | |
| 3 | 2 (9) | 3 (13) | |
| Steatosis | |||
| 0 | 11 (48) | 9 (39) | 0.780 |
| 1 | 10 (43) | 10 (43) | |
| 2 | 2 (9) | 4 (18) | |
| 3 |
Hepatic fibrosis and steatosis: After hepatic IR, the rates and grades of fibrosis and steatosis were not significantly different between the two groups (Table 4). Also, after reperfusion, the findings were identical to baseline among the participants.
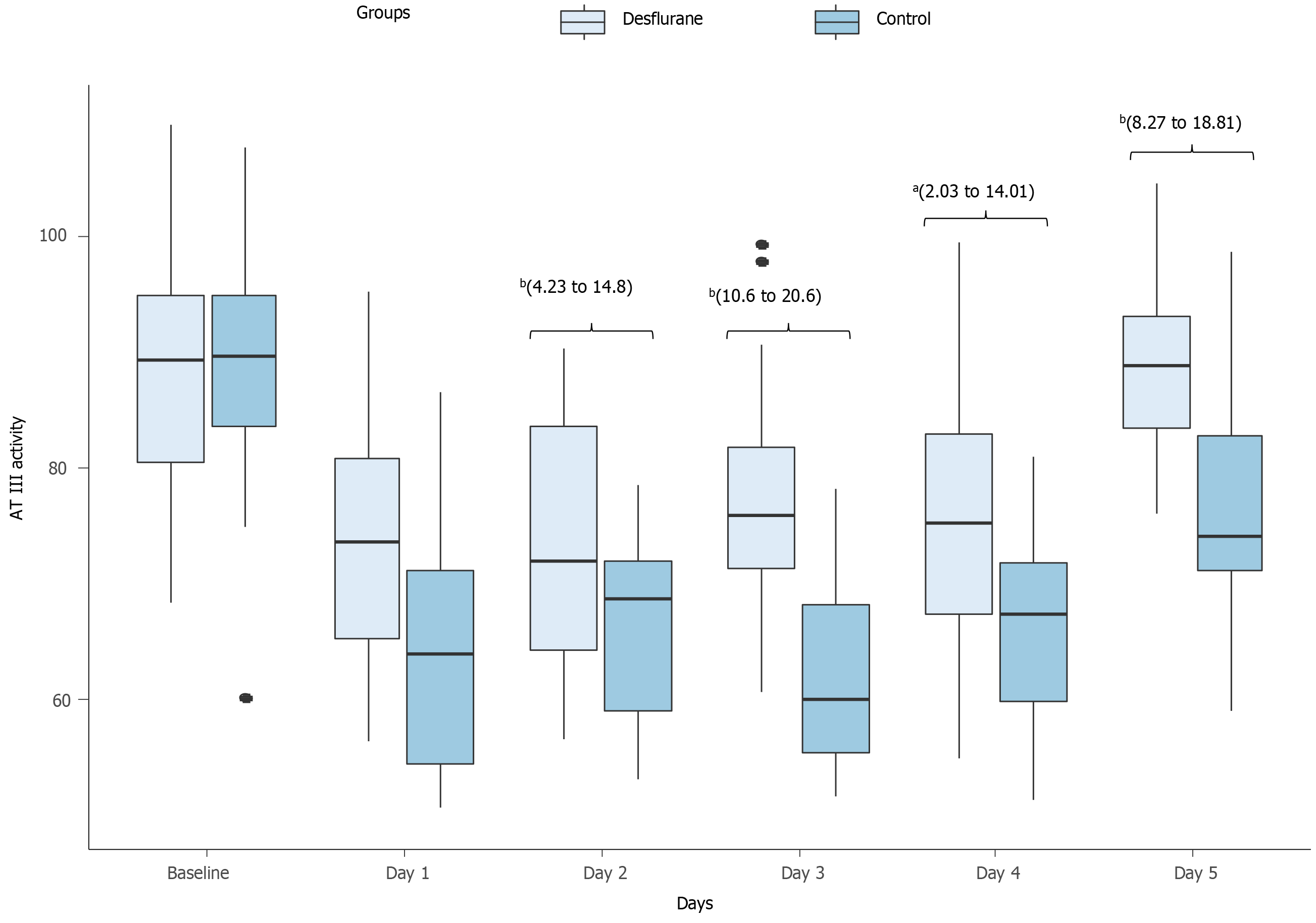
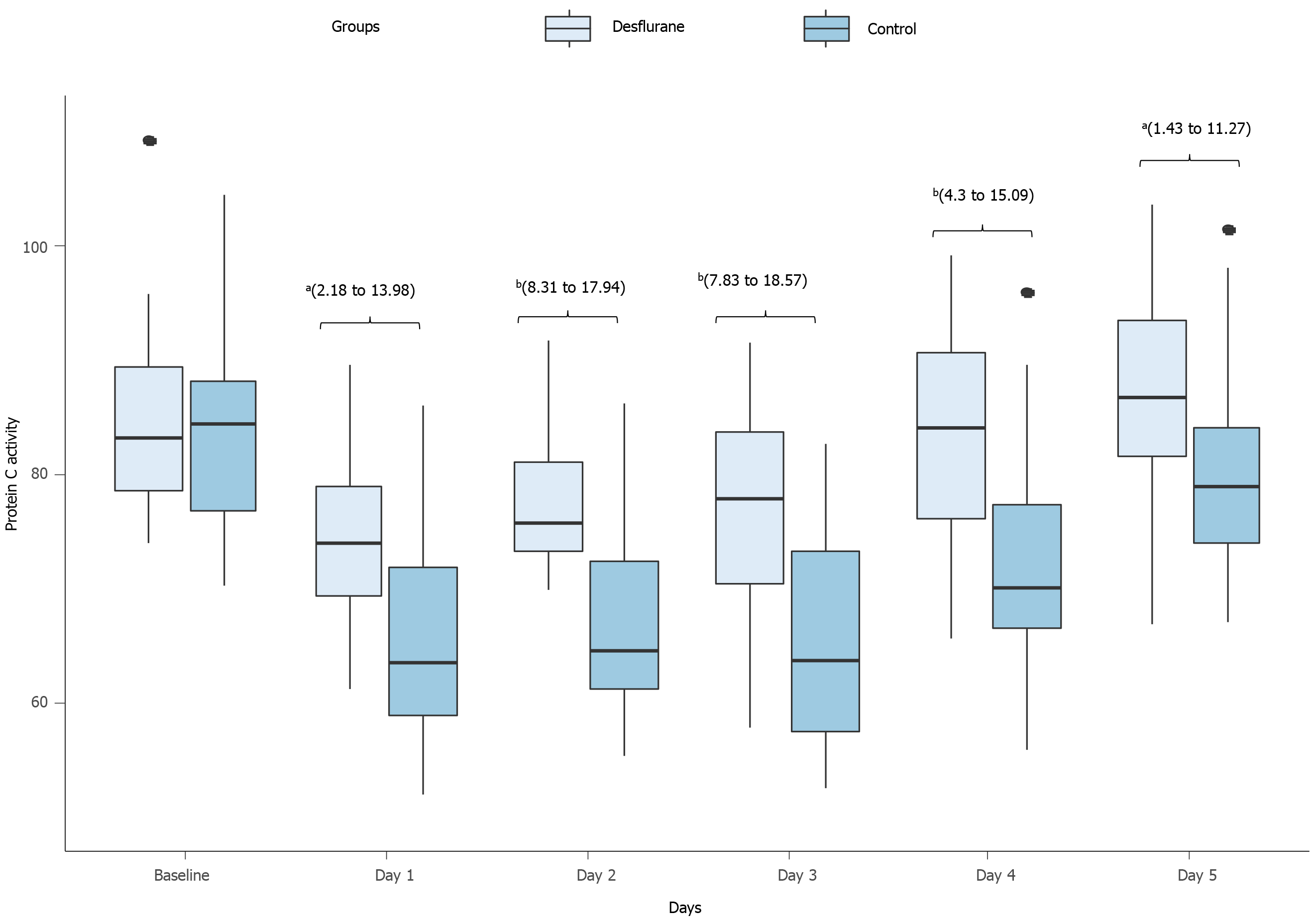
Coagulation markers: Upon intensive care unit admission, all patients had significant acquired reduction in the activity of AT III, PC and PS. However, the postoperative activity of AT III (Figure 2) and PC (Figure 3) differed significantly between the two groups from POD 1 to POD 5. The intervention group showed a postoperative moderate drop in the activities of both AT III and PC, while the control group showed a substantial drop (Table 5).
| Change, % | AT III | P (95%CI) | PC | P (95%CI) | INR | P (95%CI) |
| Day 1 | a(4.6 to 15.4) | a(2.2 to 13.5) | a(-22.6 to -7.5) | |||
| Desflurane | -17.9 ± 16.7 | -11.9 ± 13.2 | 12.5 ± 17.1 | |||
| Control | -27.9 ± 14.9 | -20.9 ± 16.1 | 27.5 ± 13.6 | |||
| Day 2 | a(5.3 to 18.5) | b(9.7 to 18.1) | b(-39.5 to -11.7) | |||
| Desflurane | -15.4 ± 16.6 | -8.2 ± 10.1 | 14.5 ± 20.1 | |||
| Control | -27.3 ± 18.9 | -22.1 ± 16.5 | 40.1 ± 31.1 | |||
| Day 3 | b(14.3 to 23.7) | b(10.2 to 18.9) | a(-27.6 to -4.2) | |||
| Desflurane | -12.1 ± 16.6 | -8.3 ± 11.9 | 17.9 ± 15.6 | |||
| Control | -31.1 ± 28.2 | -22.9 ± 15.7 | 33.6 ± 28.2 | |||
| Day 4 | a(10.9 to 18.2) | a(5.6 to 15.5) | a(-26.8 to -4.1) | |||
| Desflurane | -12.3 ± 19.8 | -1.4 ± 14.8 | 14.4 ± 20.1 | |||
| Control | -23.7 ± 16.2 | -11.9 ± 15.4 | 29.8 ±23.6 | |||
| Day 5 | b(9.8 to 22.7) | a(2.5 to 14.3) | a(-23.7 to -2.2) | |||
| Desflurane | -3.8 ± 17.7 | 3.3 ± 13.6 | 7.7 ± 18.7 | |||
| Control | -12.4 ± 17.4 | -5.5 ±11.5 | 22.2 ± 17.8 |
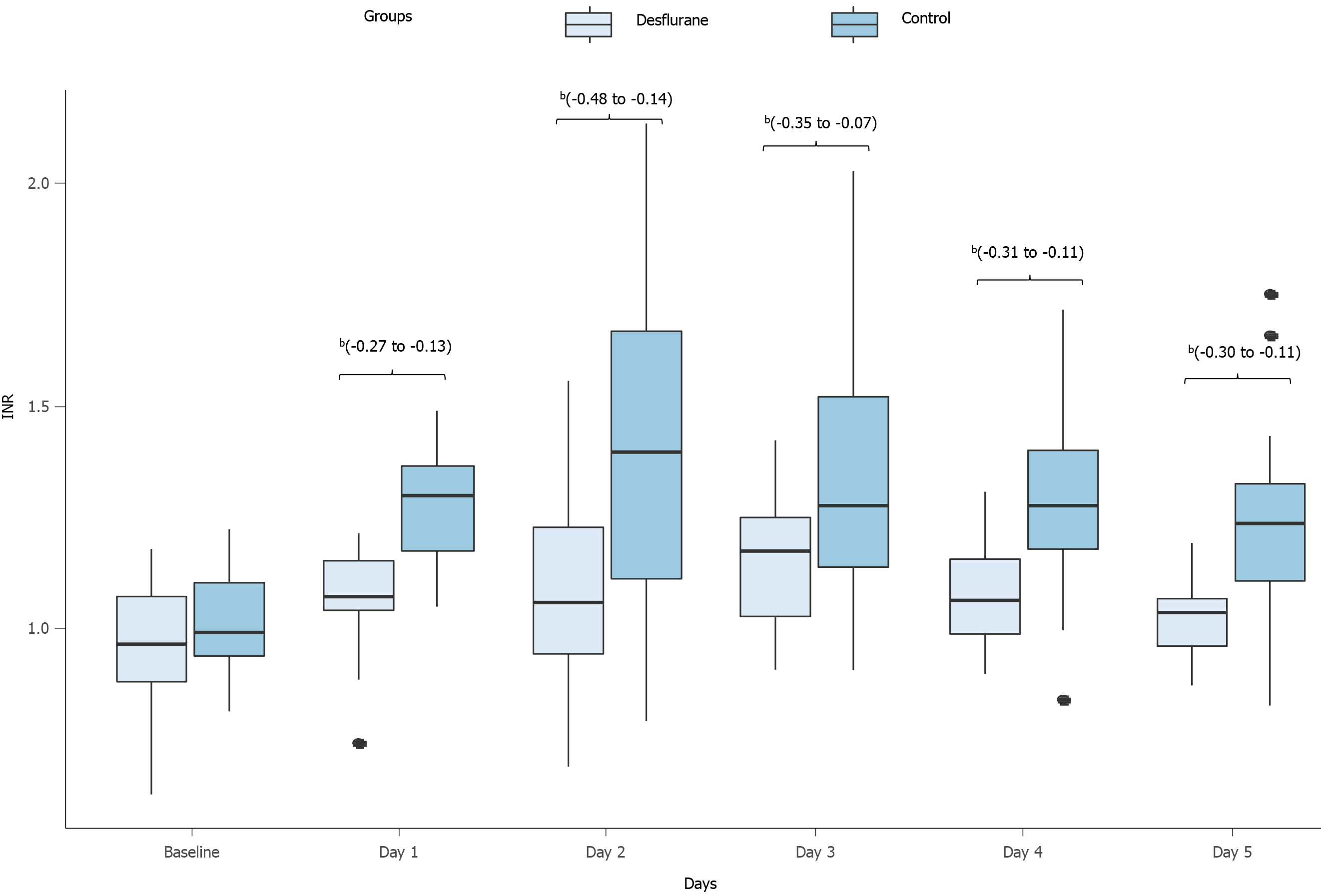
INR and aPTT: Both groups showed significantly elevated INR postoperatively. At baseline, there was no difference in INR between the two groups; however, from POD 1 to POD 5, INR in the intervention group was significantly lower than that in the control group (Figure 4, Table 5). The aPTT did not differ significantly between the two groups (Table 6).
| Day | Group | WBCs (103/μL) | SGOT (U/L) | SGPT (U/L) | γ-GT (U/L) | Tbil (mg/dL) | Fib (g/L) | Plts (109/L) | D-dimer (μg/mL) | Protein S (%) | aPTT (s) |
| 0 | Control | 6.1 ± 1.6 | 30 ± 13 | 44 ± 30 | 47 ± 26 | 0.78 ± 0.45 | 2.4 ± 0.5 | 229.2 ± 78 | 0.57 ± 0.3 | 83.5 ± 12 | 26 ± 1.4 |
| Desflurane | 5.8 ± 1.8 | 29 ± 10 | 38 ± 33 | 39 ± 24 | 0.72 ± 0.36 | 2.4 ± 0.4 | 202.5 ± 92 | 0.55 ± 0.3 | 84.1 ± 14 | 26.2 ± 1.6 | |
| P | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | |
| 1 | Control | 8 ± 2 | 32 ± 16 | 44 ± 32 | 50 ± 24 | 0.88 ± 0.48 | 3.6 ± 1 | 126.1 ± 32 | 3.8 ± 1.4 | 60.8 ± 11 | 28 ± 2 |
| Desflurane | 6 ± 1.8 | 26 ± 10 | 39 ± 23 | 37 ± 22 | 0.69 ± 0.38 | 2.7 ± 0.6 | 168.3 ± 86 | 2.6 ± 1.3 | 64.8 ± 16 | 27.8 ± 3 | |
| P | b | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | |
| 2 | Control | 11.6 ± 6.4 | 395 ± 256 | 373 ± 263 | 113 ± 96 | 1.91 ± 0.94 | 3.2 ± 0.8 | 130.5 ± 29 | 4.1 ± 0.9 | 60 ± 12 | 31 ± 4.8 |
| Desflurane | 9.2 ± 4.7 | 197 ± 83 | 227 ± 170 | 140 ± 109 | 1.65 ± 0.83 | 2.9 ± 0.5 | 160.2 ± 81 | 3.7 ± 1.1 | 65.4 ± 14 | 28 ± 3.9 | |
| P | a | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | |
| 3 | Control | 12.5 ± 3.8 | 543 ± 395 | 395 ± 326 | 129 ± 106 | 2.29 ± 1.22 | 2.9 ± 0.7 | 137.8 ± 89 | 4.2 ± 2.3 | 59.9 ± 13 | 29.5 ± 3.7 |
| Desflurane | 10.1 ± 2 | 256 ± 140 | 197 ± 83 | 131 ± 70 | 2.4 ± 1.72 | 3.06 ± 0.6 | 146.2 ± 54 | 4.9 ± 3.1 | 66.6 ± 14 | 27.2 ± 2.5 | |
| P | b | a | a | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | |
| 4 | Control | 11.7 ± 2.9 | 187 ± 109 | 304 ± 236 | 124 ± 107 | 1.31 ± 0.72 | 3 ± 0.4 | 166.4 ± 51 | 3.9 ± 1.4 | 67 ± 12 | 28 ± 2.6 |
| Desflurane | 9.9 ± 2.1 | 137 ± 102 | 177 ± 106 | 101 ± 93 | 1.6 ± 1.1 | 3.5 ± 0.9 | 157.2 ± 41 | 4.1 ± 1.1 | 77 ± 14 | 26 ± 2.7 | |
| P | b | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | |
| 5 | Control | 9.5 ± 2.8 | 105 ± 89 | 135 ± 81 | 120 ± 73 | 0.88 ± 0.48 | 3.2 ± 0.5 | 186.3 ± 31 | 3.2 ± 1.6 | 85 ± 11 | 26.7 ± 3 |
| Desflurane | 8.2 ± 2 | 84 ± 71 | 162 ± 91 | 92 ± 66 | 0.69 ± 0.38 | 4.1 ± 0.6 | 176.5 ± 76 | 3.6 ± 1.6 | 87 ± 16 | 25.9 ± 3 | |
| P | a | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 |
Platelets: From POD 1, all patients showed a reduction in platelets in comparison to preoperative levels (P < 0.05), although no differences were observed in platelet levels between the two groups (Table 6).
Fibrinogen and D-d: Although fibrinogen and D-d levels increased from POD 1 to POD 5 in all patients (P < 0.05), no statistically significant differences were observed between the two groups (Table 6).
White blood cell count: White blood cell count was significantly lower in the intervention group from POD 1 to POD 5, in comparison to that in the control group (P < 0.02) (Table 6).
Serum glutamic oxaloacetic transaminase and serum glutamic pyruvic transaminase: Both serum glutamic oxaloacetic transaminase and serum glutamic pyruvic transaminase increased in the intervention and control groups in POD 2 and remained elevated until POD 3. However, the intervention group showed a less extensive increase on those days (P < 0.05). There were no significant differences in gamma-glutamyl transferase or total bilirubin concentrations between the two groups (Table 6).
Pharmacological preconditioning as a strategy for IRI prevention in humans has been the focus of researchers in many fields of medicine[14,15], especially hepatic IRI. Pharmacological interventions that transiently subject the liver to ischemic conditions may lessen the systemic inflammatory response after surgery and enhance liver function[22,23]. The inflammatory response complicating IR leads to induction of MMPs[10], which have a key role in the inflammation process and their levels reflect the severity of that process[24-26]. Desflurane preconditioning has been promisingly associated with the upregulation of antiapoptotic molecules in an IRI model[27]. We found that levels of MMP2 and MMP9 were significantly increased in the control group, in comparison to the desflurane preconditioning intervention group. These findings indicate, for the first time in a clinical trial, that preconditioning with desflurane limits the anticipated rise in the concentration of MMPs following hepatic IRI.
In a laboratory model, MMP2 activity was found to be reduced after preconditioning in isolated rat hearts[28], while MMP2 inhibition halted IRI in a rat myocardium IR model[29]. In an inducible nitric oxide synthase knockout mouse study, researchers observed inhibition of MMP9 activity and mitigated leukocyte infiltration and liver trauma[30]; Romanic et al[31] reported similar results in MMP9 knockout mice. Taking into consideration the known physiologic and pathogenic actions of MMPs and enhanced liver function after their inhibition in research models, MMPs represent a significant topic of interest for investigation as they are directly involved in IR injury[32,33].
In the present study, we showed that the post-hepatectomy characteristic infiltration of neutrophils in the hepatic parenchyma was greater in the control group than in the desflurane preconditioning group of patients. During the inflammatory process, recruitment of neutrophils is facilitated by nuclear factor-κappa beta (NF-κβ) regulation of cytokine release [e.g., tumor necrosis factor alpha (TNFα), interleukin (IL) 1, and IL8] and the production of adhesion molecules [e.g., intercellular cell adhesion molecule (ICAM) 1 and vascular cell adhesion molecule (VCAM) 1][34]. In parallel, TNFα promotes NF-κΒ action as well as ICAM1 and MMP9 expression[35]. Desflurane can halt these pathways through its direct inhibition of expression of adhesion molecules[36], NF-κΒ and TNFα[37] all of which lead to decreased penetration of neutrophils in the hepatic tissue. Therefore, our results indicate that preconditioning with desflurane decreases the inflammatory response following hepatic IR, which in turn protects the hepatic tissue. Regarding the pathology of hepatic parenchyma, fibrosis and steatosis may be risk factors for intraoperative hemorrhage, transfusion and postoperative complications[38,39]. However, histological results in the two groups were similar.
Although, pharmacological anesthetic preconditioning, such as the administration of volatile and intravenous anesthetics has been extensively studied, no conclusive data recommend the use of a specific technique. In liver transplantation, especially in living liver donors, optimization of liver function is of outmost importance. Desflurane was questioned in this population and was found to be superior to sevoflurane and isoflurane in two studies comparing postoperative hepatic function[40,41]. Beck-Schimmer et al[20], in a randomized controlled trial of patients undergoing liver surgery, showed that ischemic preconditioning with sevoflurane before inflow occlusion dampened postoperative liver injury, even in patients with steatosis. Nguyen et al[42] also pointed that hepatectomy patients receiving sevoflurane, presented better liver function postoperatively. However, the protective effect of pharmacological preconditioning with volatile anesthetics on the remnant liver has been disputed by findings from other studies[43].
With regard to coagulation parameters, our study showed that in the desflurane preconditioning group, the activity of AT III and PC showed a moderate drop postoperatively, while the control group experienced a substantial drop. In addition, INR in the desflurane preconditioning intervention group was significantly lower in comparison to that in the control group, in the postoperative period. Coagulation system homeostasis is frequently impaired in patients undergoing major surgery, especially in those indicated for hepatectomy[44-46]. The coagulopathy present in surgical patients is associated with the marked depletion and decreased activity of the endogenous regulators of blood coagulation[47]. Numerous studies have provided evidence of the platelet count being reduced while INR, PT and aPTT are increased in the first PODs after hepatectomy[47-49], which coincides with the results of our study. Cerruti et al[48] studied the perioperative coagulation profile of living liver donors with the use of routine tests, including those for platelet count, PT-INR and aPTT, as well as testing by thromboelastogram. They reported that in the postoperative period, despite the presence of decreased platelet counts, increased PT-INR and normal aPTT values, thromboelastogram demonstrated the progressive development of hypercoagulability. The complex interaction between coagulation and inflammation may provide insight into derangements of both pathways[50]. Our findings indicate that preconditioning with desflurane may prevent coagulopathies following hepatectomy. The attenuated indices in either pathway may be at least partially attributed to the effect of desflurane on inflammatory mediators. In terms of intraoperative transfusion, the rate is relatively high in both arms compared to the literature despite the use of low central venous pressure[51]. The Pringle maneuver prevents bleeding only from portal inflow but cannot control backflow bleeding from hepatic veins. Thus, blood loss occurs during both transection and reperfusion of the liver. This may also be attributed to the characteristics of the population which includes mostly hepatic tumors and extensive resections[52].
Except for the molecular and biochemical findings, total length of hospital stay was significantly shorter in the intervention group. This may have socioeconomic implications including reduced cost of hospitalization and greater patient satisfaction. Further prospective cohorts should be designed to identify superiority of any pharmacological regimen in relation to outcomes of survival and morbidity[53].
Lastly, this is the first study to investigate the role of desflurane as a volatile preconditioning factor in liver resection. The study is a well-designed and performed randomized controlled trial with excellent allocation concealment, as the surgeon was blinded to the preconditioning intervention. Furthermore, all the hepatectomies were performed by the same expert hepatobiliary surgeon, in order to avoid bias due to inter-surgeon differences in operation techniques. In this study, hepatic IRI was assessed from different aspects, including levels of MMPs, neutrophil infiltration of hepatic parenchyma, and coagulation status. However, as with all studies, a limitation exists; that being, our inability to assess the coagulation status of the patients by thromboelastography. In addition, a larger scale study focusing on more pathways of inflammation and coagulation may be needed in order to elucidate the potential mechanisms of IRI that can be inhibited and introduce desflurane preconditioning in clinical practice.
To summarize, desflurane preconditioning was shown to decrease the inflammatory response and ameliorate the coagulation status following hepatic IRI, thereby protecting hepatic tissue in patients undergoing hepatectomy.
The primary cause of morbidity and mortality in hepatectomies is ischemia-reperfusion injury (IRI).
Understanding the pathophysiology accompanying IRI can offer novel therapeutic targets. Metalloproteinases have been identified as regulators of IRI, and ischemic preconditioning has shown promising results in attenuating IRI.
Our aim was to investigate the effect of ischemic preconditioning with desflurane, primarily on metalloproteinases and their inhibitors, and on the indices of liver and coagulation function.
Patients undergoing liver resection were randomized to receive pharmacologic preconditioning with desflurane or not. Blood samples and liver tissue specimens were collected for laboratory analysis.
Desflurane preconditioning resulted in an attenuated inflammatory response compared to the control group.
Desflurane preconditioning may be effective in ameliorating IRI in hepatectomies, as indicated by the reduction in the expression of matrix metalloproteinases observed in the intervention group. Large scale studies are needed to verify our findings, with data on long-term clinical outcomes.
Metalloproteinases may represent a useful target for managing IRI after hepatectomy.
The assistance provided by Haidich AB, the responsible statistician, was greatly appreciated.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: European Society of Anaesthesiology, 226596.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Li YY, Tan X, Luo H S-Editor: Gao CC L-Editor: Webster JR P-Editor: Wang LL
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13286] [Cited by in RCA: 13558] [Article Influence: 677.9] [Reference Citation Analysis (1)] |
| 2. | Theodoraki K, Tympa A, Karmaniolou I, Tsaroucha A, Arkadopoulos N, Smyrniotis V. Ischemia/reperfusion injury in liver resection: a review of preconditioning methods. Surg Today. 2011;41:620-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Rosen HR, Martin P, Goss J, Donovan J, Melinek J, Rudich S, Imagawa DK, Kinkhabwala M, Seu P, Busuttil RW, Shackleton CR. Significance of early aminotransferase elevation after liver transplantation. Transplantation. 1998;65:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 71] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Parks DA, Granger DN. Ischemia-induced vascular changes: role of xanthine oxidase and hydroxyl radicals. Am J Physiol. 1983;245:G285-G289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 50] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Boros P, Bromberg JS. New cellular and molecular immune pathways in ischemia/reperfusion injury. Am J Transplant. 2006;6:652-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 249] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 6. | Li CX, Ng KT, Shao Y, Liu XB, Ling CC, Ma YY, Geng W, Qi X, Cheng Q, Chung SK, Lo CM, Man K. The inhibition of aldose reductase attenuates hepatic ischemia-reperfusion injury through reducing inflammatory response. Ann Surg. 2014;260:317-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Hu C, Shen S, Zhang A, Ren B, Lin F. The liver protective effect of methylprednisolone on a new experimental acute-on-chronic liver failure model in rats. Dig Liver Dis. 2014;46:928-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Xu F, Dai CL, Peng SL, Zhao Y, Jia CJ, Xu YQ. Preconditioning with glutamine protects against ischemia/reperfusion-induced hepatic injury in rats with obstructive jaundice. Pharmacology. 2014;93:155-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Liu Z, Xu Z, Shen W, Li Y, Zhang J, Ye X. Effect of pharmacologic preconditioning with tetrandrine on subsequent ischemia/reperfusion injury in rat liver. World J Surg. 2004;28:620-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Palladini G, Ferrigno A, Richelmi P, Perlini S, Vairetti M. Role of matrix metalloproteinases in cholestasis and hepatic ischemia/reperfusion injury: A review. World J Gastroenterol. 2015;21:12114-12124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 11. | Hamada T, Fondevila C, Busuttil RW, Coito AJ. Metalloproteinase-9 deficiency protects against hepatic ischemia/reperfusion injury. Hepatology. 2008;47:186-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Cannistrà M, Ruggiero M, Zullo A, Gallelli G, Serafini S, Maria M, Naso A, Grande R, Serra R, Nardo B. Hepatic ischemia reperfusion injury: A systematic review of literature and the role of current drugs and biomarkers. Int J Surg. 2016;33 Suppl 1:S57-S70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 259] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 13. | Naim A, Pan Q, Baig MS. Matrix Metalloproteinases (MMPs) in Liver Diseases. J Clin Exp Hepatol. 2017;7:367-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 14. | Cope DK, Impastato WK, Cohen MV, Downey JM. Volatile anesthetics protect the ischemic rabbit myocardium from infarction. Anesthesiology. 1997;86:699-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 208] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 15. | Tanaka K, Ludwig LM, Kersten JR, Pagel PS, Warltier DC. Mechanisms of cardioprotection by volatile anesthetics. Anesthesiology. 2004;100:707-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 179] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 16. | Lang JD Jr, Teng X, Chumley P, Crawford JH, Isbell TS, Chacko BK, Liu Y, Jhala N, Crowe DR, Smith AB, Cross RC, Frenette L, Kelley EE, Wilhite DW, Hall CR, Page GP, Fallon MB, Bynon JS, Eckhoff DE, Patel RP. Inhaled NO accelerates restoration of liver function in adults following orthotopic liver transplantation. J Clin Invest. 2007;117:2583-2591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 177] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 17. | Jakobsson J. Desflurane: a clinical update of a third-generation inhaled anaesthetic. Acta Anaesthesiol Scand. 2012;56:420-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Tao KM, Yang LQ, Liu YT, Tao Y, Song JC, Wu FX, Yu WF. Volatile anesthetics might be more beneficial than propofol for postoperative liver function in cirrhotic patients receiving hepatectomy. Med Hypotheses. 2010;75:555-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Ko JS, Gwak MS, Choi SJ, Kim GS, Kim JA, Yang M, Lee SM, Cho HS, Chung IS, Kim MH. The effects of desflurane and propofol-remifentanil on postoperative hepatic and renal functions after right hepatectomy in liver donors. Liver Transpl. 2008;14:1150-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Beck-Schimmer B, Breitenstein S, Urech S, De Conno E, Wittlinger M, Puhan M, Jochum W, Spahn DR, Graf R, Clavien PA. A randomized controlled trial on pharmacological preconditioning in liver surgery using a volatile anesthetic. Ann Surg. 2008;248:909-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 171] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 21. | Wei X, Zheng W, Yang Z, Liu H, Tang T, Li X, Liu X. Effect of the intermittent Pringle maneuver on liver damage after hepatectomy: a retrospective cohort study. World J Surg Oncol. 2019;17:142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Jaeschke H, Woolbright BL. Current strategies to minimize hepatic ischemia-reperfusion injury by targeting reactive oxygen species. Transplant Rev (Orlando). 2012;26:103-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 224] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 23. | Zhang Y, Liu M, Yang Y, Cao J, Mi W. Dexmedetomidine exerts a protective effect on ischemia-reperfusion injury after hepatectomy: A prospective, randomized, controlled study. J Clin Anesth. 2020;61:109631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Parks WC, Wilson CL, López-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1279] [Cited by in RCA: 1414] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 25. | Manicone AM, McGuire JK. Matrix metalloproteinases as modulators of inflammation. Semin Cell Dev Biol. 2008;19:34-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 446] [Cited by in RCA: 423] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 26. | Nissinen L, Kähäri VM. Matrix metalloproteinases in inflammation. Biochim Biophys Acta. 2014;1840:2571-2580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 322] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 27. | Yi J, Zheng Y, Miao C, Tang J, Zhu B. Desflurane preconditioning induces oscillation of NF-κB in human umbilical vein endothelial cells. PLoS One. 2013;8:e66576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Lalu MM, Csonka C, Giricz Z, Csont T, Schulz R, Ferdinandy P. Preconditioning decreases ischemia/reperfusion-induced release and activation of matrix metalloproteinase-2. Biochem Biophys Res Commun. 2002;296:937-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Wang W, Schulze CJ, Suarez-Pinzon WL, Dyck JR, Sawicki G, Schulz R. Intracellular action of matrix metalloproteinase-2 accounts for acute myocardial ischemia and reperfusion injury. Circulation. 2002;106:1543-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 351] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 30. | Hamada T, Duarte S, Tsuchihashi S, Busuttil RW, Coito AJ. Inducible nitric oxide synthase deficiency impairs matrix metalloproteinase-9 activity and disrupts leukocyte migration in hepatic ischemia/reperfusion injury. Am J Pathol. 2009;174:2265-2277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 31. | Romanic AM, Harrison SM, Bao W, Burns-Kurtis CL, Pickering S, Gu J, Grau E, Mao J, Sathe GM, Ohlstein EH, Yue TL. Myocardial protection from ischemia/reperfusion injury by targeted deletion of matrix metalloproteinase-9. Cardiovasc Res. 2002;54:549-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 112] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 32. | Dejonckheere E, Vandenbroucke RE, Libert C. Matrix metalloproteinases as drug targets in ischemia/reperfusion injury. Drug Discov Today. 2011;16:762-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 33. | Viappiani S, Sariahmetoglu M, Schulz R. The role of matrix metalloproteinase inhibitors in ischemia-reperfusion injury in the liver. Curr Pharm Des. 2006;12:2923-2934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1:a001651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2497] [Cited by in RCA: 3634] [Article Influence: 227.1] [Reference Citation Analysis (0)] |
| 35. | Lee IT, Lin CC, Wu YC, Yang CM. TNF-alpha induces matrix metalloproteinase-9 expression in A549 cells: role of TNFR1/TRAF2/PKCalpha-dependent signaling pathways. J Cell Physiol. 2010;224:454-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 36. | Biao Z, Zhanggang X, Hao J, Changhong M, Jing C. The in vitro effect of desflurane preconditioning on endothelial adhesion molecules and mRNA expression. Anesth Analg. 2005;100:1007-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Li Y, Zhang X, Zhu B, Xue Z. Desflurane preconditioning inhibits endothelial nuclear factor-kappa-B activation by targeting the proximal end of tumor necrosis factor-alpha signaling. Anesth Analg. 2008;106:1473-1479, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | McCormack L, Petrowsky H, Jochum W, Furrer K, Clavien PA. Hepatic steatosis is a risk factor for postoperative complications after major hepatectomy: a matched case-control study. Ann Surg. 2007;245:923-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 249] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 39. | Aravinda KN, Nagesh NS, Sushruth CS. Evaluation of hepatic steatosis as a risk factor for post-operative outcomes following partial hepatectomy. HPB. 2019;21:S398. [DOI] [Full Text] |
| 40. | Toprak HI, Şahin T, Aslan S, Karahan K, Şanli M, Ersoy MÖ. Effects of desflurane and isoflurane on hepatic and renal functions and coagulation profile during donor hepatectomy. Transplant Proc. 2012;44:1635-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 41. | Ko JS, Gwak MS, Choi SJ, Yang M, Kim MJ, Lee JY, Kim GS, Kwon CH, Joh JW. The effects of desflurane and sevoflurane on hepatic and renal functions after right hepatectomy in living donors*. Transpl Int. 2010;23:736-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Nguyen TM, Fleyfel M, Boleslawski E, M'Ba L, Geniez M, Ethgen S, Béhal H, Lebuffe G. Effect of pharmacological preconditioning with sevoflurane during hepatectomy with intermittent portal triad clamping. HPB (Oxford). 2019;21:1194-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 43. | Rodríguez A, Taurà P, García Domingo MI, Herrero E, Camps J, Forcada P, Sabaté S, Cugat E. Hepatic cytoprotective effect of ischemic and anesthetic preconditioning before liver resection when using intermittent vascular inflow occlusion: a randomized clinical trial. Surgery. 2015;157:249-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 44. | Singh SA, Vivekananthan P, Sharma A, Sharma S, Bharathy KG. Retrospective analysis of post-operative coagulopathy after major hepatic resection at a tertiary care centre in Northern India. Indian J Anaesth. 2017;61:575-580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 45. | Mallett SV, Sugavanam A, Krzanicki DA, Patel S, Broomhead RH, Davidson BR, Riddell A, Gatt A, Chowdary P. Alterations in coagulation following major liver resection. Anaesthesia. 2016;71:657-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 46. | Russell MC. Complications following hepatectomy. Surg Oncol Clin N Am. 2015;24:73-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 47. | Bezeaud A, Denninger MH, Dondero F, Saada V, Venisse L, Huisse MG, Belghiti J, Guillin MC. Hypercoagulability after partial liver resection. Thromb Haemost. 2007;98:1252-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 48. | Cerutti E, Stratta C, Romagnoli R, Schellino MM, Skurzak S, Rizzetto M, Tamponi G, Salizzoni M. Thromboelastogram monitoring in the perioperative period of hepatectomy for adult living liver donation. Liver Transpl. 2004;10:289-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 49. | Shontz R, Karuparthy V, Temple R, Brennan TJ. Prevalence and risk factors predisposing to coagulopathy in patients receiving epidural analgesia for hepatic surgery. Reg Anesth Pain Med. 2009;34:308-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 50. | Esmon CT. The interactions between inflammation and coagulation. Br J Haematol. 2005;131:417-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 701] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 51. | Li Z, Sun YM, Wu FX, Yang LQ, Lu ZJ, Yu WF. Controlled low central venous pressure reduces blood loss and transfusion requirements in hepatectomy. World J Gastroenterol. 2014;20:303-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 65] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 52. | Latchana N, Hirpara DH, Hallet J, Karanicolas PJ. Red blood cell transfusion in liver resection. Langenbecks Arch Surg. 2019;404:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 53. | Lai HC, Lee MS, Lin C, Lin KT, Huang YH, Wong CS, Chan SM, Wu ZF. Propofol-based total intravenous anaesthesia is associated with better survival than desflurane anaesthesia in hepatectomy for hepatocellular carcinoma: a retrospective cohort study. Br J Anaesth. 2019;123:151-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |