Published online Oct 27, 2020. doi: 10.4254/wjh.v12.i10.870
Peer-review started: July 29, 2020
First decision: September 12, 2020
Revised: September 12, 2020
Accepted: September 22, 2020
Article in press: September 22, 2020
Published online: October 27, 2020
Processing time: 86 Days and 12.3 Hours
The novel coronavirus 2019 (COVID-19) pandemic has dramatically transformed the care of the liver transplant patient. In patients who are immunosuppressed and with multiple comorbidities, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has been associated with increased severity and mortality. The main objective of this report is to communicate our experience in the therapeutic management of SARS-CoV-2 infection in 3 liver transplant patients. Secondly, we stress the management and investigation of the contagious spreading into a liver transplant ward.
The patients were two women (aged 61 years and 62 years) and one man (aged 68 years), all of them having recently received a liver transplant. All three patients required intensive care unit admission and invasive mechanical ventilation. Two of them progressed severely until death. The other one, who received tocilizumab, had a good recovery. In the outbreak, the wife of one of the patients and four healthcare professionals involved in their care were also infected.
We illustrate in detail the evolution of a nosocomial COVID-19 outbreak in a liver transplant ward. We believe that these findings will contribute to a better understanding of the natural history of the disease and will improve the treatment of the liver transplant patient with COVID-19.
Core Tip: In patients who are immunosuppressed and with multiple comorbidities, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has been associated with increased severity and mortality. We report our experience in the therapeutic management of SARS-CoV-2 infection in 3 liver transplant patients and stress the management and investigation of a contagious spreading into a liver transplant ward.
- Citation: Alconchel F, Cascales-Campos PA, Pons JA, Martínez M, Valiente-Campos J, Gajownik U, Ortiz ML, Martínez-Alarcón L, Parrilla P, Robles R, Sánchez-Bueno F, Moreno S, Ramírez P. Severe COVID-19 after liver transplantation, surviving the pitfalls of learning on-the-go: Three case reports. World J Hepatol 2020; 12(10): 870-879
- URL: https://www.wjgnet.com/1948-5182/full/v12/i10/870.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i10.870
After the first cases of coronavirus-2 pneumonia (SARS-CoV-2) were detected in early December 2019 in Wuhan (Hubei, China)[1,2] a pandemic has overtaken hundreds of countries[3]. The main active sources are currently located in Europe and the United States[4]. This medical emergency has tested global healthcare systems, which have established strategic changes and protocols to prioritize healthcare and avoid overloading. The frequency of liver transplantation operations has been seriously affected. Transplant programs depend on the availability of donors, the vast majority of whom are deceased, and medical personnel normally oversee these programs in intensive care units, but these facilities are currently overcrowded. The result of these conditions has been a dramatic decrease in activity in all transplant groups around the world. In Spain, the world leader in organ donation, surgeons had access to the livers of about 100 deceased donors per week during the 3-month period before the detection of the first case of novel coronavirus (COVID-19); this number has since dropped dramatically to a level of only 15 donors per week[5,6].
In an effort to contain the pandemic, drastic community measures of social confinement and distancing have been established, and these measures extend to the healthcare environment, enhancing telematic activities for the ambulatory management of patients. At the in-hospital level, most of the preventive measures are aimed at preventing the spread of infection by healthcare professionals during the care of COVID-19 patients. The impact of nosocomial infection by COVID-19 has warranted little attention and could be especially relevant to transplant recipients during their hospitalization.
The main objective of our work is to communicate our experience in the therapeutic management of severe SARS-CoV-2 infection in three liver transplant patients who required invasive mechanical ventilation, two of whom had an infection of nosocomial origin. We also analysed some lessons learned from this experience.
Since the detection of the first case of COVID-19 in our region on March 8, 2020, and until May 31st, 2020, a total of twelve liver transplant patients have been hospitalized in our liver transplant unit.
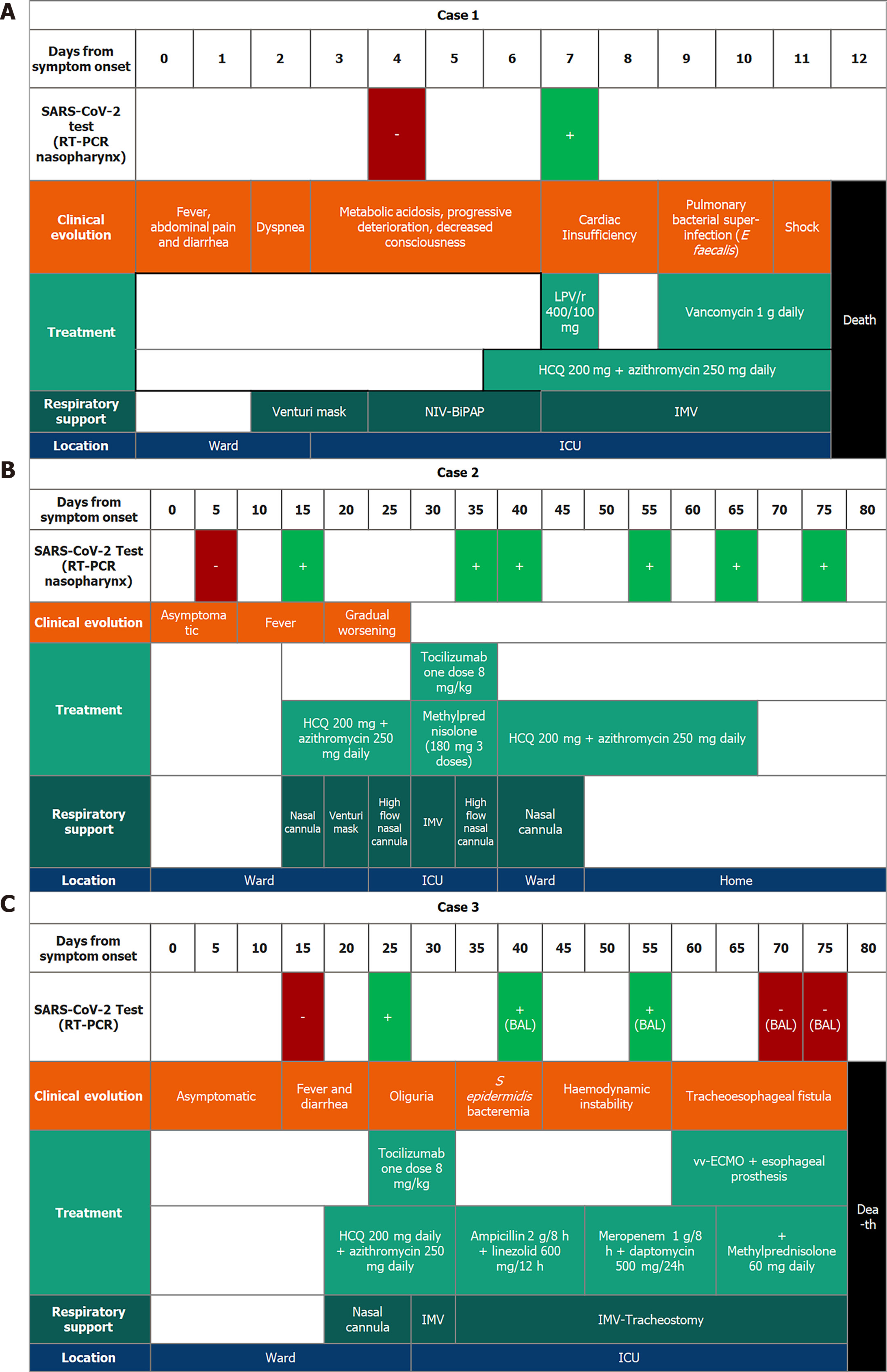
Case 1: Sixty-one-year-old woman with a liver transplant in September 2019 for cryptogenic cirrhosis. In early March, she was admitted for diarrhea, and a few days later she developed acute respiratory failure, and heart failure. A first RT-PCR of SARS-CoV-2 from throat and pharyngeal swabs was negative but became positive three days later after a second RT-PCR was conducted due to high clinical suspicion (Figure 1). Treatment with hydroxychloroquine and lopinavir/ritonavir was then initiated, adjusting the tacrolimus levels, but the patient suffered progressive clinical and analytical worsening, with the need for invasive mechanical ventilation, associated with pulmonary superinfection by Enterococcus faecalis and Enterococcus faecium detected in bronchoalveolar lavage fluid. Finally, the patient developed shock with multisystem failure and died in the third week of hospitalization.
Case 2: Sixty-eight-year-old male, transplanted on March 4, 2020, by non-alcoholic steatohepatitis. During the immediate post-transplant period, he was diagnosed with a biliary stricture and was treated endoscopically. His wife, the primary caregiver, tested positive for SARS-CoV-2 via RT-PCR from pharyngeal swabs on March 18, 2020, after reporting slightly compatible symptoms. All staff in contact with her, including the patient himself (who was initially negative), were evaluated with RT-PCR. Of a total of 40 people tested, one hepatologist was positive for SARS-CoV-2; this physician was in contact with all patients admitted at that time. Four days later, the patient, without symptoms, was discharged. Two days after discharge, the patient was readmitted for fever and cough, and the RT-PCR of SARS-CoV-2 was positive (Figure 1). Early treatment with hydroxychloroquine and azithromycin was initiated, adjusting the doses of mycophenolic acid and tacrolimus. Seven days after the positive result, the patient was admitted to the intensive care unit due to deterioration of respiratory function requiring invasive mechanical ventilation and treatment with tocilizumab. The patient progressed satisfactorily to home discharge and asymptomatic, but still with a positive RT-PCR of SARS-CoV-2 two months later.
Case 3: Sixty-two-year-old woman who received a liver transplant in February 2019 secondary to primary biliary cholangitis and was discharged in the first week of March 2020 after an episode of constitutional syndrome. On April 6, 2020, she was readmitted with fever, dyspnea, and diarrhea, with a RT-PCR positive for SARS-CoV-2. Forty-eight hours later, the patient progressively deteriorated, requiring admission to intensive care unit with invasive mechanical ventilation, and was treated with tocilizumab in addition to hydroxychloroquine, azithromycin, and methylprednisolone. Mycophenolic acid was suspended, and doses of tacrolimus were reduced to the minimum necessary. After four days of invasive mechanical ventilation, extubation was performed. In spite of the measures adopted, the patient evolved severely. Two months after the onset of the outbreak, and still with a positive RT-PCR of SARS-CoV-2, she developed a tracheoesophageal fistula. An esophageal prosthesis and an extracorporeal venovenous membrane oxygenation (vv-ECMO) were placed. Forty-five days after the first positive RT-PCR of SARS-CoV-2 the virus was negative in the RT-PCR of the bronchoalveolar lavage. Unfortunately, the patient died eighty days after the onset of the outbreak in our liver transplant unit.
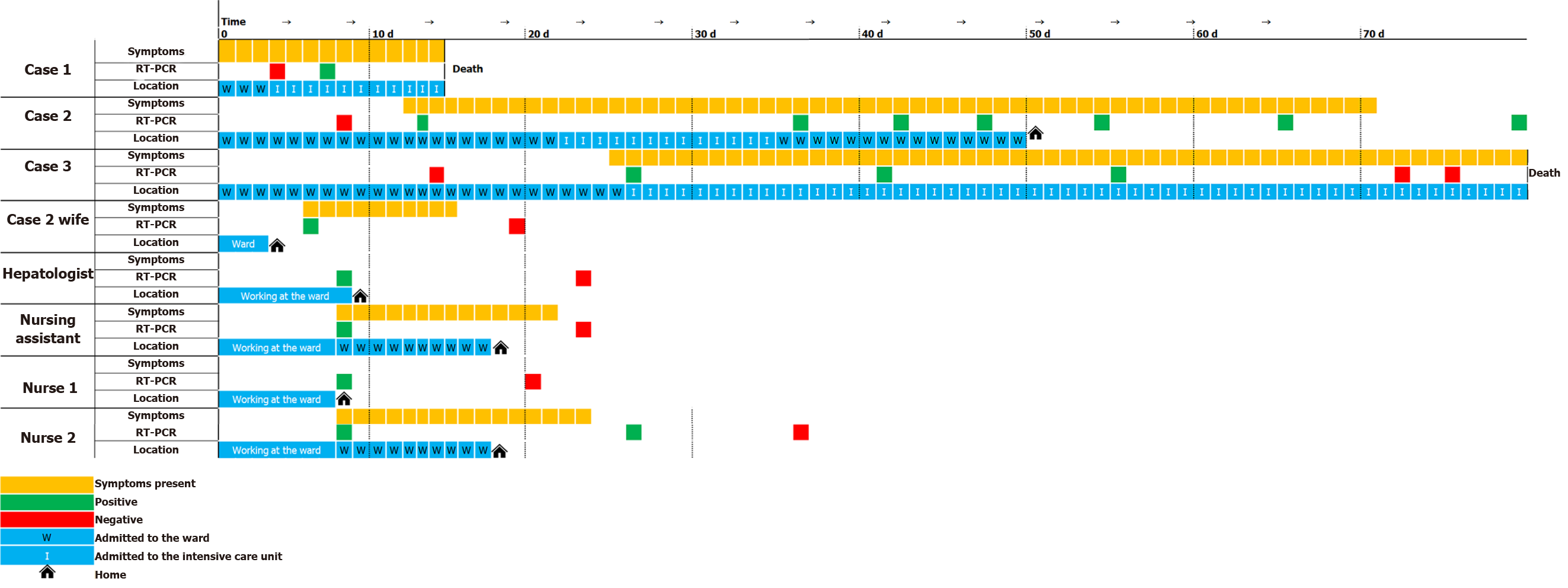
A cluster of three patients who temporarily coincided in the hospitalization ward developed a SARS-CoV-2 [reverse transcription polymerase chain reaction (RT-PCR) throat swab] infection. Given the use of anonymous clinical data and the observational approach of our paper, our work was exempt from approval from an ethics' board. Table 1 shows the main clinical features of these three patients. It is important to note that the wife of case 2, one hepatologist on the transplant team, and three nurses in the ward were also infected with the SARS-CoV-2 virus.
| Case 1 | Case 2 | Case 3 | |
| Age (yr) | 61 | 68 | 62 |
| Sex | Female | Male | Female |
| LT indication | Cryptogenic cirrhosis | NASH | Primary biliary cholangitis |
| Date of liver transplant | September 7, 2019 | March 3, 2020 | February 13, 2019 |
| Immunosuppression (per day) | Tacrolimus 5 mg and prednisone 5 mg | Tacrolimus 7 mg, mycophenolic acid 2000 mg, prednisone 20 mg | Tacrolimus 3 mg, mycophenolic acid 2000 mg, prednisone 5 mg |
| Blood concentration of tacrolimus (before COVID-19) | 7 ng/mL | 7.5 ng/mL | 5.2 ng/mL |
| Allograft function (before COVID-19) | Normal | Normal | Increased GGT and ALP |
| Comorbidities | Hypothyroidism | Diabetes, hypertension, stroke | Hypertension |
| Laboratory test1: | |||
| PaO2: FiO2 ratio (while IMV) | 237 (76-376) | 367 (337-385) | 256 (133-329) |
| White-cell count (× 103/UL) | 4.50 (2.38-9.87) | 6.61 (1.8-17.4) | 15.71 (7.02-36.14) |
| Lymphocyte count (× 103/UL) | 0.30 (0-0.65) | 0.325 (0.2-1.02) | 1 (0.54-2.01) |
| Platelet count (× 103/UL) | 9.5 (3-38) | 113.5 (37-372) | 290 (158-406) |
| Hemoglobin (g/DL) | 8.7 (6.5-9.4) | 9.2 (7-11.8) | 10.4 (7.6-12.9) |
| Il-6 (pg/mL) | 599 (400-799) | 558 (192-1000) | 54 (50-286) |
| C-reactive protein (mg/DL) | 10.3 (8.2-18.3) | 4 (0.4-13.3) | 1.8 (0.7-4.6) |
| Procalcitonin (ng/mL) | 2.2 (1.1-7.8) | 0.25 (0.12-0.43) | 0.28 (0.17-0.75) |
| Ferritin (ng/mL) | 5338 (814-9862) | 1262 (392-2095) | 2047 (1360-2297) |
| Lactate dehydrogenase (U/L) | 452 (209-649) | 265 (161-378) | 399 (165-646) |
| Aspartate aminotransferase (U/L) | 126 (29-466) | 14 (9-36) | 30 (16-44) |
| Alanine aminotransferase (U/L) | 89 (29-197) | 21 (8-31) | 24 (5-98) |
| Total bilirubin (mg/DL) | 1.2 (0.3-2.65) | 0.56 (0.39-1.23) | 1.82 (0.21-4) |
| Creatine kinase (U/L) | 14 (10-18) | 13 (7-75) | 29 (29-36) |
| Creatinine (mg/DL) | 0.69 (0.45-1.07) | 1.22 (1.04-1.93) | 0.89 (0.54-2.65) |
| D-dimer (ng/mL) | 1073 (565-1825) | 1347 (620 - 3431) | 283 (153-648) |
| Sodium (meq/L) | 137 (128-141) | 139 (136 - 163) | 141 (135-145) |
| Potassium (meq/L) | 4.6 (2.9-5.8) | 4 (3.3-4.9) | 4 (3.2-5.2) |
| Chloride (meq/L) | 102 (97-105) | 103 (100-111) | 107 (99-112) |
| RT-PCR of SARS-CoV-2 | Negative on day 3; positive on day 6 | Negative on day 8; positive on days 13, 36, 42, 47, 54, 65 and 79 | Negative on days 14, 72 and 75; positive on days 26, 42 and 55 |
| Radiologic findings | Bilateral pneumonia, pleural effusion | Bilateral pneumonia, peripheral ground-glass opacity, pleural effusion | Bilateral pneumonia, peripheral ground-glass opacity |
| Treatment | HCQ (200 mg daily), azithromycin (250 mg daily), LPV/r (one dose 400/100 mg), vancomycin (1 g daily) | HCQ (200 mg daily), azithromycin (250 mg daily), tocilizumab (one dose 8 mg/kg), methylprednisolone (180 mg three doses) | HCQ (200 mg daily), azithromycin (250 mg daily), tocilizumab (one dose 8 mg/kg), methylprednisolone (60 mg daily), vv-ECMO |
| Immunosuppressant dose reduction | Yes (low dose of tacrolimus) | Yes (mycophenolic acid suspended and low dose of tacrolimus) | Yes (mycophenolic acid suspended) |
| Rejection during or after COVID-19 | No | No | Yes |
| Complications | Secondary Enterococcus faecalis (BAL culture) lung infection | Asymptomatic intra-abdominal collection | Tracheoesophageal fistula |
In our transplant unit, the outbreak of SARS-CoV-2 began on March 18. After a positive RT-PCR result in case 1, the wife of case 2 also tested positive after reporting a fever and neck pain. Without delay, we conducted an exhaustive investigation on all the members of the unit (25 nurses and assistants, two cleaning staff, one warden, and nine physicians), with one nursing assistant testing positive for SARS-CoV-2 (hospital admission for ten days for pneumonia, without the need for intensive care unit), two nurses testing positive (one with a mild symptoms and negative RT-PCR at one month, and the other asymptomatic and negative at 13 d), and a hepatologist testing positive (negative at 15 d, asymptomatic and no admission).
The remaining healthcare personnel who tested negative for SARS-CoV-2 were placed in preventive home confinement for 14 d, with negative RT-PCR determinations of SARS-CoV-2 thereafter. In addition, the hospital ward was closed for complete disinfection.
Figure 2 shows the epidemiological timeline of the three positive liver transplant recipients as well as the four contacts in the ward (one doctor, one assistant, and two nurses) who tested positive for SARS-CoV-2.
The final diagnosis of the presented cases is severe COVID-19 after liver transplantation.
Case 1 was treated with Hydroxychloroquine (HCQ, 200 mg daily), azithromycin (250 mg daily), lopinavir/ritonavir (one dose 400/100 mg) and vancomycin (1 g daily). The patient in case 2, underwent a treatment that included HCQ (200 mg daily), azithromycin (250 mg daily), tocilizumab (one dose 8 mg/kg) and methylprednisolone (180 mg three doses). In the case of patient 3, however, the outcome was more severe and required the use of a vv-ECMO in addition to HCQ (200 mg daily), azithromycin (250 mg daily), tocilizumab (one dose 8 mg/kg) and methylprednisolone (60 mg daily) (Table 1 and Figure 1).
All three patients required intensive care unit admission and invasive mechanical ventilation (Figure 1). Two of them (cases 1 and 3) progressed severely until death. The other one (case 2), who received tocilizumab, had a good recovery. In the outbreak, the wife of one of the patients and four healthcare professionals involved in their care were also infected (Figure 2).
The most appropriate management for transplant recipients who develop COVID-19 and the impact of the infection on this population are not well known. In a previous publication on a population of 111 liver transplant patients with more than ten years of evolution and residents in lombardy (the epicentre of the pandemic in Italy), three deaths were reported due to COVID-19[7]. The three liver transplant patients were all male and over 65 years old, with a body mass index greater than 28 kg/m2 and with cardiovascular risk factors. The occurrence of co-morbidities such as older age, obesity, diabetes mellitus, use of anti-hypertensive drugs, and other cardiovascular risk factors have been associated with more severe clinical manifestations in the general population[8]. There is no consensus regarding the optimal management of immunosuppressive treatment, and most groups suggest not to modify the immunosuppression strategies in asymptomatic or mild infections, but the experience reported in the literature in severe and critical conditions is variable, and some authors with whom we agree defend the decrease of immunosuppression[9].
In the physiopathology of SARS-CoV-2, the liver appears to be a susceptible organ[10] and liver damage occurs according to three mechanisms: (1) Direct cytotoxicity of the virus itself; (2) Indirect damage from autoimmune aetiology; and (3) Hepatotoxicity of drugs used in the management of the infection (remdesivir, tocilizumab, chloroquine and its derivatives, and azithromycin, among others)[11]. Typical clinical manifestations of SARS-CoV-2 infection include fever, cough, and respiratory distress. The presence of gastrointestinal symptoms at the onset of the picture has been previously reported[12-15] and, along with the fever, were the initial manifestations in cases 1 and 3 of our series. In this initial phase, the RT-PCR determinations of SARS-CoV-2 were negative in both cases, and the patients presented gastrointestinal and non-respiratory symptoms. This pattern of symptoms was similar to that reported in a renal transplant patient with COVID-19[16,17].
The clinical situation worsened rapidly in case 1, evolving rapidly towards a fatal outcome. In this patient, elevated levels of C-reactive protein and procalcitonin were detected, as well as severe lymphopenia[18]. In addition, the presence of Enterococcus faecium and Enterococcus faecalis was found in the culture of the bronchoalveolar flush, which, in the context of SARS-CoV-2 pneumonia (Figure 1A), undoubtedly triggered the clinical course toward shock and the death of the patient. Although some authors support the hypothesis that SARS-CoV-2 may cause true sepsis of viral origin by direct attack of the virus[19], this theory is not proven in practice, and we believe that that this theory does not explain by itself the fulminant evolution of this patient. Nevertheless, the clinical evolution was favourable in case 2, in which the patient was treated with hydroxychloroquine and tocilizumab. In case 1, we opted for the use of a lopinavir/ritonavir combination, although we do not know if this was correct, but the dose of tacrolimus was closely monitored due to the interaction of these drugs through the inhibition of cytochrome P450 family 3 subfamily A[20,21]. Several studies have shown that patients infected with SARS-CoV-2 can develop a hyperinflammatory state which leads to an acute respiratory distress syndrome [acute respiratory distress syndrome (ARDS)[22]]. During ARDS pathogenesis, a "cytokine storm" including IL-6, tumor necrosis factor alpha, and IL-12 is released[23]. These data suggest, that a blockage of pro-inflammatory pathways could be a therapeutic alternative in the management of patients with severe COVID-19. Tocilizumab is a monoclonal antibody that specifically binds to the IL-6 receptor and inhibits signal transduction mediated by the binding of this receptor to its ligand. In two of our patients, tocilizumab was administered early, even before the need for invasive mechanical ventilation, achieving favorable outcomes. In contrast, in case 1, where tocilizumab was not administered, a situation of shock and multiorgan failure was triggered, resulting in the death of the patient. These findings would be in consonance with what was previously published regarding ARDS and suggest that tocilizumab could be an effective treatment for severe patients with COVID-19.
The liver transplant population has several specific peculiarities. It has been described that, after infection, more than 50% of patients with a liver transplant develop severe forms of the disease[8]. In addition, the time for virus detection tests among these patients to become negative (clearance) is longer than that among the general population, and positive RT-PCRs of SARS-CoV-2 have been described in transplant patients beyond 53 d from the first positive test[24], which carries a potentially higher risk of contagion and the need for a longer period of isolation[25].
The vast majority of measures to prevent infection in the population are focused on non-hospital settings (such as confinement and telemedicine). In hospitals, these measures are preferably designed to prevent the transmission of COVID-19 from patients to healthcare personnel, where the use of personal protective equipment is mandatory. Furthermore, in the case of patients with liver disease, additional measures must be taken. Xiao et al[26] suggest, for example, that the communication between patients and medical staff should be done online and each patient taken care of by one attending doctor and one nurse exclusively.
Preventive measures should begin as soon as the recipient is admitted, including the existence of a specific safety circuit until the result of the RT-PCR is known. For example, two of our patients who were candidates for transplant in the last week have tested positive on the day of the transplant but had no symptoms, and the donor grafts were therefore transplanted to other recipients. In addition to community transmission (case 1), nosocomial transmission of the virus must also be considered (cases 2 and 3). Once a case of nosocomial transmission is discovered, special measures should be applied not only to ward staff but also to all ward patients and facilities. In our experience after case 2, the entire ward was evacuated, and a procedure of disinfection and a quarantine of the premises and of all healthcare staff who had worked on the ward were undertaken.
Another problem in relation to the in-hospital management of SARS-CoV-2–infected transplant patients resides in the discordance between the positivity of RT-PCR and the symptoms suggestive of COVID-19, indicating a high-risk window of infection[27]. As other published works have examined[28], in the outbreak that took place in our unit, there was a period in which healthcare professionals and companions who were asymptomatic carriers of SARS-CoV-2 concurred in space and time with other patients and healthcare professionals who did not have the virus. These circumstances favored the propagation of the virus until the first positive case was detected and the necessary measures were taken.
A final, equally important aspect is the real impact of the COVID-19 pandemic on organ donation, transplant policies, and waiting list mortality, which altogether constitute the so-called “indirect mortality” from SARS-CoV-2. Most countries have implemented emergency policies to prevent contagion, ranging from issuing systematic screening tests for SARS-CoV-2 in all donors and recipients, limiting donation to far from the hospital where the graft will be implanted, restricting liver transplant activity only in acute liver failure or critical patients[29,30], and implementing telemedicine in outpatient follow-up. In these circumstances, increases in both mortality among those on the waiting list and in the number of drop-outs due to clinical worsening or tumour progression (indirect deaths from COVID-19) are to be expected. In fact, in Spain, organ donation and transplantation have decreased dramatically. Before the declaration of the state of alarm on March 13, 2020, there were 7.2 donors and 16 transplants per day on average, but since that date, the rates have fallen to an average of 1.2 donors and 2.1 transplants per day[5].
Therefore, there are several lessons learned from our experience. Firstly, early administration of anti-IL-6 monoclonal antibodies could be beneficial in slowing down the cytokine storm in critically ill patients with COVID-19. Secondly, the disease prodrome in two patients were the gastrointestinal and not the respiratory symptoms. Finally, COVID-19 is highly contagious, so drastic preventive measures and exhaustive epidemiological investigations must be conducted in the case of clinical suspected disease in the ward, even if the RT-PCR of SARS-CoV-2 has been tested negative.
Many uncertainties persist in relation to the diagnosis, treatment, and management of COVID-19 in liver transplant patients. It is certain that we will learn more about the disease and be able to treat it more effectively in the coming months. In the meantime, we are walking blind, and we must rely on our scarce previous experience, on our intuition, and on the oldest methodology in medicine: Trial and error.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pandey CK S-Editor: Zhang L L-Editor: A P-Editor: Li X
| 1. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 18866] [Article Influence: 3773.2] [Reference Citation Analysis (7)] |
| 2. | Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W; China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18987] [Cited by in RCA: 17630] [Article Influence: 3526.0] [Reference Citation Analysis (0)] |
| 3. | Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Liu M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TTY, Wu JT, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382:1199-1207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11224] [Cited by in RCA: 9313] [Article Influence: 1862.6] [Reference Citation Analysis (0)] |
| 4. | COVID-19 Map. Johns Hopkins Coronavirus Resource Center [Internet]. [Cited 2020 April 20]. Available from: https://coronavirus.jhu.edu/map.html. |
| 5. | Organización Nacional de Trasplantes (ONT). COVID-19: Impacto en la actividad de donación y trasplantes [Internet]. [Cited 2020 April 20]. Available from: http://www.ont.es/infesp/Paginas/Impacto_tx.aspx#_blank. |
| 6. | Ahn C, Amer H, Anglicheau D, Ascher NL, Baan CC, Battsetset G, Bat-Ireedui B, Berney T, Betjes MGH, Bichu S, Birn H, Brennan D, Bromberg J, Caillard S, Cannon RM, Cantarovich M, Chan A, Chen ZS, Chapman JR, Cole EH, Cross N, Durand F, Egawa H, Emond JC, Farrero M, Friend PJ, Geissler EK, Ha J, Haberal MA, Henderson ML, Hesselink DA, Humar A, Jassem W, Jeong JC, Kaplan B, Kee T, Kim SJ, Kumar D, Legendre CM, Man K, Moulin B, Muller E, Munkhbat R, Od-Erdene L, Perrin P, Rela M, Tanabe K, Tedesco Silva H, Tinckam KT, Tullius SG, Wong G. Global Transplantation COVID Report March 2020. Transplantation. 2020;104:1974-1983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 77] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 7. | Bhoori S, Rossi RE, Citterio D, Mazzaferro V. COVID-19 in long-term liver transplant patients: preliminary experience from an Italian transplant centre in Lombardy. Lancet Gastroenterol Hepatol. 2020;5:532-533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 205] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 8. | Boyarsky BJ, Po-Yu Chiang T, Werbel WA, Durand CM, Avery RK, Getsin SN, Jackson KR, Kernodle AB, Van Pilsum Rasmussen SE, Massie AB, Segev DL, Garonzik-Wang JM. Early impact of COVID-19 on transplant center practices and policies in the United States. Am J Transplant. 2020;20:1809-1818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 192] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 9. | Pons JA, Ramírez P, Revilla-Nuin B, Pascual D, Baroja-Mazo A, Robles R, Sanchez-Bueno F, Martinez L, Parrilla P. Immunosuppression withdrawal improves long-term metabolic parameters, cardiovascular risk factors and renal function in liver transplant patients. Clin Transplant. 2009;23:329-336. [RCA] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Zhong Z, Zhang Q, Xia H, Wang A, Liang W, Zhou W, Zhou L, Liu X, Rao L, Li Z, Peng Z, Mo P, Xiong Y, Ye S, Wang Y, Ye Q. Clinical characteristics and immunosuppressant management of coronavirus disease 2019 in solid organ transplant recipients. Am J Transplant. 2020;20:1916-1921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 11. | Fix OK, Hameed B, Fontana RJ, Kwok RM, McGuire BM, Mulligan DC, Pratt DS, Russo MW, Schilsky ML, Verna EC, Loomba R, Cohen DE, Bezerra JA, Reddy KR, Chung RT. Clinical Best Practice Advice for Hepatology and Liver Transplant Providers During the COVID-19 Pandemic: AASLD Expert Panel Consensus Statement. Hepatology. 2020;72:287-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 422] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 12. | Zhou Z, Zhao N, Shu Y, Han S, Chen B, Shu X. Effect of Gastrointestinal Symptoms in Patients With COVID-19. Gastroenterology. 2020;158:2294-2297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 167] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 13. | Jin X, Lian JS, Hu JH, Gao J, Zheng L, Zhang YM, Hao SR, Jia HY, Cai H, Zhang XL, Yu GD, Xu KJ, Wang XY, Gu JQ, Zhang SY, Ye CY, Jin CL, Lu YF, Yu X, Yu XP, Huang JR, Xu KL, Ni Q, Yu CB, Zhu B, Li YT, Liu J, Zhao H, Zhang X, Yu L, Guo YZ, Su JW, Tao JJ, Lang GJ, Wu XX, Wu WR, Qv TT, Xiang DR, Yi P, Shi D, Chen Y, Ren Y, Qiu YQ, Li LJ, Sheng J, Yang Y. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69:1002-1009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 944] [Cited by in RCA: 869] [Article Influence: 173.8] [Reference Citation Analysis (0)] |
| 14. | Wan Y, Li J, Shen L, Zou Y, Hou L, Zhu L, Faden HS, Tang Z, Shi M, Jiao N, Li Y, Cheng S, Huang Y, Wu D, Xu Z, Pan L, Zhu J, Yan G, Zhu R, Lan P. Enteric involvement in hospitalised patients with COVID-19 outside Wuhan. Lancet Gastroenterol Hepatol. 2020;5:534-535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 15. | Pereira MR, Mohan S, Cohen DJ, Husain SA, Dube GK, Ratner LE, Arcasoy S, Aversa MM, Benvenuto LJ, Dadhania DM, Kapur S, Dove LM, Brown RS, Rosenblatt RE, Samstein B, Uriel N, Farr MA, Satlin M, Small CB, Walsh TJ, Kodiyanplakkal RP, Miko BA, Aaron JG, Tsapepas DS, Emond JC, Verna EC. COVID-19 in solid organ transplant recipients: Initial report from the US epicenter. Am J Transplant. 2020;20:1800-1808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 541] [Cited by in RCA: 664] [Article Influence: 132.8] [Reference Citation Analysis (0)] |
| 16. | Guillen E, Pineiro GJ, Revuelta I, Rodriguez D, Bodro M, Moreno A, Campistol JM, Diekmann F, Ventura-Aguiar P. Case report of COVID-19 in a kidney transplant recipient: Does immunosuppression alter the clinical presentation? Am J Transplant. 2020;20:1875-1878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 207] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 17. | Akalin E, Azzi Y, Bartash R, Seethamraju H, Parides M, Hemmige V, Ross M, Forest S, Goldstein YD, Ajaimy M, Liriano-Ward L, Pynadath C, Loarte-Campos P, Nandigam PB, Graham J, Le M, Rocca J, Kinkhabwala M. Covid-19 and Kidney Transplantation. N Engl J Med. 2020;382:2475-2477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 594] [Cited by in RCA: 641] [Article Influence: 128.2] [Reference Citation Analysis (0)] |
| 18. | Qin J, Wang H, Qin X, Zhang P, Zhu L, Cai J, Yuan Y, Li H. Perioperative Presentation of COVID-19 Disease in a Liver Transplant Recipient. Hepatology. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 19. | Li H, Liu L, Zhang D, Xu J, Dai H, Tang N, Su X, Cao B. Hypothesis SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 772] [Cited by in RCA: 886] [Article Influence: 177.2] [Reference Citation Analysis (0)] |
| 20. | Yeh RF, Gaver VE, Patterson KB, Rezk NL, Baxter-Meheux F, Blake MJ, Eron JJ, Klein CE, Rublein JC, Kashuba AD. Lopinavir/ritonavir induces the hepatic activity of cytochrome P450 enzymes CYP2C9, CYP2C19, and CYP1A2 but inhibits the hepatic and intestinal activity of CYP3A as measured by a phenotyping drug cocktail in healthy volunteers. J Acquir Immune Defic Syndr. 2006;42:52-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 157] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 21. | Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Li H, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Wang J, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. 2020;382:1787-1799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3386] [Cited by in RCA: 3626] [Article Influence: 725.2] [Reference Citation Analysis (0)] |
| 22. | Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033-1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6366] [Cited by in RCA: 6746] [Article Influence: 1349.2] [Reference Citation Analysis (0)] |
| 23. | Teachey DT, Rheingold SR, Maude SL, Zugmaier G, Barrett DM, Seif AE, Nichols KE, Suppa EK, Kalos M, Berg RA, Fitzgerald JC, Aplenc R, Gore L, Grupp SA. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood. 2013;121:5154-5157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 494] [Article Influence: 41.2] [Reference Citation Analysis (1)] |
| 24. | Liu H, He X, Wang Y, Zhou S, Zhang D, Zhu J, He Q, Zhu Z, Li G, Sun L, Wang J, Cheng G, Liu Z, Lau G. Management of COVID-19 in patients after liver transplantation: Beijing working party for liver transplantation. Hepatol Int. 2020;14:432-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 25. | Huang JF, Zheng KI, George J, Gao HN, Wei RN, Yan HD, Zheng MH. Fatal outcome in a liver transplant recipient with COVID-19. Am J Transplant. 2020;20:1907-1910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 26. | Xiao Y, Pan H, She Q, Wang F, Chen M. Prevention of SARS-CoV-2 infection in patients with decompensated cirrhosis. Lancet Gastroenterol Hepatol. 2020;5:528-529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 27. | Gandhi M, Yokoe DS, Havlir DV. Asymptomatic Transmission, the Achilles' Heel of Current Strategies to Control Covid-19. N Engl J Med. 2020;382:2158-2160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 801] [Cited by in RCA: 742] [Article Influence: 148.4] [Reference Citation Analysis (0)] |
| 28. | Young BE, Ong SWX, Kalimuddin S, Low JG, Tan SY, Loh J, Ng OT, Marimuthu K, Ang LW, Mak TM, Lau SK, Anderson DE, Chan KS, Tan TY, Ng TY, Cui L, Said Z, Kurupatham L, Chen MI, Chan M, Vasoo S, Wang LF, Tan BH, Lin RTP, Lee VJM, Leo YS, Lye DC; Singapore 2019 Novel Coronavirus Outbreak Research Team. Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore. JAMA. 2020;323:1488-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1346] [Cited by in RCA: 1365] [Article Influence: 273.0] [Reference Citation Analysis (0)] |
| 29. | Tzedakis S, Jeddou H, Houssel-Debry P, Sulpice L, Boudjema K. COVID-19: Thoughts and comments from a tertiary liver transplant center in France. Am J Transplant. 2020;20:1952-1953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Vargas M, Iacovazzo C, Servillo G. Additional Suggestions for Organ Donation during COVID-19 Outbreak. Transplantation. 2020;104:1984-1985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |