Published online Jan 27, 2020. doi: 10.4254/wjh.v12.i1.21
Peer-review started: July 16, 2019
First decision: August 7, 2019
Revised: October 2, 2019
Accepted: December 6, 2019
Article in press: December 6, 2019
Published online: January 27, 2020
Processing time: 181 Days and 8.8 Hours
Drug-eluting bead transarterial chemoembolization (DEB-TACE) is an endovascular treatment to release chemotherapeutic agents within a target lesion, minimizing systemic exposure and adverse effects to chemotherapeutics. Therefore, identifying which patient characteristics may predict imaging response to DEB-TACE can improve treatment results while selecting the best candidates. Predictors of the response after DEB-TACE still have not been fully elucidated. This is the first prospective study performed with standardized DEB-TACE technique that aim to identify predictors of radiological response, assessing patients clinical and laboratory characteristics, diagnostic imaging and intraprocedure data of the hepatocellular carcinoma treated in the neoadjuvant context for liver transplantation.
To identify pre- and intraoperative clinical and imaging predictors of the radiological response of drug-eluting bead transarterial chemoembolization (DEB-TACE) for the neoadjuvant treatment of hepatocellular carcinoma (HCC).
This is prospective, cohort study, performed in a single transplant center, from 2011 to 2014. Consecutive patients with HCC considered for liver transplant who underwent DEB-TACE in the first session for downstaging or bridging purposes were recruited. Pre and post-chemoembolization imaging studies were performed by computed tomography or magnetic resonance. The radiological response of each individual HCC was evaluated by objective response using mRECIST and the percentage of necrosis.
Two hundred patients with 380 HCCs were examined. Analysis of the objective response (nodule-based analysis) demonstrated that HCC with pseudocapsules had a 2.01 times greater chance of being responders than those without pseudocapsules (P = 0.01), and the addition of every 1mg of chemoembolic agent increased the chance of therapeutic response in 4% (P < 0.001). Analysis of the percentage of necrosis through multiple linear regression revealed that the addition of each 1mg of the chemoembolic agent caused an average increase of 0.65% (P < 0.001) in necrosis in the treated lesion, whereas the hepatocellular carcinoma with pseudocapsules presented 18.27% (P < 0.001) increased necrosis compared to those without pseudocapsules.
The presence of a pseudocapsule and the addition of the amount of chemoembolic agent increases the chance of an objective response in hepatocellular carcinoma and increases the percentage of tumor necrosis following drug-eluting bead chemoembolization in the neoadjuvant treatment, prior to liver transplantation.
Core tip: This is the first prospective study performed with standardized drug-eluting bead transarterial chemoembolization technique that aim to identify predictors of radiological response, assessing patients clinical and laboratory characteristics, diagnostic imaging and intraprocedure data of the hepatocellular carcinoma treated in the neoadjuvant context for liver transplantation. Two hundred patients with 380 hepatocellular carcinomas were examined and we could conclude that the presence of a pseudocapsule and the addition of the amount of chemoembolic agent increases the chance of an objective response and increases the percentage of tumor necrosis in hepatocellular carcinoma following drug-eluting bead chemoembolization in the neoadjuvant treatment, prior to liver transplantation.
- Citation: Galastri FL, Nasser F, Affonso BB, Valle LGM, Odísio BC, Motta-Leal Filho JM, Salvalaggio PR, Garcia RG, de Almeida MD, Baroni RH, Wolosker N. Imaging response predictors following drug eluting beads chemoembolization in the neoadjuvant liver transplant treatment of hepatocellular carcinoma. World J Hepatol 2020; 12(1): 21-33
- URL: https://www.wjgnet.com/1948-5182/full/v12/i1/21.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i1.21
Hepatocellular carcinoma (HCC) is the most common primary cancer of the liver, the seventh most frequent malignant neoplasm and it is the third leading cause of cancer-related death in the world[1-3]. Liver transplantation remains the best treatment option for patients whose HCC falls within the Milan criteria[4,5]. Nevertheless, insufficient organ donation demands priority criteria for transplantation in many countries[5]. To avoid HCC progression while on the waiting list, patients can receive neoadjuvant treatment if they are within the criteria “bridging therapy” or not “downstaging therapy”, and remain eligible for transplantation[6,7]. In many centers, transarterial chemoembolization (TACE) is the treatment of choice for that purpose[8].
Although TACE has been proven effective for the treatment of intermediate stage HCC[4], tumor response as a neoadjuvant therapy prior to resection and liver transplantation, (BCLC stages 0, A and B) is less predictable[9]. Drug-eluting bead transarterial chemoembolization (DEB-TACE) is a novel endovascular treatment based on the use of microspheres that release chemotherapeutic agents within a target lesion, minimizing systemic exposure and adverse effects to chemotherapeutics[8-11]. Hence, identifying which patient characteristics may predict imaging response to DEB-TACE may improve treatment results when selecting the best candidates for neoadjuvant therapy.
Current publications regarding determinants of post-TACE tumor response seem to be based on published data using c-TACE, and on retrospective studies[12-14]. DEB-TACE’s predictors of response have not been completely elucidated. This is the first single-center prospective study performed using a standardized DEB-TACE technique that aimed to identify predictors of radiological response, assessing patients clinical and laboratory characteristics, diagnostic imaging and intraprocedural data of HCCs treated in the neoadjuvant context for liver transplantation.
This was a single-center, observational cohort, prospective study, conducted at the Interventional Medicine Center, and was approved by the local institutional review board (CAAE 0199.0.028.000-11). All patients signed an informed consent form.
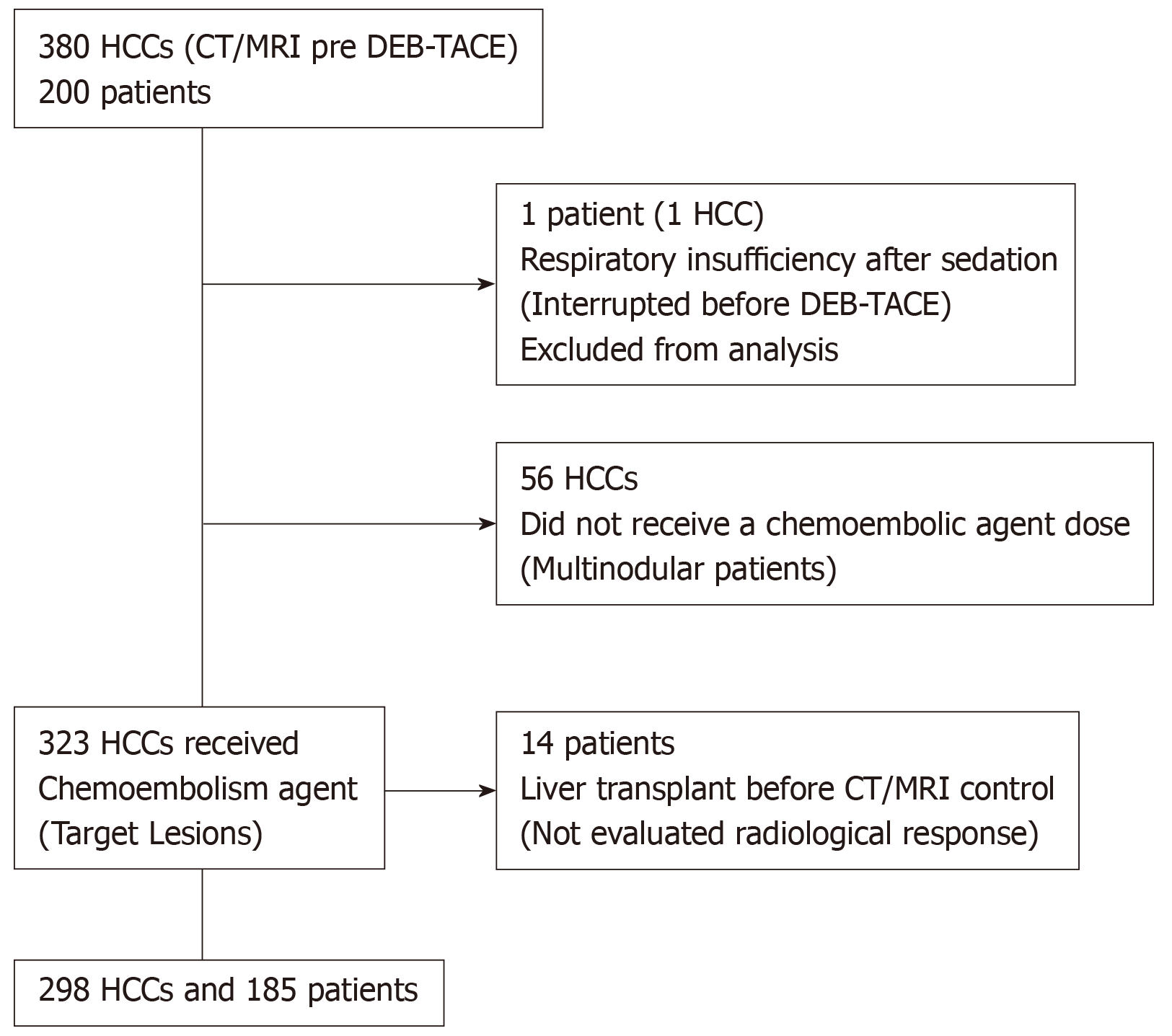
Two hundred consecutive HCC patients underwent DEB-TACE first session at our institution from April 1, 2011 until June 30, 2014, according to the outpatient treatment protocol[15]. These patients had a total of 380 tumors, and 323 of them were treated. Inclusion criteria was as it follows: patients with HCC BCLC staging 0, A or B, who took part in the liver transplantation program of the institution, in which the DEB-TACE procedure purpose was downstaging or for bridging strategy, and we assured they did not have extrahepatic spread or vascular invasion. Patient pre-treatment assessments was performed and included clinical and physical examination, imaging studies and laboratory tests — including contrast-enhanced magnetic resonance (MRI) or triple-phase computed tomography (CT). The intraoperative variables that were assessed were treatment-specific and general procedure data for each tumor. Exclusively the imaging results from the first session procedure were analyzed. The imaging was performed from 30 to 60 d after DEB-TACE and so the response evaluation.
All procedures were performed under sedation (midazolam and fentanyl), intravenous analgesia and local an aesthesia with 2% lidocainee. Catheterizations were performed via common femoral artery, followed by superior mesenteric, celiac trunk and common hepatic artery angiograms performed with a Cobra 2 5F or Simmons 2 5F (Cordis, United States). With the angiograms, it was possible to outline the hepatic artery anatomy, to delineate the tumor and the vessels that supply it, and assess portal vein patency.
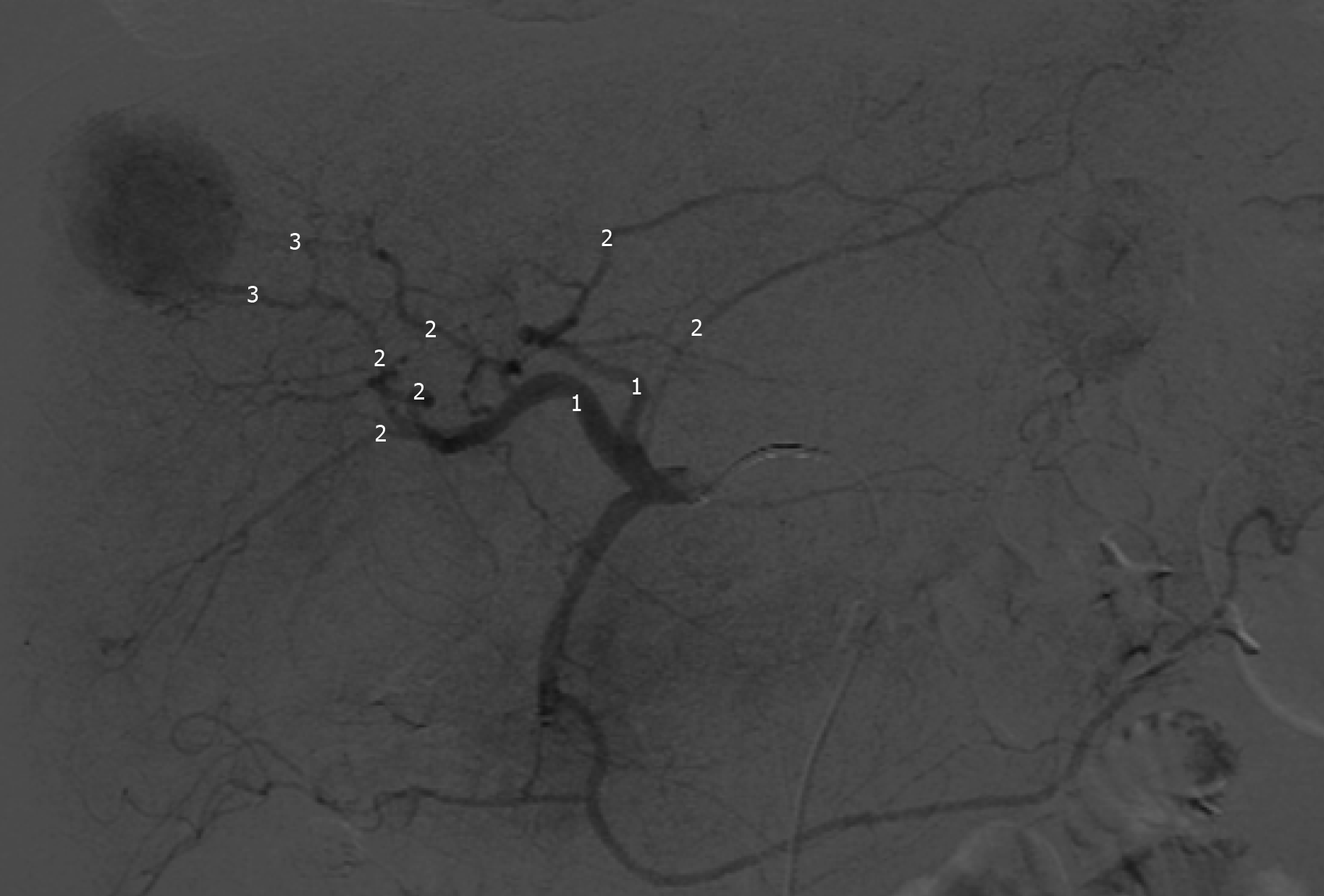
The feeding vessels previously identified were catheterized with a 2.8 F microcatheter (Progreat, Terumo, Japan), and followed by embolization of the tumors with injection of one vial of 100-300 µm DC-BEAD (Biocompatibles®, United Kingdom, LTDA) or 50-100 µm HepaSphere (Merit Medical Systems, United States) loaded with 50 mg doxorubicin mixed with iodinated contrast medium, in line with the manufacturers recommendations. Proximal embolization was defined by delivery of beads from the right or left hepatic artery; Segmental embolization by DEB-TACE delivery from segmental branches; and subsegmental embolization by the injection of beads from subsegmental or even more distal branches (Figure 1)[16].
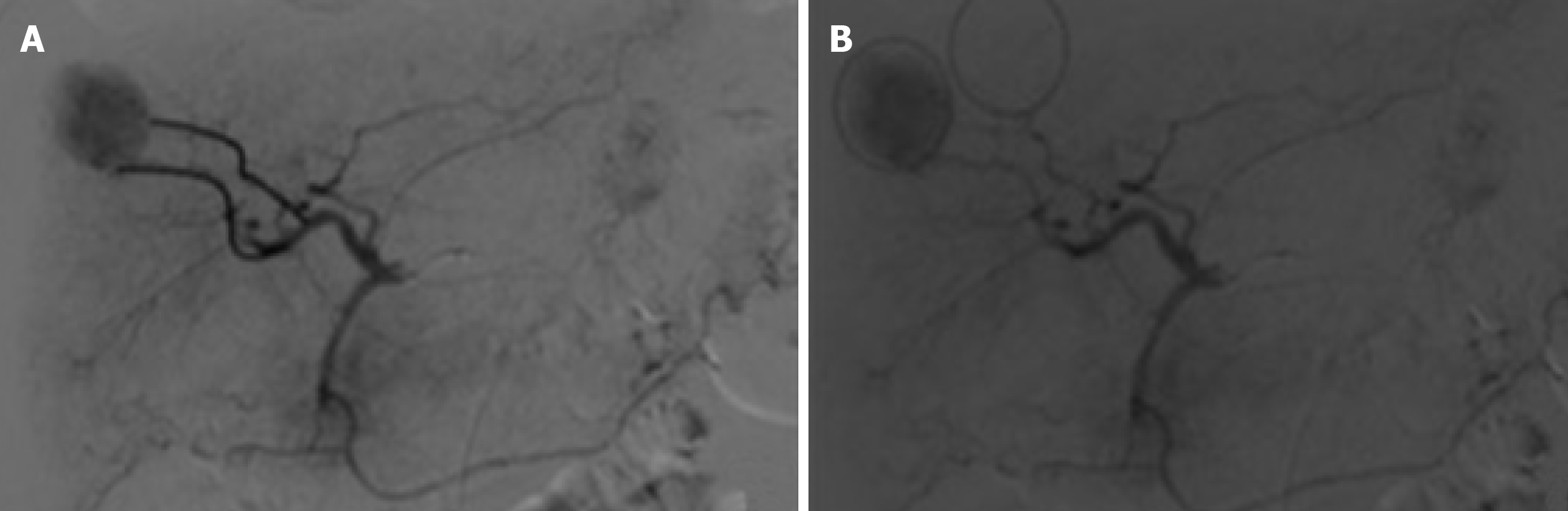
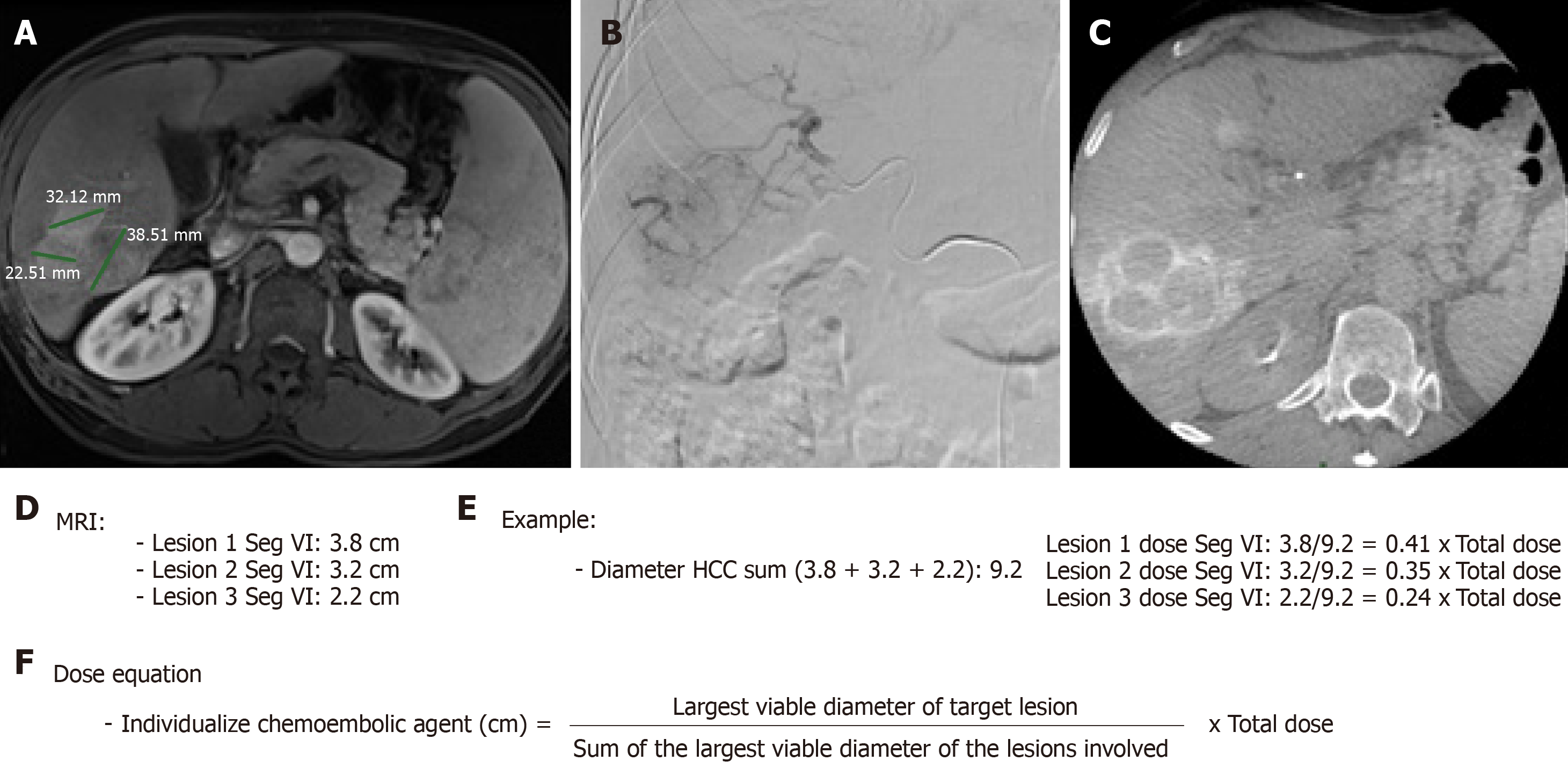
When necessary to guide catheterization and evaluate tumor vascularization cone bean computed tomography (Xper CT, Philips, Netherlands) imaging was carried out. Whether the target lesion was hyper ou hypovascular, compared to the hepatic parenchyma in angiographic presentation, was also described (Figure 2). The endpoint was reached when near stasis was observed in the arterial branch(es) supplying the tumor. If that was no accomplished after the first procedure, the same HCC was identified in the database and later in another opportunity. The dose of chemoembolic agent used in each treated lesion was quantified. In situations such as proximal/contiguous lesion involvement, or where it was not possible to perform superselective catheterization and individualization of the target lesion, the following equation was used to individualize the dose of chemoembolic agent administered to the lesion (Figure 3). A suturing device (Perclose Proglide, Abbott, United States) was used for access closure in all patients.
The primary outcome of the study was to determine the radiological objective response (OR) of HCC to DEB-TACE therapy, as assessed by mRECIST guidelines[17,18]. The secondary endpoint of the study was to determine radiological response using the percentage of HCC necrosis after DEB-TACE therapy. Tumor response was evaluated in three manners.
Nodule-based analysis: Response of each treated tumor was evaluated and the baseline diameter prior to DEB-TACE was compared to the same tumor diameter after DEB-TACE, as stated by the mRECIST guidelines[17]. Complete response (CR) was defined as the absence of intratumoral contrast enhancement, and partial response (PR) when at least 30% decrease in diameter of the viable tumor was reached. Any case that did not meet for either partial response or progressive disease was considered as stable disease (SD), and progressive disease (PD) was defined as an increase of 20% or more in diameter of the viable tumor. OR was characterized as responder (RE) when the nodule reached CR and PR, and non-responder (NR) when the nodule reached SD and PD.
Target lesion response: The response of treated nodules was evaluated by comparing the baseline sum of diameters of target lesions previous to DEB-TACE with the sum of diameters of viable target lesions after DEB-TACE in each patient, according to mRECIST guidelines CR was defined as the absence of intratumoral contrast enhancement in all target lesions, and PR when at least 30% decrease in the sum of the diameters of the viable tumor was reached. Any case that did not meet for either partial response or progressive disease was considered as SD, and PD was defined as an increase of 20% or more in the sum of the diameters of the viable target lesions. OR was characterized as RE when the target lesion reached CR and PR, and NR when the target lesion reached SD and PD.
Individual response of treated HCC (% necrosis): Analysis of individual necrotic percentage response of each of the 298 treated HCCs was assessed by comparing the largest axial diameter of necrosis of each tumor with the largest diameter of the same tumor post-DEB-TACE imaging.
Statistical analysis was performed using SPSS software, version 20.0 (IBM, Armonk, NY, United States). Differences between the means of continuous variables were compared according to the OR using Student's t-tests. Qualitative variables were described according to OR, and the association with chi-square test or exact tests (Fisher's exact test or likelihood ratio test) was verified. Pearson's correlations with quantitative variables were calculated for necrosis assessment and necrosis percentages were compared according to qualitative characteristics using Student's t-test or analysis of variance (ANOVA). To evaluate prognostic factors for OR, multivariate logistic regression analysis was performed and multiple linear regression analysis was used to evaluate prognostic factors of the percentage of tumor necrosis. For both models, the univariate analysis variables that were statistically significant for the outcomes were inserted, using the stepwise backward selection method with 5% input and output cryethium. The ROC (receiver operating characteristics) curve was constructed for the OR model to evaluate the quality of fit of the model. A p-value of less than or equal to 0.05 was considered significant.
In this study, 200 patients were included, with a total of 380 tumors detected at baseline imaging examinations. Of the 380 nodules, 323 were defined as target lesions and underwent DEB-TACE. The procedure was interrupted before embolization in one patient, with a single tumor, because he presented respiratory failure after sedation. Prior to the control imaging tests, 14 patients underwent hepatic transplant and were excluded. Therefore, the tumor radiological response was evaluated in 185 patients and the remaining 298 HCCs (Figure 4). The mean time between baseline CT/MRI and DEB-TACE was 40.2 d.
According to OR for the target lesions (mRECIST; Target Lesion Response), no difference was observed between groups, concerning clinical characteristics, pre procedure laboratory and intraoperative information,as shown in Tables 1-3. Higher indirect bilirubin alone suggested a lower mean value in RE patients (P = 0.05) (Table 2).
| Variable | NR (n = 44) | RE (n = 141) | Total (n = 185) | P value |
| Gender (male), n (%) | 39 (88.6) | 118 (83.7) | 157 (84.9) | 0.424 |
| Age (yr), mean ± SD | 58.7 ± 9.2 | 57.4 ± 8.1 | 57.7 ± 8.4 | 0.3631 |
| BMI, mean ± SD | 27.2 ± 4.2 | 27 ± 4.8 | 27.1 ± 4.7 | 0.7171 |
| Systemic hypertension, n (%) | 19 (43.2) | 60 (42.6) | 79 (42.7) | 0.941 |
| DM, n (%) | 17 (38.6) | 46 (32.6) | 63 (34.1) | 0.463 |
| DLP, n (%) | 1 (2.3) | 9 (6.4) | 10 (5.4) | 0.4562 |
| Smoker (%) | 12 (27.3) | 28 (19.9) | 40 (21.6) | 0.297 |
| Coagulopathy3, 4, n (%) | 21 (47.7) | 63 (44.7) | 84 (45.4) | 0.723 |
| Thrombocitopenia5, n (%) | 28 (63.6) | 93 (66) | 121 (65.4) | 0.778 |
| CHILD, n (%) | 0.7056 | |||
| A | 19 (45.2) | 72 (52.6) | 91 (50.8) | |
| B | 20 (47.6) | 56 (40.9) | 76 (42.5) | |
| C | 3 (7.1) | 9 (6.6) | 12 (6.7) | |
| MELD, mean ± SD | 12.1 ± 3.5 | 11.9 ± 3.5 | 12 ± 3.5 | 0.6051 |
| Downstaging7 - Milan Criteria, n (%) | 9 (20.5) | 45 (31.9) | 54 (29.2) | 0.144 |
| Multinodular HCC, n (%) | 19 (43.2) | 68 (48.2) | 87 (47) | 0.558 |
| Variable | NR (n = 44) | RE (n = 141) | Total (n = 185) | P value |
| Hb (g/dL) | 12.9 ± 2.5 | 12.9 ± 2 | 12.9 ± 2.1 | 0.6671 |
| Ht (%) | 37.1 ± 6.7 | 37.4 ± 5.6 | 37.3 ± 5.9 | 0.8621 |
| Creatinine (mg/dL) | 0.95 ± 0.81 | 0.92 ± 0.86 | 0.93 ± 0.84 | 0.4591 |
| Albumin (g/dL) | 3.43 ± 0.53 | 3.34 ± 0.55 | 3.37 ± 0.54 | 0.5751 |
| Alphafetoprotein (UI/mL) | 200.3 ± 463 | 435.1 ± 2307.2 | 379.3 ± 2027.7 | 0.3201 |
| Direct bilirubin (mg/dL) | 0.73 ± 0.49 | 0.65 ± 0.41 | 0.67 ± 0.43 | 0.6481 |
| Indirec bilirubina (mg/dL) | 1.43 ± 0.87 | 1.14 ± 0.71 | 1.21 ± 0.76 | 0.0501 |
| Total bilirubin (mg/dL) | 1.9 ± 1.11 | 1.78 ± 0.89 | 1.81 ± 0.94 | 0.8421 |
| INR | 1.31 ± 0.19 | 1.31 ± 0.21 | 1.31 ± 0.2 | 0.7781 |
| Platelets (x 103/mm3) | 85 ± 42.9 | 91.7 ± 54.8 | 90.1 ± 52.1 | 0.8101 |
| AST (U/L) | 87 ± 68.1 | 84.4 ± 59 | 85.1 ± 61.3 | 0.8421 |
| ALT (U/L) | 71.4 ± 52.4 | 71.5 ± 49.7 | 71.5 ± 50.2 | 0.8251 |
The individual preoperative radiological characteristics and intraoperative variables of HCCs were classified according to OR (mRECIST; nodule-based analysis). Upon univariate analysis, large HCC diameter (P < 0.001), the presence of a pseudocapsule (P < 0.001), increasing levels of chemoembolic agent delivered (P < 0.001) and larger numbers of feeding vessels (P = 0.041) were found to be predictive factors for OR (Table 4).
| Variable | NR (n = 93) | RE (n = 205) | Total (n = 298) | P value |
| Preoperative | ||||
| HCC diameter (cm) | 2.2 (1; 8.5) | 2.7 (1.1; 8) | 2.5 (1; 8.5) | < 0.0011 |
| Liver segment2, n (%) | 0.9613 | |||
| 1 | 2 (2.2) | 4 (2) | 6 (2) | |
| 2 | 8 (8.6) | 24 (11.7) | 32 (10.7) | |
| 3 | 5 (5.4) | 15 (7.3) | 20 (6.7) | |
| 4 | 10 (10.8) | 18 (8.8) | 28 (9.4) | |
| 5 | 9 (9.7) | 23 (11.2) | 32 (10.7) | |
| 6 | 19 (20.4) | 35 (17.1) | 54 (18.1) | |
| 7 | 23 (24.7) | 46 (22.4) | 69 (23.2) | |
| 8 | 17 (18.3) | 40 (19.5) | 57 (19.1) | |
| Pseudocapsule, n (%) | 47 (50.5) | 149 (72.7) | 196 (65.8) | < 0.001 |
| Intraoperative | ||||
| Chemoembolic dosis (mg) | 11.4 (1.5; 50) | 22.5 (2.4; 100) | 18.23 (1.5; 100) | < 0.0011 |
| Feeding vessels | 0.0411 | |||
| mean ± SD | 1.2 ± 0.4 | 1.3 ± 0.5 | 1.2 ± 0.5 | |
| median (min; max) | 1 (1; 3) | 1 (1; 3) | 1 (1; 3) | |
| Selective catheterization@, n (%) | 0.1973 | |||
| Proximal | 3 (3.4) | 9 (5.2) | 12 (4.6) | |
| Segmental | 33 (37.9) | 47 (27.2) | 80 (30.8) | |
| Subsegmental | 51 (58.6) | 117 (67.6) | 168 (64.6) | |
| Hypervascular4, n (%) | 70 (75.3) | 156 (78.8) | 226 (77.7) | 0.502 |
| End-point5, n (%) | 88 (94.6) | 187 (91.2) | 275 (92.3) | 0.308 |
By multivariate logistic regression analysis, among variables that showed relevance alone, only the dose of the chemoembolic agent (OR = 1.04; 95%CI: 1.02-1.06, P < 0.001) and the presence of a pseudocapsule (OR = 2.01; 95%CI: 1.18-3.42) were jointly prognostic factors for OR (Table 5). For each milligram of chemoembolic agent solution administered, there was a 4% increase chance of the chemoembolized tumor being RE. The variables of number of feeding vessels and diameter of HCC lost statistical significance in the presence of the variables of the chemoembolic agent dose and pseudocapsule presence. Once a target tumor received the full dose of the 50 mg chemoembolic agent, it was 58.9% more likely to be RE than a tumor that received 1 mg chemoembolic agent. The chance of the tumor being RE when in the presence of a pseudocapsule was 2.01 times greater (95%CI: 1.18-3.42, P = 0.01) the chance of tumors without a pseudocapsule being RE (Table 5).
| Variable | OR | 95%CI | P value | |
| Inferior | Superior | |||
| Chemoembolic dose (mg) | 1.04 | 1.02 | 1.06 | < 0.001 |
| Pseudocapsule | 2.01 | 1.18 | 3.42 | 0.01 |
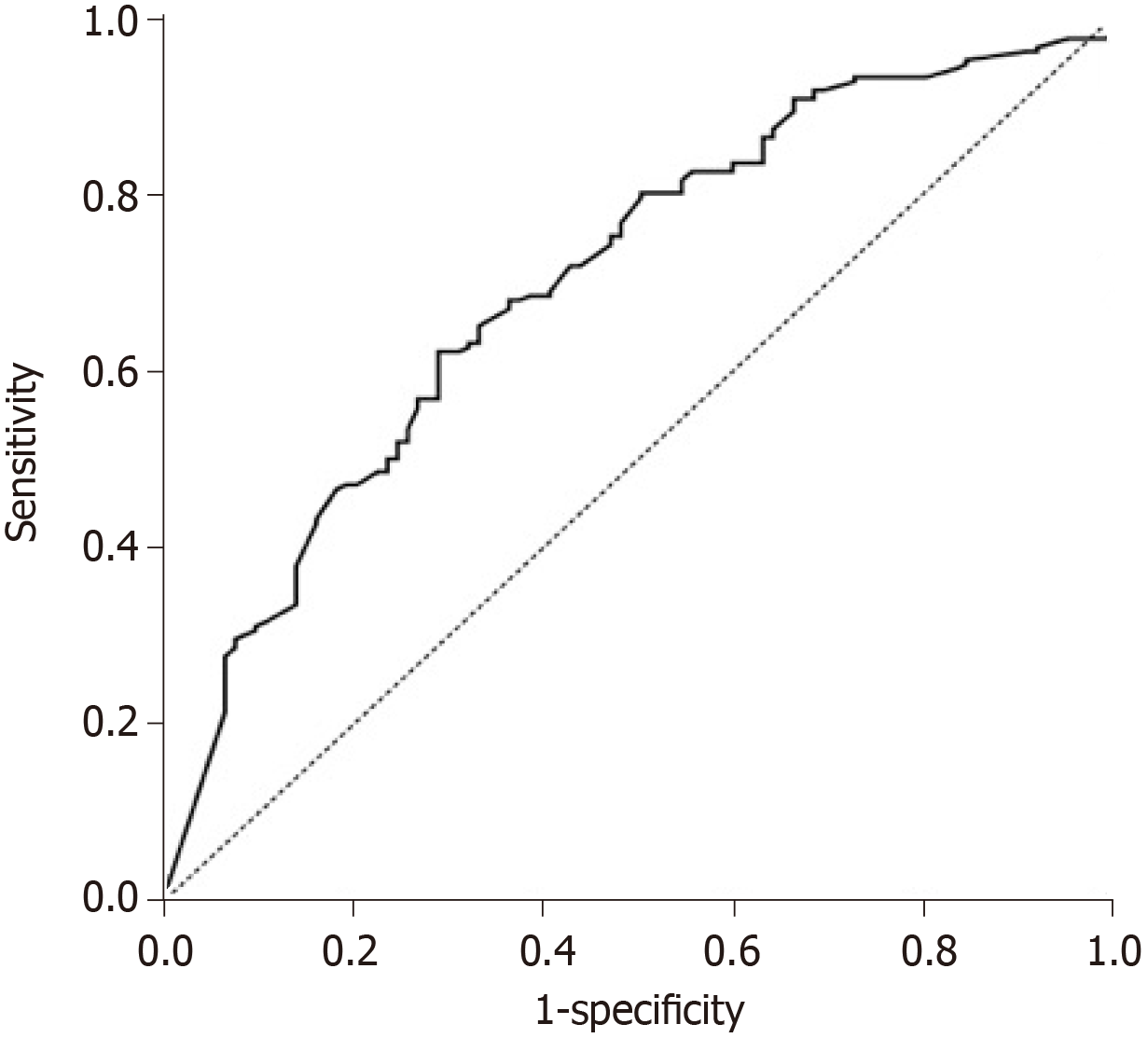
Despite the adjustments found for the dose of chemoembolic agent (mg) and pseudocapsule as explanatory variables for OR, the area under the curve (AUC) was 70.5% (Figure 5) indicating that other characteristics not evaluated in this study are also important additional factors that explain OR. Nevertheless, according to the ROC curve, these two variables presented an acceptable adjustment for OR.
When the necrosis rate was evaluated with respect to each of the tumor characteristics (Table 6), necrosis increased as HCC diameter increased (r = 0.210; P < 0.001) and as the dose of chemoembolic agent increased (r = 0.310, P < 0.001). The presence of a pseudocapsule conveyed, on average, a higher percentage of necrosis (P < 0.001). Regarding arterial catheterization, tumors chemoembolized through subsegmental branches presented a higher percentage of necrosis than tumors chemoembolized through segmental branches (P = 0.038).
| Variable | Description | P value |
| HCC diameter (cm)1 | 0.21 | < 0.001 |
| Chemoembolic dose (mg)1 | 0.31 | < 0.001 |
| Feeding vessel1 | 0.093 | 0.11 |
| Liver segment2 | 0.7093 | |
| 1 | 41.7 ± 49.2 | |
| 2 | 60.9 ± 43.6 | |
| 3 | 64 ± 38.9 | |
| 4 | 53 ± 42.4 | |
| 5 | 47.3 ± 42 | |
| 6 | 50 ± 43.6 | |
| 7 | 56.2 ± 41.3 | |
| 8 | 59.3 ± 43.2 | |
| Pseudocapsule | < 0.0014 | |
| No | 39.2 ± 43.8 | |
| Yes | 63.4 ± 39.2 | |
| Selective catheterization5 | 0.0383 | |
| Proximal | 70.4 ± 43.9 | |
| Segmental | 46.3 ± 41.6 | |
| Subsegmental | 59.1 ± 41.9 | |
| Hypervascular6 | 0.9883 | |
| No | 54.4 ± 43.6 | |
| Yes | 54.3 ± 42.2 | |
| End point7 | 0.1983 | |
| No | 66.1 ± 37.7 | |
| Yes | 54.2 ± 42.6 |
However, according to multiple linear regression (Table 7), when evaluated together, only the dose of the chemoembolic agent and presence of a pseudocapsule were related to the percentage of necrosis. The addition of each 1 milligram of the chemoembolic agent resulted in an average increase of 0.65% in necrosis in the treated lesion, whereas HCCs with a pseudocapsule presented 18.27% more necrosis than CHCs without a pseudocapsule. On average, HCCs that did not receive mg of chemoembolic agent and did not have a pseudocapsule, presented 27.8% necrosis. This radiological response, as a percentage of HCC necrosis treated through the DEB-TACE, can be expressed by the equation:
| Variable | Coefficient | Standard-error | t value | P value |
| Constant | 27.83 | 4.64 | 6 | < 0.001 |
| Chemoembolic dose (mg) | 0.65 | 0.14 | 4.58 | < 0.001 |
| Pseudocapsule | 18.27 | 5 | 3.66 | < 0.001 |
Radiological response to neoadjuvant HCC treatments is a fundamental method of evaluation for the decision to maintain and meet the criteria necessary for execution of a hepatic transplant[7,11]. Although the pseudocapsule was considered a predictor of radiological response in our study, this radiological characteristic of HCC cannot be differentiated from the true tumor capsule by imaging tests, requiring histopathological evaluation[19-21]. However, Ishigami et al[19] were able to correlate radiological findings of the pseudocapsule through histopathological analysis, evidencing that it was composed of prominent sinusoids and/or peritumoral fibrosis connecting to Glisson capsule fibrosis. Pseudocapsule HCCs, according to the same author, can be considered similar to those with a true fibrotic capsule histologically in terms of tumor invasiveness because the incidence of vascular invasion and degrees of cellular differentiation of evaluated tumors were similar[19].
In a previous study, evaluating 23 patients submitted to DEB-TACE in the same clinical stage of the current study who were submitted to liver transplantation, the presence of true capsule tumor was an independent predictor of histological response[22]. Similarly, studies evaluating changes in the histopathological architecture of HCCs treated with cTACE found that unencapsulated tumors have a worse response to cTACE than capped tumors, suggesting that unencapsulated lesions are primarily nourished by the portal vein[20]. Unlike our study, however, none of the cited studies were able to quantify the importance of the presence of pseudocapsules with respect to radiological response of HCC to DEB-TACE.
When we consider only those studies that used the DEB-TACE technique, we find Vesselle et al[23], a prospective cohort studying BCLC stage A and B patients[11,23], who were not candidates for curative therapy and used a heterogeneous caliber of embolic agent, identified that HCCs smaller than 5 cm were associated with a greater chance of CR and that tumors located in the hepatic segments I and IV presented worse radiological results[23]. In our study, the location of HCC in the hepatic segments, based on the hepatic segmentation of Couinaud, was not a predictor of radiological response (P = 0.961 OR, nodule-based analysis (mRECIST) and HCC treated P = 0.709, percentage of necrosis).
Odisio et al[22] evaluated histopathological response in a similar population with the same standardized DEB-TACE technique, dividing HCCs into two groups according to the diameter, 3.2 cm (95%CI: 2.55 -3.85) and 2.1 cm (95%CI: 1.79-2.48), and found a higher percentage of necrosis in HCCs with larger diameters. In our study, although the mean diameter of HCCs was related to radiological response, both according to OR of the individual HCC response, NR: 2.2 cm (SD: 1; 8.5 cm) and RE: 2.7 cm (P < 0.001), as well as the percentage of HCC necrosis treated (P < 0.001), when evaluated together with the dose of the chemoembolic agent and pseudocapsule, it did not retain statistical significance.
There have been no published studies that related the dose of the chemotherapeutic agent used individually in each HCC in neoadjuvant DEB-TACE procedures with radiological response. In a study by Odisio et al[22], cumulative dose of the chemoembolic agent in all DEB-TACE sessions in which the HCC was submitted, was examined using histopathological results, and no statistical significance was observed. The elapsed time of more than one DEB-TACE procedure in addition to the waiting period for LT may have influenced the outcome of the histopathological evaluation. However, another factor that may justify the absence of this correlation in the study of Odisio et al[22], is the fact that the HCCs that did not reach vascular stasis until the end of the chemoembolic agent with carrier microspheres were administered the complementary embolization with microspheres (300-500 µm Bead Block, Biocompatibles, United Kingdom Ltd.) until reaching vascular stasis. In our study, the dose of the standardized chemoembolic solution of carrier microspheres was not supplemented with non-carrier microspheres, except in cases of tumor rupture, increasing the reliability of the method in reaching the same dose ratio in all HCCs of the chemoembolic/cm of viable HCC until vascular stasis is reached. HCCs that did not reach vascular stasis were identified and tested for radiological response as described in Tables 4 and 6. According to our study, the dose of chemoembolic agent administered individually in each HCC is directly related to radiological response when evaluated by the mRECIST OR method[18], as well as the percentage of necrosis.
Analysis of randomized univariate associations of demographic, laboratory, and comorbid data, according to Target Lesion Response (mRECIST)[17,18], showed that patients with OR showed lower values of indirect bilirubin (P = 0.05), indicating a possibility that the greater clinical severity of these patients may have influenced the worse performance of their radiological response. However, CHILD and MELD scores, specific scores for clinical liver function evaluation, were tested and were not statistically significance. Understanding the relationship of these severity criteria to DEB-TACE results in the neoadjuvant scenario to liver transplantation becomes important because the addition of neoadjuvant procedures to liver transplant brings with it an additional risk known for surgical procedures of patients staged in more advanced severity classes. Thus, the benefit of the use of DEB-TACE in this scenario should be evaluated in a rigorous and standardized way, to identify patients who can obtain the maximum radiological response with the lowest clinical risk.
In relation to intraoperative variables [duration of the procedure (min), radioscopy time (min) and contrast volume (mL)] tested according to OR - Target Lesion Response (mRECIST) (Table 3), no statistical significance was observed with radiological response. Thus, possible assumptions were made that longer procedures, with a longer radioscopy time or requiring a greater volume of contrast medium during the DEB-TACE that were, therefore, more difficult, could present worse radiological results but were not confirmed.
Intraoperative variable for arterial catheterization, when evaluated by the percentage of HCC necrosis treated, suggested that tumors chemoembolized through subsegmental branches had a higher percentage of necrosis than tumors chemoembolized through segmental branches (P = 0.038). However, in a multiple linear regression analysis, this variable did not maintain statistical significance. Even when we evaluated the variable arterial selectivity according to OR - target lesion response (mRECIST), it was not statistically relevant. Thus, the perception that the best radiological response in transarterial procedures is obtained with the maximum superselecttion of the target HCC was not confirmed in this study.
The limitations of this study include that data analysis was related only to the first treatment of HCC by DEB-TACE, and there were some HCCs that did not achieve vascular stasis in this first session of DEB-TACE (not achieved the end point). Furthermore, use of the 50-100 µm Hepasphere carrier microsphere (Merit Medical Systems, United States) only occurred in 28 HCCs, while the 100-300 µm DC Beads, Biocompatible, United Kingdom Ltd. was used in the remaining 270 HCCs, so it was not possible to identify differences between these materials used in the radiological results.
Analysis of predictors of radiological response of DEB-TACE for the neoadjuvant treatment of HCC showed that a pseudocapsule increases the chance of HCCs being responders by 2.01 times, and every milligram of chemoembolic agent administered causes a 4% increase in the chance of HCC being responders. The addition of each 1 mg of the chemoembolic agent resulted in an average increase of 0.65% in necrosis, and the presence of a pseudocapsule causeds 18.27% more necrosis in treated HCCs.
Drug-eluting bead transarterial chemoembolization (DEB-TACE) minimizes systemic exposure and adverse effects to chemotherapeutics in hepatocellular carcinoma (HCC) patients.
Predictors of the response after DEB-TACE still have not been fully elucidated.
Identifying characteristics which may predict imaging response can improve treatment results and select the best candidates.
This was a single center, observational cohort prospective study.
Pseudocapsule increases by 2.01 times the chance of HCC to be responder and 18.27% more necrosis in treated HCCs. Every milligram of the chemoembolic agent administered causes a 4% increase the chance of HCC to be a responder and increase of 0.65% in necrosis.
Pseudocapsule and the addition of the amount of chemoembolic agent are imaging response predictors following drug eluting beads chemoembolization in the neoadjuvant liver transplant treatment of hepatocellular carcinoma.
Identify what other criteria not evaluated in this study may also be important additional factors explaining the post-DEB-TACE radiological response in the neodjuvant treatment of hepatocarcinoma.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Akbulut S, Chiu KW, Govindarajan GK, Rubbini M S-Editor: Ma YJ L-Editor: A E-Editor: Wu YXJ
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55757] [Article Influence: 7965.3] [Reference Citation Analysis (132)] |
| 2. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4086] [Article Influence: 583.7] [Reference Citation Analysis (6)] |
| 3. | Raoul JL, Sangro B, Forner A, Mazzaferro V, Piscaglia F, Bolondi L, Lencioni R. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev. 2011;37:212-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 409] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 4. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5304] [Article Influence: 182.9] [Reference Citation Analysis (0)] |
| 5. | Majno P, Lencioni R, Mornex F, Girard N, Poon RT, Cherqui D. Is the treatment of hepatocellular carcinoma on the waiting list necessary? Liver Transpl. 2011;17 Suppl 2:S98-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Frangakis C, Geschwind JF, Kim D, Chen Y, Koteish A, Hong K, Liapi E, Georgiades CS. Chemoembolization decreases drop-off risk of hepatocellular carcinoma patients on the liver transplant list. Cardiovasc Intervent Radiol. 2011;34:1254-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Graziadei IW, Sandmueller H, Waldenberger P, Koenigsrainer A, Nachbaur K, Jaschke W, Margreiter R, Vogel W. Chemoembolization followed by liver transplantation for hepatocellular carcinoma impedes tumor progression while on the waiting list and leads to excellent outcome. Liver Transpl. 2003;9:557-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 336] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 8. | Massarollo PC, Coppini AZ, Salzedas-Netto AA, Coelho FF, Minami T, Gonzalez AM. Favorable Long-term Outcome in Patients Submitted to Liver Transplantation After Downstaging of Hepatocellular Carcinoma According to a Brazilian Selection Protocol. Transplant Proc. 2016;48:2338-2340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Abrams P, Marsh JW. Current approach to hepatocellular carcinoma. Surg Clin North Am. 2010;90:803-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Lencioni R, Chen XP, Dagher L, Venook AP. Treatment of intermediate/advanced hepatocellular carcinoma in the clinic: how can outcomes be improved? Oncologist. 2010;15 Suppl 4:42-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 11. | European Association For The Study Of The Liver. European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4517] [Article Influence: 347.5] [Reference Citation Analysis (2)] |
| 12. | Matsui O, Kadoya M, Kameyama T, Yoshikawa J, Takashima T, Nakanuma Y, Unoura M, Kobayashi K, Izumi R, Ida M. Benign and malignant nodules in cirrhotic livers: distinction based on blood supply. Radiology. 1991;178:493-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 371] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 13. | Yao FY, Kerlan RK, Hirose R, Davern TJ, Bass NM, Feng S, Peters M, Terrault N, Freise CE, Ascher NL, Roberts JP. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology. 2008;48:819-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 409] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 14. | Angelico M. TACE vs DEB-TACE: Who wins? Dig Liver Dis. 2016;48:796-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Nasser F, Cavalcante RN, Galastri FL, de Rezende MB, Felga GG, Travassos FB, De Fina B, Affonso BB. Safety and feasibility of same-day discharge of patients with hepatocellular carcinoma treated with transarterial chemoembolization with drug-eluting beads in a liver transplantation program. J Vasc Interv Radiol. 2014;25:1012-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Cavalcante RN, Nasser F, Motta-Leal-Filho JM, Affonso BB, Galastri FL, De Fina B, Garcia RG, Wolosker N. Occurrence of Vascular Lake Phenomenon as a Predictor of Improved Tumor Response in HCC Patients That Underwent DEB-TACE. Cardiovasc Intervent Radiol. 2017;40:1044-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3353] [Cited by in RCA: 3292] [Article Influence: 219.5] [Reference Citation Analysis (36)] |
| 18. | Gillmore R, Stuart S, Kirkwood A, Hameeduddin A, Woodward N, Burroughs AK, Meyer T. EASL and mRECIST responses are independent prognostic factors for survival in hepatocellular cancer patients treated with transarterial embolization. J Hepatol. 2011;55:1309-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 277] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 19. | Ishigami K, Yoshimitsu K, Nishihara Y, Irie H, Asayama Y, Tajima T, Nishie A, Hirakawa M, Ushijima Y, Okamoto D, Taketomi A, Honda H. Hepatocellular carcinoma with a pseudocapsule on gadolinium-enhanced MR images: correlation with histopathologic findings. Radiology. 2009;250:435-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 143] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 20. | Higuchi T, Kikuchi M, Okazaki M. Hepatocellular carcinoma after transcatheter hepatic arterial embolization. A histopathologic study of 84 resected cases. Cancer. 1994;73:2259-2267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 21. | Ishizaki M, Ashida K, Higashi T, Nakatsukasa H, Kaneyoshi T, Fujiwara K, Nouso K, Kobayashi Y, Uemura M, Nakamura S, Tsuji T. The formation of capsule and septum in human hepatocellular carcinoma. Virchows Arch. 2001;438:574-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Odisio BC, Galastri F, Avritscher R, Afonso BB, Segatelli V, Felga GE, Salvalaggio PR, Ensor J, Wallace MJ, Nasser F. Hepatocellular carcinomas within the Milan criteria: predictors of histologic necrosis after drug-eluting beads transarterial chemoembolization. Cardiovasc Intervent Radiol. 2014;37:1018-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Vesselle G, Quirier-Leleu C, Velasco S, Charier F, Silvain C, Boucebci S, Ingrand P, Tasu JP. Predictive factors for complete response of chemoembolization with drug-eluting beads (DEB-TACE) for hepatocellular carcinoma. Eur Radiol. 2016;26:1640-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |