Published online Jul 27, 2019. doi: 10.4254/wjh.v11.i7.586
Peer-review started: April 23, 2019
First decision: June 5, 2019
Revised: June 19, 2019
Accepted: July 4, 2019
Article in press: July 5, 2019
Published online: July 27, 2019
Processing time: 95 Days and 5 Hours
Acute liver failure (ALF) is a life-threatening syndrome with varying aetiologies requiring complex care and multidisciplinary management. Its changing incidence, aetiology and outcomes over the last 16 years in the Australian context remain uncertain.
To describe the changing incidence, aetiology and outcomes of ALF in South Eastern Australia.
The database of the Victorian Liver Transplant Unit was interrogated to identify all cases of ALF in adults (> 16 years) in adults hospitalised between January 2002 and December 2017. Overall, 169 patients meeting criteria for ALF were identified. Demographics, aetiology of ALF, rates of transplantation and outcomes were collected for all patients. Transplant free survival and overall survival (OS) were assessed based on survival to discharge from hospital. Results were compared to data from a historical cohort from the same unit from 1988-2001.
Paracetamol was the most common aetiology of acute liver failure, accounting for 50% of cases, with an increased incidence compared with the historical cohort (P = 0.046). Viral hepatitis and non-paracetamol drug or toxin induced liver injury accounted for 15% and 10% of cases respectively. Transplant free survival (TFS) improved significantly compared to the historical cohort (52% vs 38%, P = 0.032). TFS was highest in paracetamol toxicity with spontaneous recovery in 72% of cases compared to 31% of non-paracetamol ALF (P < 0.001). Fifty-nine patients were waitlisted for emergency liver transplantation. Nine of these died while waiting for an organ to become available. Forty-two patients (25%) underwent emergency liver transplantation with a 1, 3 and 5 year survival of 81%, 78% and 72% respectively.
Paracetamol toxicity is the most common aetiology of ALF in South-Eastern Australia with a rising incidence over 30 years. TFS has improved, however it remains low in non-paracetamol ALF.
Core tip: Acute liver failure (ALF) is a life-threatening syndrome with varying aetiologies based on geographic location. Paracetamol is the most common cause of ALF in South Eastern Australia with a rising incidence over 30 years. Despite this, transplantation for paracetamol induced ALF is lower than other large centres at 4% with a comparable overall survival. Non-paracetamol ALF however portends a poor prognosis with less than one third surviving without emergency liver transplantation.
- Citation: Hey P, Hanrahan TP, Sinclair M, Testro AG, Angus PW, Peterson A, Warrillow S, Bellomo R, Perini MV, Starkey G, Jones RM, Fink M, McClure T, Gow P. Epidemiology and outcomes of acute liver failure in Australia. World J Hepatol 2019; 11(7): 586-595
- URL: https://www.wjgnet.com/1948-5182/full/v11/i7/586.htm
- DOI: https://dx.doi.org/10.4254/wjh.v11.i7.586
Acute liver failure (ALF) is a clinical syndrome characterised by severe liver injury associated with the development of coagulopathy and hepatic encephalopathy in the absence of known pre-existing liver disease[1]. The causes of ALF differ markedly based on geographic location[2]. In developing countries viral hepatitis is the most common aetiology, whereas in the developed world, the majority of ALF cases are due to paracetamol poisoning and / or other drug reactions[3-5].
Historically, ALF was associated with low rates of spontaneous survival and in regions with transplant programs, the majority of eligible patients underwent emergency liver transplantation (ELT)[6]. However, more recently, transplant-free survival (TFS) has improved[3,7,8]. In the United States, rates of liver transplantation for ALF have been reported to be as low as 20% with an overall survival to hospital discharge of 75%[8]. For non-paracetamol aetiologies, however, TFS is less than 30%, and ELT has an established survival benefit[9]. In contrast, for paracetamol induced ALF, TFS is much higher and the survival advantage of transplantation is less clear [10].
The Victorian Liver Transplant Unit (VLTU) provides liver transplantation and quaternary hepatology services for a population of 6.7 million in South-East Australia, representing 27% of the total Australian population[11]. Essentially all cases of ALF are referred to the VLTU, with only very few patients either not referred or dying prior to transfer. King’s College Criteria is used to determine suitability for liver trans-plantation.
The primary aim of this study was to report the aetiology, incidence and outcomes of all adult cases of ALF presenting to the VLTU over the last 16 years. Our secondary aim was to compare current data to historical data by the same unit[12]. We hypothesised that the incidence and underlying causes of ALF across the population would be unchanged, but that outcomes would be improved despite relatively low utilization of ELT.
All adult patients with ALF aged greater than 16 years managed at the VLTU between January 01, 2002 and December 31, 2017 were included in this study. ALF was defined as acute liver injury with the development of coagulopathy and hepatic encephalopathy within 26 wk of onset of symptoms, in the absence of known chronic liver disease[13]. Data were extracted from the VLTU database for comparison with a previously published historical cohort[12]. Patients with previous liver transplantation and primary non-function of the graft were excluded.
Information was collected from the unit database and crosschecked against medical records. The aetiology of ALF was determined by the treating team based on clinical history, paracetamol levels, viral and autoimmune serology, metabolic testing and, if available, histology from liver biopsy or explant specimens. Indeterminate ALF, also known as non-A non-B hepatitis or seronegative hepatitis, was diagnosed when all other aetiologies had been excluded.
Demographics, aetiology of ALF, rates of transplantation and outcomes were collected for all patients. Transplant free survival and overall survival (OS) were assessed based on survival to discharge from hospital. Patients who recovered without the need for liver transplantation were typically discharged back to the care of the referring health service and therefore, long-term outcomes are unknown. Medical records of patients who undergo liver transplantation, however, are regularly updated and were used to calculate post-transplant survival.
This study was approved through the Austin Health Human Research Ethics Committee.
The incidence of ALF was calculated based on annual local population data available from the Australian Bureau of Statistics. Where data was normally distributed (by Shapiro-Wilk analysis), two-tailed student t-tests were used to compare for significance. Data not meeting normality criteria were reported as median (IQR) and compared using the Mann-Whitney U test. To assess differences in endpoints and changes in aetiology over time, data were first analysed by comparison of the two cohorts, and then as a continuous set. For categorical data, comparison was made using chi-square test. Time-series analysis was completed using linear regression.
Records of 221 patients who had a diagnosis of acute liver injury on the liver transplant database were reviewed. Twenty-one patients were excluded due to the absence of hepatic encephalopathy. Twenty-two cases were excluded as they had underlying cirrhosis with acute decompensation and four cases because they had been transplanted at other centres. Five patients were excluded because they suffered ALF secondary to primary graft non-function after liver transplantation for non-ALF indications.
One hundred and sixty-nine patients met the inclusion criteria. One hundred and thirty-two cases (78%) were female and 37 (22%) were male. The median age at presentation was 41 years[31;52] for females and 37 years[27;49] for males. The median rate of referral over the study period was 9 cases per year, or 1.6 cases per million population per year. Twenty-two patients (13%) presented directly to our centre, 118 cases (70%) were referred from other metropolitan hospitals and 29 (17%) from regional and rural hospitals. One hundred and fifty-seven patients (92.9%) required support in the intensive care unit during their admission.
Paracetamol toxicity was the most common cause of ALF accounting for a total of 84 cases (50%) (Table 1). The median rate of paracetamol induced ALF referred to the centre over the 16-year study period was 0.7 cases per million population per year[11]. Non-paracetamol drug or toxin induced liver injury was the cause of ALF in 17 patients (10%). Viral hepatitis was the cause of ALF in 26 cases (15%). This included 20 cases of fulminant hepatitis B virus (HBV) infection and two from reactivated chronic HBV following systemic chemotherapy. Hepatitis A virus (HAV) was identified in four cases. Other viral aetiologies included herpes simplex virus-2 (HSV) and varicella zoster virus both in isolated cases.
| Drug/Toxins | Cases of ALF | Waitlisted for ELT | ELT |
| (n = 101) | (n = 21) | (n = 13) | |
| Paracetamol | 84 | 11 | 3 |
| Antibiotics | 4 | 2 | 2 |
| Amoxicillin/clavulanate (2), clarithromycin, isoniazid | |||
| Infliximab | 2 | 2 | 2 |
| Illicit drugs | 4 | 1 | 1 |
| LSD, injected buprenorphine, amphetamines, MDMA | |||
| NSAIDs | 1 | 1 | 1 |
| Amanita phalloides | 1 | 1 | 1 |
| Herbal medicines | |||
| Black cohosh herb, kava kava | 2 | 2 | 2 |
| Other | |||
| Moxonidine, fenofibrate, chlorambucil | 3 | 1 | 1 |
Fulminant autoimmune hepatitis was diagnosed in nine patients (5%). The diagnosis was made based on a combination of positive auto-antibody testing, elevated IgG levels and histology results either during the episode of ALF or after recovery. ALF secondary to malignancy was identified in four patients. Veno-occlusive disease caused ALF in one case following allogeneic stem cell trans-plantation. Wilson’s disease was the cause of ALF in two cases. Severe ischemic hepatitis due to hepatic artery injury and extensive portal vein thrombosis resulted in ALF in four patients.
ALF occurred in five patients during pregnancy. Aetiologies included acute fatty liver disease of pregnancy, HSV hepatitis and pregnancy-precipitated liver failure from a urea-cycle disorder[14]. Unintentional paracetamol poisoning causing ALF occurred in two cases in the context of hyperemesis and malnutrition. One of these cases presented with foetal death in utero in the third trimester of pregnancy, however in all other pregnancies, the infants survived. Twenty-one cases (12%) were classified as indeterminate ALF after exclusion of other aetiologies.
Over the study period the TFS was 52% and OS to discharge from hospital was 72%. TFS was significantly higher in patients with paracetamol induced ALF at 74% compared to non-paracetamol aetiologies at 31% (P < 0.0001). OS however did not differ significantly at 77% and 67% for paracetamol and non-paracetamol aetiologies respectively (P = 0.13). ALF caused by indeterminate hepatitis had the lowest TFS at 10%.
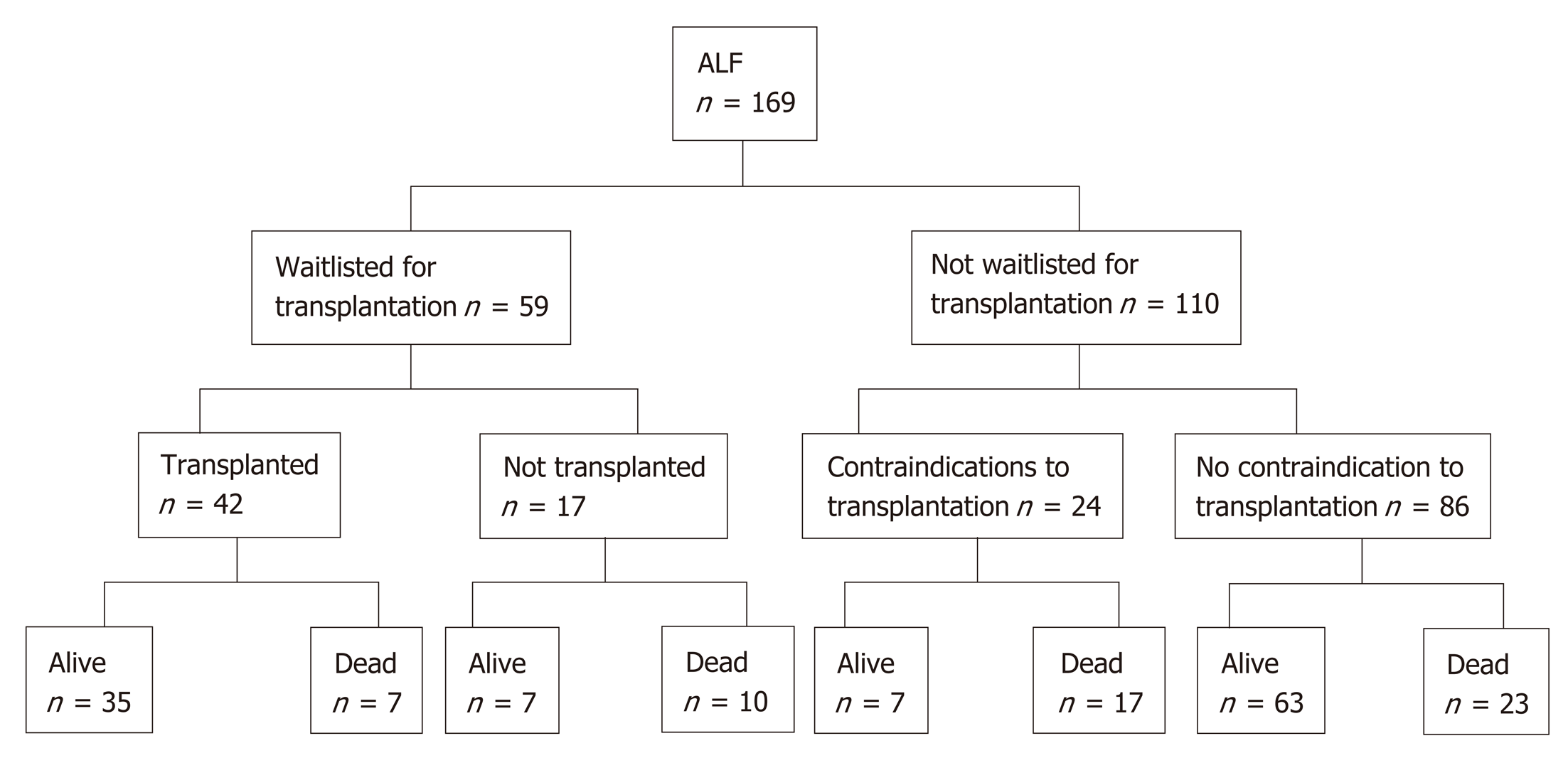
Fifty-nine patients (35%) were waitlisted for transplantation (Figure 1). Eight patients improved and were delisted, however one subsequently died of sepsis. One patient was delisted following an intraoperative finding of ischemic gut and eight patients died while waiting for a donor liver (14%). 42 patients (25%) underwent liver transplantation for ALF. Rates of liver transplantation were highest in patients with indeterminate hepatitis at 67%. Only three patients with paracetamol induced ALF were transplanted (4%).
After medical and psychosocial assessment, liver transplantation was con-traindicated in 24 patients. Of these, fifteen cases were deemed medically unsuitable for transplantation. Medical contraindications included uncontrolled sepsis, ischemic bowel, intracranial events, active malignancy and known severe coronary artery disease. Only three (20%) of these patients recovered and survived to discharge. Six patients had psychiatric or psychosocial contraindications and three patients had concurrent heavy alcohol intake rendering them unsuitable for transplantation. Paracetamol was the cause of ALF in eight of these patients (89%). Four (44%) recovered and were discharged from hospital.
For patients undergoing liver transplantation for ALF, 1, 3 and 5-year survival was 81%, 78% and 72% respectively. Seven patients (17%) died within 30 d of transplantation. The aetiology of ALF for such patients included indeterminate hepatitis (2), AIH (2), drug and toxins (2) and hepatic arterial injury leading to ischamic heptitis (1). Causes of death post operatively included sepsis in three cases, cardiovascular or cerebral events in three patients and hepatic artery thrombosis in one case.
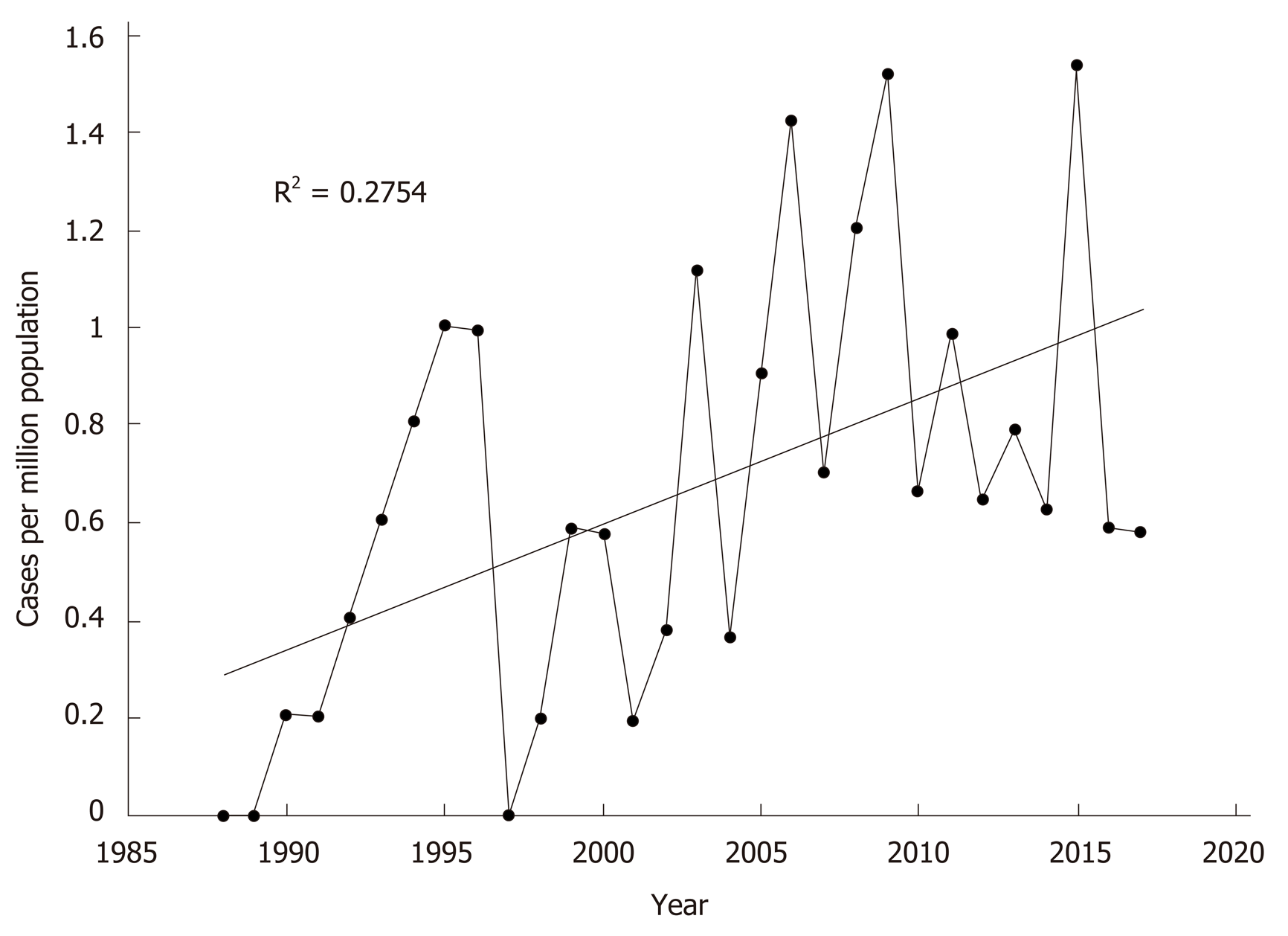
Compared to historical data published by the unit from 1988-2001[12]. there was no difference in age at presentation or gender distribution (Table 2). Paracetamol poisoning remained the most common aetiology over the two time periods. There was a significant increase in the rate of paracetamol-induced ALF in this current data set compared to the historical cohort (36% vs 50%, P = 0.046). When presented as continuous data, linear regression analysis also identified a significant increase in paracetamol-induced ALF per capita across the 30 years (R2 = 0.275; F(1,28) = 10.643, P = 0.003) (Figure 2). Overall ALF referrals to the unit also increased significantly over the time period (R2 = 0.178; F(1,28) = 6.074, P = 0.020). Comparing this dataset to historical data (1988-2001) also identified no differences in rates of viral hepatitis.
| 1988-20011 (n = 80) | 2002-2017 (n = 169) | P value | |||
| n | % | n | % | ||
| Referral rate2, median [IQR] | 1.2 [0.6;1.6] | 1.6 [1.3;1.7] | 0.020 | ||
| Age, yr, median [IQR] | 36 [27.0;48.0] | 40 [30.0;52.0] | 0.168 | ||
| Gender | |||||
| Male | 16 | 20.0 | 37 | 21.9 | |
| Female | 64 | 80.0 | 132 | 78.1 | 0.733 |
| Aetiology | |||||
| Paracetamol | 29 | 36.3 | 84 | 49.7 | 0.046 |
| Viral hepatitis | 11 | 13.8 | 26 | 15.3 | 0.734 |
| Hepatitis B | 8 | 10.0 | 20 | 11.8 | 0.669 |
| Hepatitis A | 3 | 3.8 | 4 | 2.4 | 0.538 |
| Varicella zoster | 0 | 0.0 | 1 | 0.1 | 0.491 |
| Herpes simplex | 0 | 0.0 | 1 | 0.1 | 0.491 |
| Non-paracetamol drug/toxin | 5 | 6.3 | 17 | 10.1 | 0.323 |
| Indeterminate | 27 | 33.8 | 21 | 12.4 | <0.001 |
| Autoimmune hepatitis | 0 | 0.0 | 9 | 5.3 | 0.036 |
| Other | 8 | 10.0 | 12 | 7.1 | 0.432 |
| Waitlisted for transplantation | 35 | 43.8 | 59 | 33.5 | 0.179 |
| Waitlist mortality | 9 | 25.7 | 8 | 13.6 | 0.139 |
| Liver transplantation | 26 | 32.5 | 42 | 24.9 | 0.206 |
| Transplant-free survival | 30 | 37.5 | 88 | 52.1 | 0.032 |
| Overall hospital survival | 50 | 62.5 | 122 | 72.2 | 0.122 |
TFS to discharge from hospital improved in the present study compared to the historical cohort from 38% to 52% (P = 0.032). An improvement in TFS was observed in both paracetamol toxicity (69% vs 74%, P = 0.614) and non-paracetamol aetiologies (20% vs 31%, P = 0.160) although neither met significance (Table 3). Overall, there was no statistically significant difference in hospital survival (63% to 72%, P = 0.122) or transplantation rates for ALF (33% to 25%, P = 0.206).
| Aetiology | TFS (%) | OS (%) | ||||||||
| 1988-20011 | 2002-2017 | P value | 1988-20011 | 2002-2017 | P value | |||||
| n | % | n | % | n | % | n | % | |||
| Paracetamol | 20 | 69.0 | 62 | 73.8 | 0.614 | 21 | 72.4 | 65 | 77.4 | 0.589 |
| Non-paracetamol | 10 | 19.6 | 26 | 30.6 | 0.160 | 29 | 56.9 | 57 | 67.1 | 0.230 |
| Viral hepatitis | 2 | 18.2 | 10 | 38.4 | 0.228 | 5 | 45.5 | 18 | 69.2 | 0.173 |
| HBV | 2 | 25.0 | 7 | 35.0 | 0.609 | 4 | 50.0 | 14 | 70.0 | 0.318 |
| HAV | 0 | 0.0 | 3 | 75.0 | 0.047 | 1 | 0.33 | 3 | 75.0 | 0.270 |
| Drug/toxin induced | 3 | 60.0 | 5 | 29.4 | 0.211 | 4 | 80.0 | 12 | 70.6 | 0.678 |
| Autoimmune hepatitis | 0 | 0.0 | 3 | 33.3 | - | 0 | 0.0 | 6 | 66.7 | - |
| Indeterminate hepatitis | 5 | 18.5 | 2 | 9.5 | 0.381 | 15 | 55.5 | 14 | 66.7 | 0.435 |
| Other | 0 | 0.0 | 6 | 50.0 | 0.017 | 5 | 62.5 | 7 | 58.3 | 0.852 |
This cohort study represents the largest modern series of patients presenting with ALF in Australia. As the sole liver transplant unit responsible for a population of 6.7 million, the VLTU captured both the incidence and outcomes of this rare but life-threatening syndrome. We identified paracetamol toxicity as the commonest cause of ALF, responsible for 50% of presentations. We also found an increasing rate of paracetamol induced ALF referrals to our centre over a 30-year period. Finally, we found that transplant-free survival for both paracetamol toxicity and non-paracetamol aetiologies has improved, but that spontaneous survival for non-paracetamol ALF remains low. This is particularly the case for indeterminate hepatitis and non-paracetamol drug-induced liver injury, highlighting the need for early referral to a transplant centre.
Our reported prevalence of paracetamol-induced ALF is similar to series from the United Kingdom and United States with rates ranging from 37%-79%[3,4,15]. However, of concern, this is the only cohort to show an overall steady increase in its incidence. These results differ from local Victorian data demonstrating a decrease in hospital admissions for non-ALF paracetamol poisoning from 2000 to 2007[16]. In the United States, rates of ALF due to paracetamol poisoning have also fallen following the introduction of paracetamol sales restrictions in 1998[3,17]. These regulations limited pharmacy sales to a maximum of 32 tablets per pack. While restrictions in non-pharmacy sales of paracetamol have been in place in Australia since 2013, packets of up to 100 tablets are still readily available in pharmacies. Risk factors for ALF from paracetamol include prolonged fasting and malnutrition often resulting in inadvertent toxicity[18] and combination pain relief products can also result in accidental overdose[16]. Improved awareness and public health strategies to address these factors may curtail this trend in life-threatening paracetamol toxicity.
Viral hepatitis was the second most common cause of ALF in this series. HBV accounted for 12% of all cases of ALF with no change compared to the historical cohort. This is in contrast to the declining incidence of HBV-induced ALF in other developed countries[3,19,20]. This may in part reflect the fact that many older Australians are still not vaccinated against the virus[21]. Also, Australia has high rates of immigration from countries in Asia where HBV remains endemic. Fulminant flares of HBV in the setting of inappropriately ceasing anti-viral therapy was a common background scenario in this patient group. Additionally, two patients in this cohort developed ALF from HBV infection during treatment of haematological malignancies with systemic chemotherapy. In both cases, reactivation of HBV was fatal. This emphasises the importance of early identification of patients at risk, monitoring and prophylaxis with nucleoside analogues where appropriate[22].
Hepatitis A was the cause of ALF in just four (2%) of our cases. HAV vaccination is not routine in Australia and is recommended only in high-risk populations including men who have sex with men and travellers to endemic areas. However, outbreaks do occur in a predominantly non-immune population[23]. Of note, three of the HAV induced ALF cases occurred in the same year, coinciding with a large food-born outbreak of HAV in Victoria [24]. While HAV and HBV were uncommon causes of ALF, mortality and morbidity were high with less than 40% of this patient population surviving without transplantation.
In this series we demonstrated that patients with paracetamol induced ALF have high rates of spontaneous survival and are unlikely to require transplantation. Indeed, rates of transplantation for paracetamol poisoning in this cohort are lower than reported by other large centres at 8%-25% but with comparable overall survival[3,15,18]. The role of ELT in paracetamol induced ALF has been questioned for more than 25 years[25]. King’s College Hospital have been the world leaders in defining patients with paracetamol induced ALF who will die without transplantation and these criteria have been debated and refined over the last 30 years[1,10,26-28]. More recently the King’s group have questioned whether transplantation plays any role in paracetamol induced ALF[28]. Our own policy is to consider transplantation only for the small subgroup of patients who have progressive coagulopathy and hyperlactatemia despite 48-72 hours of supportive treatment.
Over the 30-year period under review, TFS for ALF improved significantly. This finding has been observed in other large cohorts[3,4,8,15]. Early recognition of ALF and referral to specialised transplant centres may be one factor that has improved TFS. Supportive care for ALF within intensive care units has also evolved. This is particularly the case in the monitoring and management of cerebral oedema. For the last decade, we have had a protocolised approach to the management of this patient group with the aim of minimizing deaths from cerebral oedema. This combination approach, termed “quadruple-H therapy” comprises hyperventilation, hyper-natremia, hypothermia and hemodiafiltration[29]. This multimodal approach has minimised the use of invasive intracranial pressure monitoring devices. Death attributed to cerebral oedema occurred in just one patient (0.6%) in this series.
Despite improvements in supportive care, transplantation still plays a pivotal role in this condition. Organ allocation in Australia is typically State based. However, patients with ALF who are ventilated in intensive care are classified as a category 1 and are allocated the next suitable organ within Australia or New Zealand[30]. Despite this approach, waitlist mortality remained high. There is often a brief window period where patients with ALF can be transplanted before they develop refractory multiple organ failure. Therefore, timely availability of a suitable donor organ for waitlisted patients remains critical.
This is the largest Australian cohort to report on the changing incidence and aetiology of ALF. We identified a concerning rise in paracetamol toxicity causing ALF. However, despite the high incidence of paracetamol induced ALF, we report on our very low use of ELT in this group of patients despite a comparable overall survival to other large international centres.
This study has several limitations. Firstly, this is a retrospective study, which interrogated prospectively recorded data. The diagnosis of ALF requires the presence of hepatic encephalopathy, which relied on the adequate documentation of this clinical finding in medical records. Additionally, these data did not include patients who were treated at other centres. It is possible that death prior to transfer, suicidal intent, substance abuse or significant comorbidities deemed unsuitable for transplantation may have prevented some patients being referred to the VLTU.
In conclusion, paracetamol toxicity remains the most common aetiology of ALF, with increasing rates over time, highlighting the need for public health measures to reduce this preventable cause. TFS has improved which may reflect advances in supportive care measures. Regardless, the majority of cases of non-paracetamol ALF still require transplantation and therefore early referral to a specialised transplant centre remains imperative.
Acute liver failure (ALF) is a rare clinical syndrome with varying aetiologies based on geographic location. This condition is associated with high morbidity and mortality, and emergency liver transplantation is often life-saving.
In Australia, published data from 1988-2001 demonstrated that paracetamol toxicity was the major cause of ALF, followed by non-A non-B hepatitis. An updated analysis of aetiologies and outcomes in an Australian context is therefore required.
This study aimed to provide a description of the aetiologies and outcomes of acute liver failure presenting to a large Australian liver transplant centre. We also aimed to describe changes over the past thirty years since the availability of liver transplantation for this condition.
This is a retrospective cohort study of all patients admitted to the Victorian Liver Transplant Unit from 2001-2017. Data were compared to previous published series from the unit from 1988-2001, and as continuous data, to assess changes in aetiologies and outcomes over the past 30 years.
Paracetamol toxicity accounted for half of all cases of ALF, with a rise in the incidence of this condition over the past 30 years. Despite this observation, rates of liver transplantation for this condition are low at 4%, with an excellent overall survival. Rates of emergency liver transplantation were highest in indeterminate hepatitis and non-paracetamol drug induced liver injury. Transplant-free survival improved in this cohort compared to the historical cohort, however there was no significant change in overall survival.
Paracetamol represents the major cause of ALF in South-Eastern Australia with a concerning rise in its incidence over the past 30 years. Transplant-free survival has improved but remains low for ALF due to non-paracetamol causes.
This study shows a concerning rise in the incidence of paracetamol induced ALF in Australia, raising important questions regarding awareness and public health strategies to curb this rise. Larger multi-centre studies are required to confirm this observation. Transplant-free survival improved in this population similar to reports from other large international series, highlighting advances in supportive care.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Auzinger G, He SQ, Zheng YW S-Editor: Ma YJ L-Editor: A E-Editor: Zhang YL
| 1. | O'Grady JG, Alexander GJ, Hayllar KM, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97:439-445. [PubMed] |
| 2. | Ichai P, Samuel D. Etiology and prognosis of fulminant hepatitis in adults. Liver Transpl. 2008;14 Suppl 2:S67-S79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 3. | Bernal W, Hyyrylainen A, Gera A, Audimoolam VK, McPhail MJ, Auzinger G, Rela M, Heaton N, O'Grady JG, Wendon J, Williams R. Lessons from look-back in acute liver failure? A single centre experience of 3300 patients. J Hepatol. 2013;59:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 306] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 4. | Ostapowicz G, Fontana RJ, Schiødt FV, Larson A, Davern TJ, Han SH, McCashland TM, Shakil AO, Hay JE, Hynan L, Crippin JS, Blei AT, Samuel G, Reisch J, Lee WM; U. S. Acute Liver Failure Study Group. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947-954. [PubMed] |
| 5. | Khuroo MS, Kamili S. Aetiology and prognostic factors in acute liver failure in India. J Viral Hepat. 2003;10:224-231. [PubMed] |
| 6. | Schiodt FV, Atillasoy E, Shakil AO, Schiff ER, Caldwell C, Kowdley KV, Stribling R, Crippin JS, Flamm S, Somberg KA, Rosen H, McCashland TM, Hay JE, Lee WM. Etiology and outcome for 295 patients with acute liver failure in the United States. Liver Transpl Surg. 1999;5:29-34. [PubMed] |
| 7. | Koch DG, Tillman H, Durkalski V, Lee WM, Reuben A. Development of a Model to Predict Transplant-free Survival of Patients With Acute Liver Failure. Clin Gastroenterol Hepatol. 2016;14:1199-1206.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 8. | Reuben A, Tillman H, Fontana RJ, Davern T, McGuire B, Stravitz RT, Durkalski V, Larson AM, Liou I, Fix O, Schilsky M, McCashland T, Hay JE, Murray N, Shaikh OS, Ganger D, Zaman A, Han SB, Chung RT, Smith A, Brown R, Crippin J, Harrison ME, Koch D, Munoz S, Reddy KR, Rossaro L, Satyanarayana R, Hassanein T, Hanje AJ, Olson J, Subramanian R, Karvellas C, Hameed B, Sherker AH, Robuck P, Lee WM. Outcomes in Adults With Acute Liver Failure Between 1998 and 2013: An Observational Cohort Study. Ann Intern Med. 2016;164:724-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 301] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 9. | Kremers WK, van IJperen M, Kim WR, Freeman RB, Harper AM, Kamath PS, Wiesner RH. MELD score as a predictor of pretransplant and posttransplant survival in OPTN/UNOS status 1 patients. Hepatology. 2004;39:764-769. [PubMed] |
| 10. | O'Grady J. Timing and benefit of liver transplantation in acute liver failure. J Hepatol. 2014;60:663-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 11. | Australian Bureau of Statistics. 3101.0 - Australian Demographic Statistics, Dec 2017. [Cited 2018 Mar. 3] Available from: http://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/3101.0Dec%202017. |
| 12. | Gow PJ, Jones RM, Dobson JL, Angus PW. Etiology and outcome of fulminant hepatic failure managed at an Australian liver transplant unit. J Gastroenterol Hepatol. 2004;19:154-159. [PubMed] |
| 13. | Lee WM, Stravitz RT, Larson AM. Introduction to the revised American Association for the Study of Liver Diseases Position Paper on acute liver failure 2011. Hepatology. 2012;55:965-967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 330] [Cited by in RCA: 355] [Article Influence: 27.3] [Reference Citation Analysis (35)] |
| 14. | Sinclair M, Ket S, Testro A, Gow PJ, Angus PW. Acute hepatic decompensation precipitated by pregnancy-related catabolic stress: a rare mimic of acute liver failure. Obstet Gynecol. 2014;123:480-483. [PubMed] |
| 15. | Donnelly MC, Davidson JS, Martin K, Baird A, Hayes PC, Simpson KJ. Acute liver failure in Scotland: changes in aetiology and outcomes over time (the Scottish Look-Back Study). Aliment Pharmacol Ther. 2017;45:833-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Sood S, Howell J, Sundararajan V, Angus PW, Gow PJ. Paracetamol overdose in Victoria remains a significant health-care burden. J Gastroenterol Hepatol. 2013;28:1356-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Hawton K, Simkin S, Deeks J, Cooper J, Johnston A, Waters K, Arundel M, Bernal W, Gunson B, Hudson M, Suri D, Simpson K. UK legislation on analgesic packs: before and after study of long term effect on poisonings. BMJ. 2004;329:1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 155] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 18. | Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiødt FV, Ostapowicz G, Shakil AO, Lee WM; Acute Liver Failure Study Group. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1326] [Cited by in RCA: 1282] [Article Influence: 64.1] [Reference Citation Analysis (0)] |
| 19. | Manka P, Verheyen J, Gerken G, Canbay A. Liver Failure due to Acute Viral Hepatitis (A-E). Visc Med. 2016;32:80-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 20. | Daniels D, Grytdal S, Wasley A; Centers for Disease Control and Prevention (CDC). Surveillance for acute viral hepatitis - United States, 2007. MMWR Surveill Summ. 2009;58:1-27. [PubMed] |
| 21. | The Kirby Institute. HIV, viral hepatitis and sexually transmissible infections in Australia: annual surveillance report 2017. Sydney: Kirby Institute, UNSW Sydney. Available from: https://kirby.unsw.edu.au/report/annual-surveillance-report-hiv-viral-hepatitis-and-stis-australia-2017. |
| 22. | European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2323] [Cited by in RCA: 2401] [Article Influence: 184.7] [Reference Citation Analysis (0)] |
| 23. | Wasley A, Fiore A, Bell BP. Hepatitis A in the era of vaccination. Epidemiol Rev. 2006;28:101-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 181] [Article Influence: 9.5] [Reference Citation Analysis (2)] |
| 24. | Donnan EJ, Fielding JE, Gregory JE, Lalor K, Rowe S, Goldsmith P, Antoniou M, Fullerton KE, Knope K, Copland JG, Bowden DS, Tracy SL, Hogg GG, Tan A, Adamopoulos J, Gaston J, Vally H. A multistate outbreak of hepatitis A associated with semidried tomatoes in Australia, 2009. Clin Infect Dis. 2012;54:775-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 25. | Gow PJ, Angus PW, Smallwood RA. Transplantation in patients with paracetamol-induced fulminant hepatic failure. Lancet. 1997;349:651-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Craig DG, Ford AC, Hayes PC, Simpson KJ. Systematic review: prognostic tests of paracetamol-induced acute liver failure. Aliment Pharmacol Ther. 2010;31:1064-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Bailey B, Amre DK, Gaudreault P. Fulminant hepatic failure secondary to acetaminophen poisoning: a systematic review and meta-analysis of prognostic criteria determining the need for liver transplantation. Crit Care Med. 2003;31:299-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 28. | O'Grady JG. Transplant in haste, repent at your leisure? Liver Transpl. 2015;21:570-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Warrillow SJ, Bellomo R. Preventing cerebral oedema in acute liver failure: the case for quadruple-H therapy. Anaesth Intensive Care. 2014;42:78-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | The Transplantation Society of Australia and New Zealand: Clinical Guidelines for Organ Transplantation from Deceased Donors May 2017. Available from: https://www.tsanz.com.au/organallocationguidelines/documents/ClinicalGuidelinesV1.1May2017.pdf. |