Published online Oct 27, 2020. doi: 10.4254/wjh.v12.i10.829
Peer-review started: May 25, 2020
First decision: June 12, 2020
Revised: July 6, 2020
Accepted: September 4, 2020
Article in press: September 4, 2020
Published online: October 27, 2020
Processing time: 151 Days and 11.5 Hours
Malnutrition is frequently encountered in patients with cirrhosis and appears to significantly impact their prognosis. While evaluating the burden of malnutrition in cirrhosis is gathering momentum, as suggested by multiple recently published reports, there is still a persistent scarcity of solid data in the field, especially with regards to the role of nutritional interventions.
To assess the prevalence of malnutrition in patients with advanced cirrhosis and to evaluate its impact on survival.
One hundred and one consecutive patients with advanced cirrhosis were screened for malnutrition using the Subjective Global Assessment (SGA) criteria and the mid-arm circumference (MAC). Malnutrition was defined as SGA class B and C and MAC < 10th percentile. All patients were interviewed regarding their food intake using an adapted questionnaire. Subsequently, total energy intake was calculated and further subdivided in main nutrients. The data were then compared to the available recommendations at the time of analysis to assess adherence.
54/79 patients (68.4%) in the decompensated group had malnutrition, while only 3/22 patients (13.6%) were malnourished in the compensated group. After a median follow-up time of 27 mo (0-53), the overall mortality was 70%. Survival was significantly lower among patients with malnutrition. The mortality rates were 50% at 1 year and 63% at 2 years for the patients with malnutrition, compared to 21% at 1 year and 30% at 2 years for patients without malnutrition (P = 0.01). On multivariate analysis, the factors independently associated with mortality were age, creatinine level and adherence to the protein intake recommendations. The mortality was lower in patients with the appropriate protein intake: 8% at 1 year and 28% at 2 years in the adherent group, compared to 47% at 1 year and 56% at 2 years in the non-adherent group.
The prevalence of malnutrition is high among patients with advanced cirrhosis and might be related in part to a low adherence to nutritional recommendations, especially with regards to protein intake.
Core Tip: It is already known that the patients having cirrhosis can be affected by malnutrition and this status can impact the survival. This article studied the prevalence of malnutrition in advanced stages of cirrhosis, and its influence on survival. Our results showed that the prevalence of malnutrition is high in patients with advanced cirrhosis and is related in part to a low adherence to nutritional recommendations. Appropriate protein intake could increase the survival.
- Citation: Crisan D, Procopet B, Epure A, Stefanescu H, Suciu A, Fodor A, Mois E, Craciun R, Crisan N. Malnutrition and non-compliance to nutritional recommendations in patients with cirrhosis are associated with a lower survival. World J Hepatol 2020; 12(10): 829-840
- URL: https://www.wjgnet.com/1948-5182/full/v12/i10/829.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i10.829
Poor nutritional status has a high prevalence in patients with advanced liver disease and has significant influence on prognosis[1]. At the initial development of the Child-Turcotte score, the nutritional status was included in the evaluation of patients submitted to porto-caval shunting surgery and the score had significant prognostic relevance[2]. Currently, the Child-Pugh-Turcotte score is still one of the most utilized systems to stage cirrhosis and to assess the prognosis of these patients. Malnutrition has proved to be independently correlated with survival and the addition of mid-arm muscle circumference (MAMC) and triceps skinfold thickness (TST) to the Child-Pugh score increases the prognostic accuracy[3].
The mechanism of malnutrition in cirrhosis is multifactorial, including lower nutrient intake (loss of appetite, impaired digestion/absorption or low protein diet), hypercatabolic status and decreased liver protein synthesis[4]. However, data regarding the evaluation of lifestyle and nutritional intake in patients with advanced liver disease and their adherence to nutritional recommendations is limited.
Even if malnutrition is more prevalent in advanced stages of cirrhosis, the assessment of nutritional status in these patients may be difficult mainly because of sodium-water retention. Therefore, tracking weight change or body mass index most likely does not accurately reflect the nutritional status of a patient. The latest available guidelines at the time of data collection were the 2006 ESPEN (European Society for Parenteral and Enteral Nutrition) guidelines, which recommended the use of Subjective Global Assessment (SGA) or anthropometry to identify those at risk for malnutrition in patients with cirrhosis[5]. These recommendations were reinforced in the 2019 revised edition of the guidelines[6]. As the anthropometric parameters should not be influenced by ascites or peripheral oedema and, MAMC or mid-arm circumference (MAC) and TST can be confidently used. However, the applicability of these parameters in decompensated cirrhotic patients has not been extensively validated. In order to avoid malnutrition and its negative consequences for patients with cirrhosis, the aforementioned guidelines recommend a daily energy intake of 30–35 kcal/kgBW/d (125–146 kJ/kgBW/d) and a protein intake of 1.2–1.5 g/kgBW/d[6]. However, the adherence to these recommend-ations is not known.
The aims of this study were: (1) To assess the adherence of patients with advanced cirrhosis to nutritional recommendations; (2) To evaluate the prevalence of malnutrition in patients with cirrhosis stratified according to their clinical stage (compensated or decompensated); and (3) To assess the influence of malnutrition and adherence to nutritional recommendation on survival.
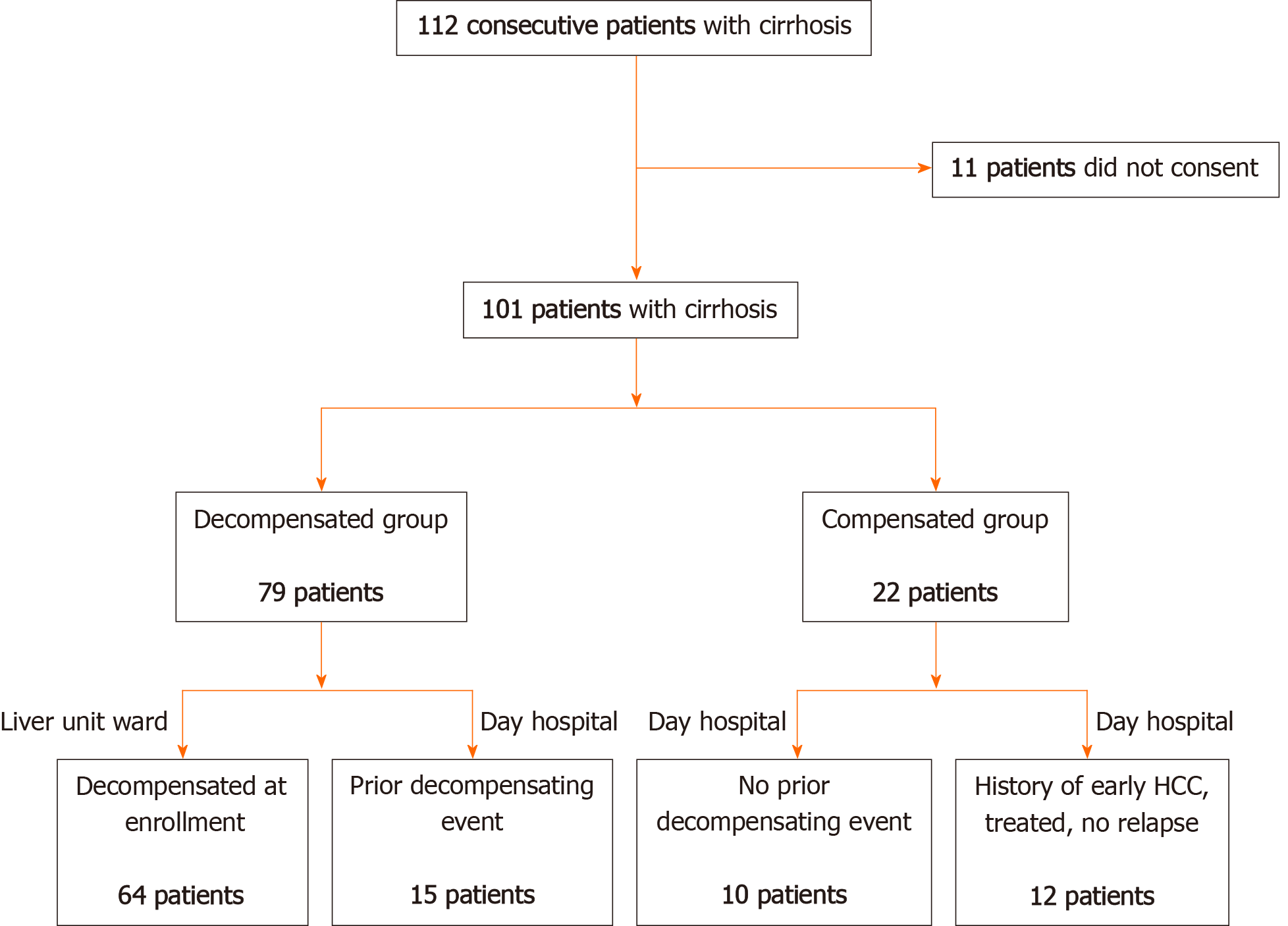
This is a prospective observational study that included 101 consecutive patients with cirrhosis of any aetiology hospitalized in our tertiary Health Care Centre. Sixty-four patients who were recruited while being hospitalized for clinical decompensation in the inpatient ward between the 1st of March and the 30th of June 2013 were considered for inclusion. Patients were included in the decompensated cirrhosis group based on the presence of ascites (and related complications: Spontaneous bacterial peritonitis, hepatic hydrothorax), portal hypertension related bleeding (variceal bleeding), diagnosis of acute on-chronic liver failure, overt hepatic encephalopathy (grade 2 to 4 in West Haven scale) or other specified events (kidney dysfunction, hepato-renal syndrome, bacterial infections and cardiopulmonary complications of portal hypertension). All consecutive patients presented to our centre during the study period were considered primarily eligible for inclusion. Eleven patients did not consent and were not included. Furthermore, a cohort of 37 consecutive patients with liver cirrhosis, completing the regular follow-up in June 2013 in the outpatient ward, was included. Among them, 15 (40.5%) had previous decompensation and were included in the decompensated group and 12 patients (32.4%) had early stage of hepatocellular carcinoma (BCLC-0 and A) previously treated by percutaneous radiofrequency ablation and, at the moment of inclusion, were in the follow-up stages. There was no significant difference between patients with or without HCC in the control group and none of the patients with HCC had previous decompensation events. All patients that agreed to participate signed an informed consent and the Ethical Committee of our University approved the protocol. The present study was designed with respect to the ethical guidelines issued by the 2000 revision (Edinburgh) of the 1975 Declaration of Helsinki. The flowchart of patient enrolment is illustrated in Figure 1.
The diagnosis of cirrhosis was based on specific findings in the patient’s medical history, clinical examination, laboratory findings and imaging examinations. Physical examination including anthropometric measurements, blood tests, abdominal ultrasonography and a complete nutritional evaluation were done at the inclusion of all patients.
All included patients together with the closest family members were questioned about their detailed alimentary intake in the last 2 wk and were asked to approximate the food habits in the last 3 mo. For this purpose, the National Health and Nutrition Examination Survey (NHANES) Food Questionnaire (https://wwwn.cdc.gov/nchs/nhanes/) was modified and adapted to the general food habits for our country (we eliminated questions referring to some foods that are rarely consumed in our country). Apart from the reduction of the number of questions we also reduced the period of assessment to 3 mo and, thus, some questions regarding seasonal and out-seasonal consumption of some seasonal foods were either merged or eliminated. Finally, the questionnaire comprised 80 questions and was based on the approximation of the quantity of main foods in the last 3 mo. The mean time for the application of the questionnaire was 30-40 min. The list of principal foods and the calculation of the energy and principal nutrients (proteins, lipids, carbohydrates and salt) intake was made using the “Healthy alimentation guidelines” published by the National Ministry of Health on their official website. These guidelines include lists of principal foods and their main nutrients content expressed by 100g. Therefore, for each patient the food intake was calculated and expressed as: kcal/kg body weight/d; protein g/kgBW/d, lipid g/kgBW/d, carbohydrates g/kgBW/d intake and salt g/kgBW/d intake.
The food intake was compared with the 2019 ESPEN (European Society for Parenteral and Enteral Nutrition) recommendations for patients with liver cirrhosis (30–35 kcal/kgBW/d energy daily intake and a protein intake of 1.2–1.5 g/kgBW/d)[6]. Patients were considered adherent if their caloric or protein intake was within the proposed recommendations.
The nutritional status evaluation was based on SGA assessment and MAC. Because of the lack of a specific measuring tool, TST was not possible and, therefore, MAMC was also unavailable.
SGA was assessed according to the Detsky et al[7] protocol, which included the history of the weight curve in the last 6 mo and during the last 2 wk, dietary intake, gastro-intestinal symptoms, functional capacity and clinical examination, including the evaluation of subcutaneous fat, muscle wasting, oedema and ascites. In this particular clinical scenario, the presence of ascites and oedema was considered as a sign of decompensation rather than a sign of malnutrition. According to the SGA evaluation the patients were classified as: Well-nourished SGA-A, mild to moderate malnutrition SGA-B and severe malnutrition SGA-C. In decompensated patients the SGA ranking was completed by consensus between the nutritionist and the hepatologist using adapted recommendations[8].
MAC was measured in the non-dominant arm at the midway between the acromion and olecranon process. Malnutrition was defined as a MAC < 10th percentile and severe malnutrition if MAC < 5th percentile using as a reference values adapted for age and gender from the Bishop’s study[9].
Because not all the cohort was prospectively followed-up in our centre after discharge, data regarding survival at 1st August 2017 and date of death of the included population were obtained from the National Population Register.
The study complies with the STROBE guidelines.
Data was expressed as mean ± SD or median and range and qualitative variables as frequencies. The t-student or Mann-Whitney tests were used for quantitative variables for the comparison between groups and the Chi-square and Fisher’s exact tests were used for qualitative data where appropriate. For multivariate analysis, a binary logistic regression using the backward LR model was used in order to determine the parameters associated with decompensation and malnutrition. Concordance between SGA and MAC regarding malnutrition diagnosis was evaluated by the weighted (two categories) kappa method. Concordance coefficient (k) was graded by the scale proposed by Landis and Koch[10]: 0%-10% = poor, 10%-20% = slight, 21%-40% = fair, 41%-60% = moderate, 61%–80% = substantial and 81%-100% = almost perfect.
For survival analysis, the cox regression was used to identify independent risk predictors (OR 95%CI) for death. Only variables significantly associated with the judgment criteria in the univariate analysis were considered for the multivariate analysis. For multivariate analysis, a cox regression using the backward LR model was used in order to determine the parameters associated with death. Actuarial rates of survival were calculated using the Kaplan–Meier plots and compared by the Log rank test. P < 0.05 was considered as the level of significance. The statistical review of the study was performed by a biomedical statistician. Statistical analysis was performed using the SPSS software version 20 (SPSS Inc. Chicago, IL, United States).
Among the 79 patients included in the decompensated group, 42 (53.2%) were male and the mean age was 61.3 ± 10. There were no differences between groups regarding age and gender. The most prevalent aetiology in the decompensated group was alcohol-related liver disease – 32 patients (51.9%), while in the compensated group viral cirrhosis was predominant – 16 patients (72.7%). The differences between the two groups were statistically significant (P = 0.005). Fifty patients (63.2%) from the decompensated group were on beta-blocker treatment for primary or secondary prophylaxis of variceal bleeding. The full baseline characteristics of the included population are listed in Table 1.
| Variablen (%) or mean ± SD | Decompensated, n = 79 | Compensated, n = 22 | P value |
| Gender M/F | 42 (53.2)/ 37 (46.8) | 11 (50)/ 11 (50) | NS |
| Age | 61.3 ± 10 | 64.5 ± 10.9 | NS |
| Child-Pugh A/B/C | 8(10.1%)/32(40.5%)/39(49.4%) | 19(86.4%)/3(13.6%)/0(0%) | < 0.001 |
| Aetiology | 0.005 | ||
| Viral | 30 (38.0) | 16 (72.7) | |
| Alcohol | 32 (51.9) | 3 (13.6) | |
| Other | 5 (10.1) | 3 (13.6) | |
| Platelet count (109/L) | 122.9 ± 86.9 | 123.1 ± 60.9 | NS |
| INR | 1.82 ± 0.53 | 1.27 ± 0.25 | < 0.001 |
| Total bilirubin (mg/dL) | 5.4 ± 6.9 | 1.2 ± 0.7 | 0.006 |
| Albumin (g/L) | 3.1 ± 0.6 | 3.9 ± 0.7 | < 0.001 |
| AST (U/L) | 83.3 ± 67.4 | 56.6 ± 29.5 | 0.08 |
| ALT (U/L) | 42.3 ± 42.7 | 46.6 ± 31.2 | NS |
| Haemoglobin (g/dL) | 10.9 ± 3.9 | 12.7 ± 2.2 | 0.05 |
| Na (mEq/L) | 134.5 ± 7.0 | 139.9 ± 3.3 | < 0.001 |
| Creatinine (mg/dL) | 1.19 ± 0.88 | 0.77 ± 0.19 | < 0.001 |
| Cholesterol (mg/dL) | 120.5 ± 51.4 | 154.7 ± 39.1 | 0.01 |
| Triglycerides (mg/dL) | 80.9 ± 42.6 | 78.1 ± 22.5 | NS |
| Child Pugh score | 9.6 ± 2.6 | 5.8 ± 1.2 | < 0.001 |
| MELD score | 19.2 ± 7.6 | 11.2 ± 5.6 | < 0.001 |
Ascites was the most frequent decompensation event - 59 (74.6%) patients, followed by hepatic encephalopathy (n = 29; 36.7% of patients), and variceal bleeding (n = 20; 25.3%).
Overall, the adherence to the current nutritional guidelines for cirrhosis was extremely low. Only 21 (20.8%) patients had the recommended alimentary intake of 30-35 kcal/kgBW/d and 57 (56.4%) patients had a suboptimal energy intake (<30 kcal/kgBW/d). Regarding the proportion of protein intake, only 26 (25.7%) patients had the recommended amount of protein (1.2-1.5 g/kgBW/d), while the rest had either superior (40%) or lower (34.3%) protein intake. When we analysed the patients from the compensated group, we observed that 11 out of 22 patients (50%) had <30 kcal/kgBW/d energy intake.
Because the majority of the patients had ascites as the main decompensation event, we also evaluated the salt intake and compared it with the actual recommendation[11] of 4.6-6.9 g/d salt intake in patients with ascites, namely a “no added salt diet”. Among all patients, only 26 (25.7%) had a salt intake < 6.9 g/d and 7 (6.9%) < 4.6 g/d, which demonstrated a very poor adherence to the “no added salt diet”. In the decompensated group, only 20 (25.3%) patients had a salt intake < 6.9 g/d.
When comparing patients adherent or not to the no added salt diet (< 6.9 g/d) regarding the food intake, those adherent to the diet had significantly lower protein, lipid, carbohydrates and caloric intake (Supplementary Table 1).
When comparing decompensated to the compensated group, there was no difference regarding the main nutrients intake (Table 2).
| Variable | Decompensated, n = 79 | Compensated, n = 22 | P value |
| MAC (cm) | 25.8 ± 3.9 | 27.9 ± 2.9 | 0.02 |
| MAC < 10th | 48 (60.8) | 6 (27.3) | 0.005 |
| MAC < 5th | 30 (38.0) | 4 (18.2) | 0.08 |
| SGA | < 0.001 | ||
| A (normal) | 25 (31.6) | 19 (86.4) | |
| B (mild-moderate) | 35 (44.3) | 3 (13.6) | |
| C (severe) | 19 (24.1) | 0 (0) | |
| BMI | 26.6 ± 4.7 | 27.1 ± 3.3 | NS |
| Kilocalories/d | 2080 ± 419 | 2200 ± 601 | NS |
| Kcal/kg/d | 29 ± 8 | 30.1 ± 9.2 | NS |
| Proteins (g/kg/d) | 1.4 ± 0.4 | 1.4 ± 0.5 | NS |
| Proteins 1.2-1.5 g/kg/d | 20 (25.3) | 6 (27.3) | NS |
| Proteins < 1.2 g/kg/d | 27 (34.2) | 6 (27.3) | NS |
| Salt (mmol/d)1 | 147 ± 4.2 | 147 ± 4.8 | NS |
| < 120mmol/d | 20 (25.3) | 6 (27.3) | NS |
According to the SGA classification, in the decompensated group, 54 (68.4%) patients had malnutrition and, among them, 19 (24.1%) had severe malnutrition (SGC-C). In the compensated group, only 3 (13.6%) were malnourished and no patient had severe malnutrition. In comparison, six (40%) out of 15 patients with prior history of decompensation admitted to or day hospital had malnutrition according to the SGA criteria (P = 0.06). Using the MAC criteria, in the decompensated group, 48 (60.8%) had malnutrition, whereas in the compensated group, 6 patients (27.3%) were malnourished. Details about nutritional markers are found in Table 2. Regarding the malnutrition diagnosis, there was only a slight-fair agreement between SGA and MAC (k = 0.261, P = 0.009).
As expected, comparing decompensated and compensated groups in univariate analysis (Table 1), variables related to liver function [Child-Pugh and model for end-stage liver disease (MELD) scores, international normalized ratio (INR), albumin, serum bilirubin, serum sodium] and variables related to the nutritional status (MAC and SGA score, cholesterol level) were associated with current or prior history of liver disease decompensation. These findings were further confirmed in multivariate analysis, where decompensation was independently associated with the MELD score (HR 1.43; 95%CI: 1.16-1.76) and the malnutrition diagnosis based on SGA (HR 7.15; 95%CI: 1.63-31.29).
In the univariate analysis, the variables associated with malnutrition were: Child-Pugh score, MELD score, haemoglobin levels, INR, albumin, total protein levels, cholesterol levels, sodium level and gender.
In the multivariate analysis, the variables independently associated with malnutrition were: Lower cholesterol levels (HR 0.97, 95%CI: 0.95-0.98, P = 0.001), lower sodium (HR = 0.71, 95%CI: 0.67-0.93, P = 0.005) and male sex (HR = 0.24, 95%CI: 0.05-1.02, P = 0.054).
Neither patients with or without malnutrition demonstrated a better adherence to an energy intake recommendation: 10 (17.5%) in malnourished patients and 11 (25%) well-nourished patients. However, the percentage of patients adherent to the recommended protein intake (1.2-1.5 g/kgBW/d) tended to be lower in malnourished patients, 11 (19%) malnourished patients vs 15 (34%) patients with normal nutritional values (P = 0.09).
Seventy-one (70%) patients died after the median time of 27 mo (0-53). Five patients (7.8%) died within one month after the inclusion. Among them, 41 (57.7%) had < 30 kcal/kgBW/d and 25 (35%) had < 1.2 g/kgBW/d protein intake.
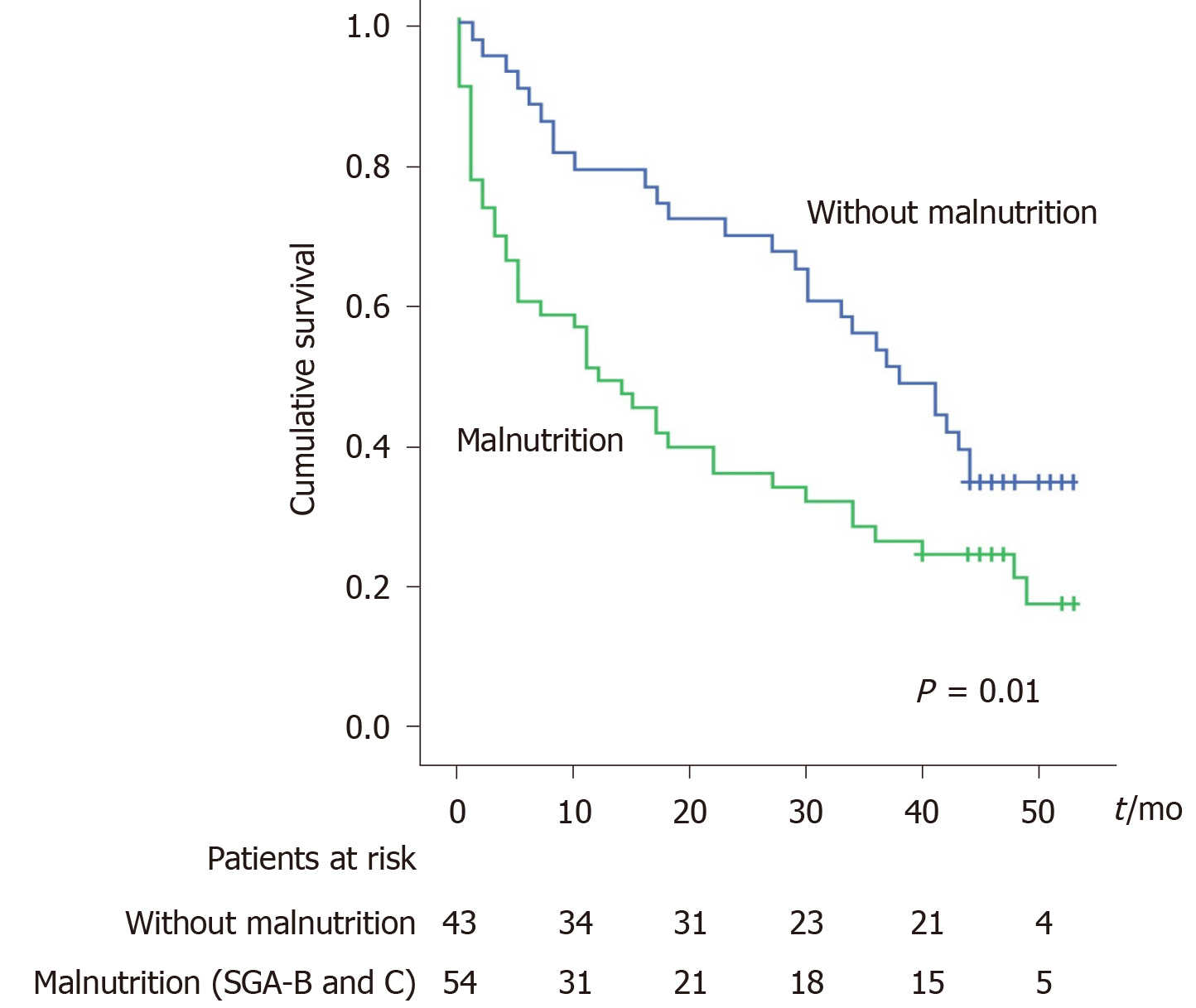
The survival is lower in patients with malnutrition when the SGA definition is used: 50% at 1 year and 63% at 2 years of patient with malnutrition died vs 21% at 1 year and 30% at 2 years patients without malnutrition (P = 0.01, Figure 2). However, using the MAC < 10th criteria for malnutrition definition, there is no difference in survival (
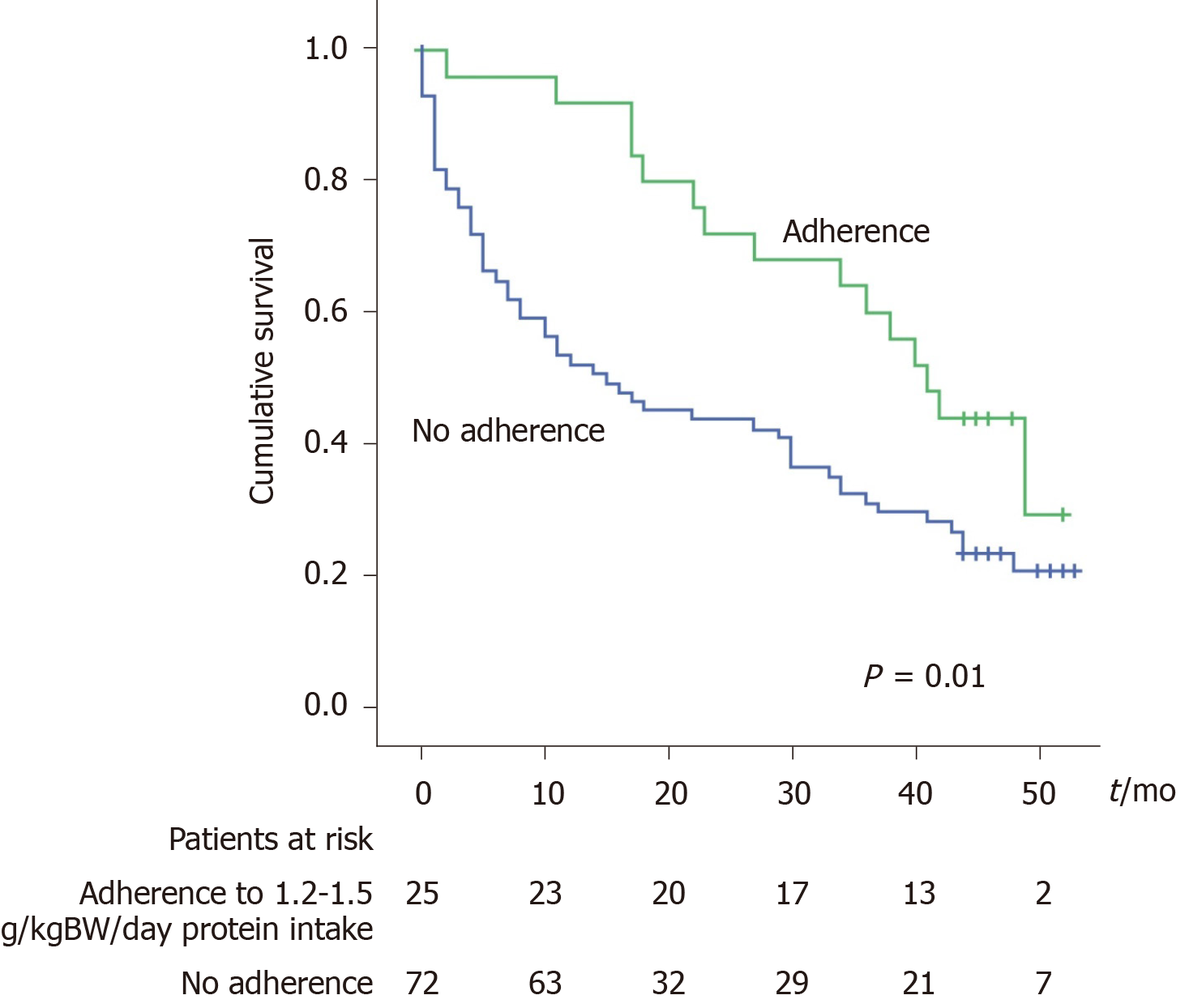
According to the multivariate analysis (Table 3), the factors independently associated with death are age, creatinine level and the adherence to the protein intake recommendation (as a protective factor). When the adherence to this recommendation was analysed with the Kaplan-Meier curves the results showed that adherent patients have a significantly better survival. The mortality rate was 8% at 1 year and 28% at 2 years in the adherent group vs 47% at 1 year and 56% at 2 years in the non-adherent group (P = 0.01, Figure 3).
| Variable | Univariate analysis; OR (95%CI) | P value | Multivariate analysis1; OR (95%CI) | P value |
| Age | 1.03 (1.01-1.06) | 0.004 | 1.03 (1.00-1.06) | 0.002 |
| Child-Pugh score | 1.13 (1.04-1.23) | 0.002 | 1.09 (0.98-1.20) | 0.09 |
| MELD score | 1.06 (1.03-1.09) | < 0.001 | ||
| Presence if malnutrition2 | 1.82 (1.12-2.94) | 0.01 | ||
| Creatinine (mg/dL) | 1.94 (1.46-2.57) | < 0.001 | 2.13 (1.31-3.45) | 0.03 |
| INR | 1.60 (1.05-2.43) | 0.02 | ||
| Albumin (g/L) | 0.45 (0.29-0.68) | 0.01 | ||
| Cholesterol (mg/dL) | 0.99 (0.98-0.99) | 0.02 | ||
| Serum sodium (mEq/L) | 0.94 (0.90-0.98) | 0.003 | ||
| Protein intake 1.2-1.5 g/kg/d3 | 0.51 (0.28-0.90) | 0.02 | 0.40 (0.20-0.77) | 0.007 |
The presence of malnutrition is very prevalent in patients with cirrhosis and very relevant to the prognosis of these patients before and after liver transplantation[12,13]. In the present study, we confirmed that malnutrition has a high prevalence among patients with advanced cirrhosis, especially in the decompensated group, and is independently associated with decompensation. The low adherence to the protein intake recommendations is independently associated with a lower survival. A possible explanation for high prevalence of malnutrition is the bad adherence to nutritional recommendation. Indeed, we found that 56.4% of the patients had a suboptimal energy intake (< 30 kcal/kgBW/d) and 33% had a suboptimal protein intake (< 1.2 g/kgBW/d), even in the fully compensated phase of the disease. Low caloric intake in patients with advanced cirrhosis was confirmed also by other reports, which found that 80% of patients Child-Pugh C and 52% in Child-Pugh B had < 30 kcal/kgBW/d intake[14]. Interestingly, in fully compensated patients, even if the prevalence of malnutrition is much lower than re-compensated and decompensated patients, 50% had a lower energy intake than recommended. Therefore, probably one of the main mechanisms of malnutrition in cirrhosis is a lower nutrient’s intake. More efforts should be made to identify patients with inappropriate adherence to nutritional recommendation and to correct the nutrition earlier, before malnutrition and decompensation occur. Among other factors that may contribute to malnutrition, the decrease in liver function probably has an important role since malnutrition is associated with higher MELD scores, bilirubin and INR and lower albumin levels.
One of the major concerns is the low adherence to the no added salt diet in our advanced cirrhosis subgroup (92% of the patients with decompensation had ascites). The first step in the treatment of sodium retention in cirrhosis is salt restriction to a level between 4.6 and 6.9 g/d[11]. Of all patients, only 25% had a salt intake < 6.9 g/d. Recently, other group confirmed these findings in an outpatient cohort with ascites, closely followed within a “Care Management Program” aiming to better manage patients with cirrhosis[15]. In their cohort, only 30% of the patients followed a low salt diet, in spite of almost half of the patients believing that they were adherent to salt consumption recommendations. Moreover, the adherent patients had a lower caloric intake. In our cohort, we also found that patients with no added salt diet had significantly lower energetic (calories/kg/d), protein, lipid and carbohydrates intake. All these data sustain the hypothesis that the no added salt diet may contribute to the loss of appetite and a lower food intake, consequently further deteriorating the nutritional status.
One of the most difficult issues is to establish which is the most appropriate method to diagnose malnutrition in cirrhosis. At the time of data collection, ESPEN recommended the use of SGA as well as anthropometric measurements for the diagnosis of malnutrition in patients with cirrhosis[5]. However, there was no distinction between different stages of cirrhosis (compensated vs decompensated) for these recommendations and none of the methods was considered as standard. In our cohort, the concordance between SGA and MAC was only slightly significant. The best concordance was in the compensated group. In the decompensated group, using SGA criteria, 75% of the patients had malnutrition whereas using MAC < 10th only 64% were malnourished. In the compensated group, when using SGA, 24% patients had malnutrition whereas for MAC < 10th, 35% of the patients were malnourished. In light of these results, we may conclude that SGA and MAC do not have the same applicability in different stages of cirrhosis. However, in the absence of a standard method for malnutrition diagnosis, it is difficult to conclude which of the methods has the best performance. In a study of 50 compensated cirrhotic patients, the handgrip test, another validated method to assess nutritional status based on muscular strength, had a superior predictive performance compared to SGA with regards decompensation within one year[16]. In this cohort, 28% patients had malnutrition according to SGA and 63% according to the handgrip test. However, the rate of decompensation at one year is very high (42%) and the authors do not report sufficient details regarding the variables related to liver function. In another study, using MAMC < 5th the prevalence of malnutrition was 45% in Child-Pugh C patients and 25% in Child-Pugh A[14]. All these divergent findings suggest that probably none of these methods is very well adapted to cirrhotic patients. By including weight curve, dietary intake and presence of ascites and oedema among the diagnostic criteria, SGA overestimates the prevalence of malnutrition in the decompensated group since all these variables are influenced by the liver function. At the same time, for some anthropometric parameters that are not influenced so much by sodium- water retention, the standard population for generating the percentiles and analysing the results was a historical American cohort of healthy subjects[9]. Probably, the best way to assess the nutritional status in cirrhosis is to apply different methods and look for concordance and interpret according to the clinical stage of the disease.
There was an independent association between adherence to protein intake recommendation (1.2-1.5 g/kg/d) and a better survival. Although it was historically thought that patients with advanced cirrhosis should follow a protein-restricted diet due to the risk of hepatic encephalopathy, it has long-been proven that the amount of protein intake does not influence the course of hepatic encephalopathy[17]. In our study we found that 74% of the patients had a lower protein intake than ESPEN’s recommendations. These findings are reproducing the results of a large Canadian cohort of patients on liver transplantation list where only 24% had appropriate protein intake[18]. The group also found that a lower protein intake is associated with transplant waiting list mortality. Low protein intake could be the cause of muscular depletion that was also associated with high mortality[19]. Although survival was lower in patients with malnutrition (based on SGA criteria, Figure 1), in multivariate analysis the presence of malnutrition was not independently associated with survival, losing its significance in the face of variables related to liver and kidney functions. Probably, the small sample size precludes obtaining a strong conclusion regarding the relation between the presence of malnutrition and survival. Moreover, in advanced stages of disease the presence of complications related to portal hypertension or liver failure weights more in the prognosis of these patients.
There are some limitations in our study. Undoubtedly, we are aware of the low number of patients, which did not allow a more detailed subgroup analysis (in different decompensation scenarios or aetiologies) as well as it hindered the discriminant significance of our multivariate analysis. Secondly, we are well aware of the possible errors in the patient’s reporting of food intake during the application of our questionnaire. Moreover, in our study we did not assess the impact of previously dietary counselling and its efficiency. We tried to overcome this form of subjectivity in assessment by also interviewing family members and by using a food questionnaire adapted to the alimentary habits of our country. Third, the food questionnaire adapted from NHANES Survey Food Questionnaire had no previous validation. Nevertheless, our questionnaire maintains the structure of the NHANES Survey Food Questionnaire, which is a well validated instrument. Fourth, in the analysis of the adherence of the low salt diet we did not use the 24 h natriuresis for the objective assessment of adherence and. However, given a cautious approach with emphasis on the core trend, rather than an adamant focus on specifics, the key findings should withstand scrutiny.
Correlations with other validated tools could further expand the knowledge on the topic. Amino acid intake profile, as well as branched-chained amino acids blood-levels, body composition assessment and imaging studies for the evaluation of sarcopenia were not analysed. Metrics developed and thoroughly validated after the design of our study, such as skeletal muscle area, skeletal muscle index or muscle radiation attenuation[20] could certainly add valuable insights. Furthermore, validation of our results on other cohorts and in comparison to the aforementioned tools could add strength to our findings.
In conclusion, given the main findings of this study, namely that prevalence of malnutrition is high in patients with cirrhosis and that adherence to the nutritional recommendations in this population is very low, intensive efforts should be initiated to identify patients at risk for malnutrition (dietary assessment) and to correct their dietary habits as early as possible. Appropriate protein intake should be strongly recommended because this may improve survival, with a low risk to benefit ratio.
While often understated when compared to other complications of chronic liver disease, malnutrition appears to be a silent but key contributor to survival and quality of life in patients with cirrhosis. Although the field is currently gathering momentum, the available data are still scarce and there is a dire need for standardized evaluation and therapeutic approach.
The focus of our research was to assess the real life impact of malnutrition on survival in a group of cirrhotic patients and to observe whether adherence to current nutritional recommendations alters their outcome.
The aims of the current research were to determine the prevalence of malnutrition in a consecutive series of cirrhotic patients and to determine its impact on survival. Furthermore, we wanted to evaluate whether adherence to current nutritional recommendations improves their outcome. By answering these clinical questions, we tried to set a working baseline, hoping to provide a solid starting point for future research in the field.
Malnutrition was assessed using the Subjective Global Assessment criteria and the mid-arm circumference. These are easy-to-use, cost efficient, bedside methods with extensive prior validation and standardization. Furthermore, dietary habits were evaluated using a comprehensive food intake questionnaire adapted to the specifics of our culture. Total energy and main nutrient intake were calculated based on their response. Patients were followed-up for a median of 27 mo and factors associated with their prognosis were accounted for in uni- and multivariate analysis.
Malnutrition was highly prevalent in patients with cirrhosis and a current or prior decompensating event (68.4%). In comparison, only 13.6% of patients with no history of decompensation were malnourished (P < 0.001). While the overall mortality in our whole group was 70% after a median follow-up of 27 mo, patients with malnutrition had a significantly worse outcome: 50% mortality at 1 year and 63% at 2 years for the patients with malnutrition, compared to 21% at 1 year and 30% at 2 years for patients without malnutrition (P = 0.01). On multivariate analysis, adherence to nutritional recommendations was associated with a better prognosis.
Our results reflect the important burden of malnutrition in patients with advanced liver disease, especially in the setting of a decompensating event. Consequently, a more attentive approach to nutrition should complement pharmacologic and interventional therapy in patients with cirrhosis, as it appears to have a significant impact on survival.
Further research should try to translate more basic research findings into clinical practice, while clinical studies should try to provide solid grounding for guideline recommendations. In this light, there is a dire need for large scale high-quality, multicentric studies on easy-to-use, non-invasive and cost-efficient methods to screen for and grade malnutrition. Not least, dietary habits of patients with advanced liver disease should be thoroughly examined, in order to provide realistic, easy to follow nutritional recommendation in order to increase adherence.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Romania
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Moriya K, Wang K S-Editor: Zhang H L-Editor: A P-Editor: Li JH
| 1. | Kondrup J. Nutrition in end stage liver disease. Best Pract Res Clin Gastroenterol. 2006;20:547-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Child C, Turcotte J. Surgery and portal hypertension. In: Child C, editor. The liver and portal hypertension. Philadelphia: W. B. Saunders Co.; 1964: 50. |
| 3. | Alberino F, Gatta A, Amodio P, Merkel C, Di Pascoli L, Boffo G, Caregaro L. Nutrition and survival in patients with liver cirrhosis. Nutrition. 2001;17:445-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 340] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 4. | Tsiaousi ET, Hatzitolios AI, Trygonis SK, Savopoulos CG. Malnutrition in end stage liver disease: recommendations and nutritional support. J Gastroenterol Hepatol. 2008;23:527-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 5. | Plauth M, Cabré E, Riggio O, Assis-Camilo M, Pirlich M, Kondrup J; DGEM (German Society for Nutritional Medicine); Ferenci P; Holm E; Vom Dahl S; Müller MJ; Nolte W; ESPEN (European Society for Parenteral and Enteral Nutrition). ESPEN Guidelines on Enteral Nutrition: Liver disease. Clin Nutr. 2006;25:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 405] [Article Influence: 21.3] [Reference Citation Analysis (1)] |
| 6. | Plauth M, Bernal W, Dasarathy S, Merli M, Plank LD, Schütz T, Bischoff SC. ESPEN guideline on clinical nutrition in liver disease. Clin Nutr. 2019;38:485-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 403] [Article Influence: 67.2] [Reference Citation Analysis (3)] |
| 7. | Detsky AS, McLaughlin JR, Baker JP, Johnston N, Whittaker S, Mendelson RA, Jeejeebhoy KN. What is subjective global assessment of nutritional status? JPEN J Parenter Enteral Nutr. 1987;11:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1939] [Cited by in RCA: 1955] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 8. | Pikul J, Sharpe MD, Lowndes R, Ghent CN. Degree of preoperative malnutrition is predictive of postoperative morbidity and mortality in liver transplant recipients. Transplantation. 1994;57:469-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 164] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 9. | Bishop CW, Bowen PE, Ritchey SJ. Norms for nutritional assessment of American adults by upper arm anthropometry. Am J Clin Nutr. 1981;34:2530-2539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 220] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159-174. [PubMed] |
| 11. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1777] [Cited by in RCA: 1818] [Article Influence: 259.7] [Reference Citation Analysis (2)] |
| 12. | Gunsar F, Raimondo ML, Jones S, Terreni N, Wong C, Patch D, Sabin C, Burroughs AK. Nutritional status and prognosis in cirrhotic patients. Aliment Pharmacol Ther. 2006;24:563-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 126] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 13. | Merli M, Giusto M, Gentili F, Novelli G, Ferretti G, Riggio O, Corradini SG, Siciliano M, Farcomeni A, Attili AF, Berloco P, Rossi M. Nutritional status: its influence on the outcome of patients undergoing liver transplantation. Liver Int. 2010;30:208-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 204] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 14. | Campillo B, Richardet JP, Scherman E, Bories PN. Evaluation of nutritional practice in hospitalized cirrhotic patients: results of a prospective study. Nutrition. 2003;19:515-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 104] [Article Influence: 4.7] [Reference Citation Analysis (2)] |
| 15. | Morando F, Rosi S, Gola E, Nardi M, Piano S, Fasolato S, Stanco M, Cavallin M, Romano A, Sticca A, Caregaro L, Gatta A, Angeli P. Adherence to a moderate sodium restriction diet in outpatients with cirrhosis and ascites: a real-life cross-sectional study. Liver Int. 2015;35:1508-1515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 16. | Alvares-da-Silva MR, Reverbel da Silveira T. Comparison between handgrip strength, subjective global assessment, and prognostic nutritional index in assessing malnutrition and predicting clinical outcome in cirrhotic outpatients. Nutrition. 2005;21:113-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 317] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 17. | Córdoba J, López-Hellín J, Planas M, Sabín P, Sanpedro F, Castro F, Esteban R, Guardia J. Normal protein diet for episodic hepatic encephalopathy: results of a randomized study. J Hepatol. 2004;41:38-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 260] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 18. | Ney M, Abraldes JG, Ma M, Belland D, Harvey A, Robbins S, Den Heyer V, Tandon P. Insufficient Protein Intake Is Associated With Increased Mortality in 630 Patients With Cirrhosis Awaiting Liver Transplantation. Nutr Clin Pract. 2015;30:530-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Tandon P, Ney M, Irwin I, Ma MM, Gramlich L, Bain VG, Esfandiari N, Baracos V, Montano-Loza AJ, Myers RP. Severe muscle depletion in patients on the liver transplant wait list: its prevalence and independent prognostic value. Liver Transpl. 2012;18:1209-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 435] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 20. | van der Werf A, Langius JAE, de van der Schueren MAE, Nurmohamed SA, van der Pant KAMI, Blauwhoff-Buskermolen S, Wierdsma NJ. Percentiles for skeletal muscle index, area and radiation attenuation based on computed tomography imaging in a healthy Caucasian population. Eur J Clin Nutr. 2018;72:288-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 208] [Article Influence: 26.0] [Reference Citation Analysis (0)] |