Published online Jun 27, 2019. doi: 10.4254/wjh.v11.i6.531
Peer-review started: May 10, 2019
First decision: June 3, 2019
Revised: June 9, 2019
Accepted: June 17, 2019
Article in press: June 17, 2019
Published online: June 27, 2019
Processing time: 51 Days and 5.5 Hours
Hepatic encephalopathy (HE) is a complication of liver cirrhosis and can result in neuropsychological and neuromuscular dysfunctions in patients. Rifaximin, an antibiotic, has been reported to decrease the occurrence of overt HE and also improve cognitive function in studies from Europe and the United States of America. There is not enough evidence of the relationship between the long-term use of rifaximin and its clinical effects in the Japanese.
To determine the clinical effects of long-term rifaximin therapy in decompensated liver cirrhosis patients, with overt HE or hyperammonemia.
In this single-center retrospective observational cohort study, we reviewed the data of 38 patients who had taken rifaximin at the dose of 1200 mg/d for more than 24 wk. The primary outcome measured was the efficacy of long-term rifaximin use, and secondary outcome measured was the safety of its long-term use as determined by its influence on portosystemic shunts as well as Escherichia coli-related infections. Moreover, we compared the prognosis between the rifaximin group and control cases, matched for hepatic elasticity assessed by magnetic resonance ela-stography, age, and Child-Pugh classification.
Of the 38 patients included in the study, 12 (31.6%) had overt HE, 27 (71.1%) had complications of esophageal varices, and 9 (23.7%) had hepatocellular carcinoma (HCC). The control group was matched for age, Child-Pugh classification, liver stiffness, and presence of HCC. The median of serum ammonia level before treatment was 104 μg/dL (59-297), and 2 wk after treatment, it significantly decreased to 85 μg/dL (34-153) (P = 0.002). A significantly low value of 80.5 μg/dL (44-150) was maintained 24 wk after treatment. The long-term use of rifaximin did not cause a decline in liver function. Diarrhea occurred in 2 patients, who improved with the administration of probiotics, and there were no cases of aborted rifaximin therapy owing to adverse events. In patients with Child C, the survival was short, but there was no significant difference compared with that of the control group.
Rifaximin therapy improves overt HE. The long-term use of rifaximin in the Japanese is effective and safe.
Core tip: This study evaluated the efficacy and safety of long-term rifaximin therapy for hepatic encephalopathy in the Japanese population. Serum ammonia level was maintained at a significantly low median value of 80.5 μg/dL (range, 44-150 μg/dL) at 24 wk after treatment, and the long-term use of rifaximin did not worsen liver function.
- Citation: Nishida S, Hamada K, Nishino N, Fukushima D, Koyanagi R, Horikawa Y, Shiwa Y, Saitoh S. Efficacy of long-term rifaximin treatment for hepatic encephalopathy in the Japanese. World J Hepatol 2019; 11(6): 531-541
- URL: https://www.wjgnet.com/1948-5182/full/v11/i6/531.htm
- DOI: https://dx.doi.org/10.4254/wjh.v11.i6.531
Hepatic encephalopathy (HE) is a complication seen in liver cirrhosis patients, which causes neuropsychological and neuromuscular dysfunction in varying degrees[1].
HE is classified into two types: minimal HE that presents as a slight abnormality and cannot be proved except by a neuromuscular examination, and overt HE that causes obvious disorders of consciousness and motor activity[1,2].
It has been reported that once overt HE occurs, the prognosis is so poor that the mortality rate within 1 year is 64% and that within 5 years is 85%. Overt HE as well as minimal HE, which are present in 80% of liver cirrhosis patients, can impair the prognosis and increase the risk of hospitalization[3].
Conventionally in Japan, the first-choice treatment for HE is synthesized disaccharides or branched-chain amino acids, and poorly absorbed antibiotics have not been approved by the health insurance. Rifaximin has been available for treatment since November 2016. Rifaximin is an oral antibiotic which decreases the ammonia-producing enteric bacteria[4,5].
Studies in Europe and the United States of America have reported on the clinical effects of rifaximin. It prevents the occurrence and recurrence of overt HE and decreases the rate of hospitalization because of HE as a long-term effect[6,7]. Also, a study on minimal HE reported that the use of rifaximin 550 mg for 8 wk improved cognitive function test performance as well as white matter integrity, as found on functional magnetic resonance imaging[8]. A meta-analysis that compared rifaximin with synthesized disaccharides reported that both were effective to the same extent in improving HE[9]. A randomized, double-blind controlled trial that compared the results of treatment with rifaximin plus lactulose therapy with lactulose therapy alone showed that significantly more cases experienced a complete recovery from HE in the rifaximin plus lactulose group than that in the lactulose only group. Moreover, rifaximin plus lactulose decreased the mortality rate and death from sepsis, and shortened hospitalization[10]. Various factors influence the development of HE[11], and spontaneous portosystemic shunts are also related to its occurrence. Moreover, it has been noted that larger the size of the shunt, the higher the possibility for HE to recur[12].
The efficacy of rifaximin therapy for 10 wk has been proved in the Japanese population[13]. However, there are no reports on the relationship between the long-term use of rifaximin and its clinical effects and the size of the shunts. In this retrospective cohort study, we clarified the efficacy and safety of long-term use of rifaximin, and investigated the influence of shunts, Escherichia coli (E. coli)-related infection, and the prognosis in the rifaximin group compared with that in the control group comprising cases of liver cirrhosis matched for magnetic resonance elastography (MRE), age, Child-Pugh classification, and the presence of hepatocellular carcinoma (HCC). We evaluated the risk factors due to which the serum ammonia levels remained above 80 μg/dL at 24 wk after initiating rifaximin treatment.
This was a single-center retrospective observational cohort study. The subjects were 38 consecutive patients with liver cirrhosis who were administered rifaximin continuously for more than 24 wk at our institute between April 2017 and July 2018. The primary outcome evaluated was the efficacy of long-term rifaximin use, and the secondary outcomes were the influences of spontaneous portosystemic shunts and E. coli-related infection. It was reported that liver stiffness measured by MRE was related to the recurrence of HCC, liver decompensation, and overall survival, and we compared the clinical prognosis between the rifaximin and control groups, pair-matched from 451 patients with chronic hepatic disease who were not given rifaximin, and measured the MRE from September 2016 to April 2018 using the covariates for liver stiffness based on MRE, age, Child-Pugh classification, and the presence of HCC.
The mortality rate as well as the incidence of complications of liver cirrhosis is influenced by whether the maximum size of the portosystemic shunt is over 8 mm or under 8 mm[14].
Moreover, the smallest size of a symptomatic portosystemic shunt reported in the literature is 8 mm[15]. Based on these reports, we classified the portosystemic shunts over 8 mm into the large group, and those under 8 mm into the small group. There was no set standard for initiating treatment with rifaximin.
This study was approved by the Ethics Committee of the Southern-Tohoku General Hospital and was conducted according to the guidelines of the 1975 Declaration of Helsinki, as revised in 2008. Informed consent was obtained from all patients for being included in the study.
3.0-T imagers (GE Healthcare, Milwaukee, WI) were used for all MRE examinations, and the condition was set as the report[16-18]. We fused the T2 image and the elastogram and measured the stiffness in 3 regions where the wave image was good and the region of interest (ROI) was set as broad as possible avoiding the liver surface, vessels, tumor, cross-hatching, hot spots, and dark spots.
MRE was measured at the beginning of the rifaximin treatment.
Contrast-enhanced CT images taken during the follow-up of HCC were assessed. The instrument used was the 64-detector MDCT Light Speed VCT (GE Healthcare, UK Ltd.) and the medium was Iomeprol 350 1.8 mL/kg (630 mg I/kg); the time taken for injecting the medium was 30 s, and the range was from the supradiaphragmatic to the subpubic region with 0.5-mm slices in the portal phase. The measurement was assessed with 5-mm slices in the axial dimension, and coronal reconstructed images in 40-mm partial maximum intensity projection (MIP). When a case had a plural system of shunts, the maximum diameter of the largest shunt was measured.
Fisher’s exact test or the Kruskal-Wallis exact test was used to compare categorical variables, and the Mann-Whitney U-test was used to compare median values of continuous variables. Wilcoxon signed-rank test was used to compare paired continuous variables. To evaluate the overall survival in the rifaximin group, the control group was chosen using MRE as the measure of comparison. As the outcome parameters may be influenced by patient selection in the rifaximin group and/or the control group, we performed propensity score matching. A multivariable logistic regression model was used to develop the propensity score, which included the following parameters: MRE, age, Child-Pugh classification, and the presence of HCC. Death was calculated using the Kaplan-Meier method and compared using the log-rank test. Survival duration was measured from the time of index date until either death or the date of the last follow-up visit for patients who remained alive. Statistical comparisons were performed using the IBM SPSS software program (IBM Corp., Armonk, NY, United States) and P–values of < 0.05 by a two-tailed test were considered statistically significant.
The background of the patients is summarized in Table 1. Rifaximin treatment was given to 38 patients with decompensated liver cirrhosis. Of these, 12 patients (31.6%) had a history of overt HE, 27 patients (71.1%) had complications associated with esophageal varices, and 9 patients (23.7%) had HCC. In 27 cases (71.1%), rifaximin was added to lactulose, and in 24 cases, branched-chain amino acid supplementation was used in combination. We compared the control group matched for age, Child-Pugh classification, liver stiffness measured by MRE, and the presence of HCC with the rifaximin group.
| Rifaximin (n = 38) | Control (n = 38) | P value | |
| Age (yr) | 69 (40-85) | 69.5 (42-90) | 0.934 |
| Sex (female/male) | 17 (44.7%)/21 (55.3%) | 11 (28.9%)/27 (71.1%) | 0.234 |
| BMI (kg/m2) | 24.78(18.67-35.88) | 23.1 (15.5-30.1) | 0.035 |
| Etiology (HBV/HCV/Alcohol/NASH) | 7 (18.4%)/5 (13.2%)/13 (34.2%)/5 (13.2%) | 4 (10.5%)/14 (36.8%)/9 (23.7%)/3 (7.9%) | 0.107 |
| Child-Pugh A/B/C | 15 (39.5%)/15 (39.5%)/8 (21.1%) | 20 (52.6%)/ 17 (44.7%)/1 (2.6%) | 0.051 |
| ALBI Grade | 1(2.6%) /27 (71.1%) /10 (26.3%) | 22 (57.9%)/15 (39.5%)/1(2.6%) | < 0.001 |
| MELD Score1 | 6 (2-17) | 9 (0-22) | 0.205 |
| ALT (IU/L)1 | 24.5 (8.0-105.0) | 31.5 (8.0-1490) | 0.109 |
| AST (IU/L)1 | 39.5 (20.0-136.0) | 45 (15-1041) | 0.426 |
| GGTP (IU/L)1 | 35.5 (11.0-558.0) | 64 (14-506) | 0.055 |
| Serum ammonia | 104 (59-297) | 52 (23-89) | < 0.001 |
| Albumin (g/dL)1 | 3.0 (2.1-4.4) | 4.05 (2.4-5.0) | < 0.001 |
| Total bilirubin (mg/dL)1 | 1.65 (0.4-4.7) | 1.0 (0.4-8.6) | 0.002 |
| Platelet count (×103/μL)1 | 8.7 (4.8-27.9) | 12.3 (2.3-30.2) | 0.023 |
| Prothrombin activity (%) | 60 (38-133) | 69 (14-108) | 0.066 |
| AFP (ng/mL)1 | 4.3 (1.0-3375) | 6.25 (1.3-385.5) | 0.065 |
| DCP (mAU/mL)1 | 37 (15-3178) | 25 (11-11688) | 0.383 |
| Fibrosis 4 index1 | 5.61 (1.71-15.1) | 4.25 (1.2-33.4) | 0.117 |
| MRE (kPa)1 | 5.04 (3.00-11.11) | 4.86 (3.36-12.50) | 0.748 |
| SWE (kPa)1 | 23.1 (8.3-56) | 16.8 (7.0-36.8) | < 0.001 |
| History of overt hepatic encephalopathy | 12 (31.6%) | 0 (0%) | < 0.001 |
| HCC | 9 (23.7%) | 12 (31.6%) | 0.442 |
| Ascites | 11 (29.0%) | 10 (26.3%) | 0.834 |
| Esophageal varices | 27 (71.1%) | 23 (60.5%) | 0.469 |
| Max diameter of portosystemic shunts (mm) | 7.6 (1.7-18.5) | 4.2 (1.6-10.9) | < 0.001 |
| Number of portosystemic shunts (0,1/2-) | 8 (22.2%)/28 (77.8%) | 24 (63.2%)/14 (36.8%) | 0.001 |
| Lactulose | 29 (76.3%) | 6 (15.8%) | < 0.001 |
| BCAA | 24 (63.2%) | 14 (36.8%) | 0.038 |
| Sarcopenia | 17 (44.7%) | 17 (44.7%) | 1 |
| Follow up period (wk)1 | 61 (24-91) | 91 (5-120) | < 0.001 |
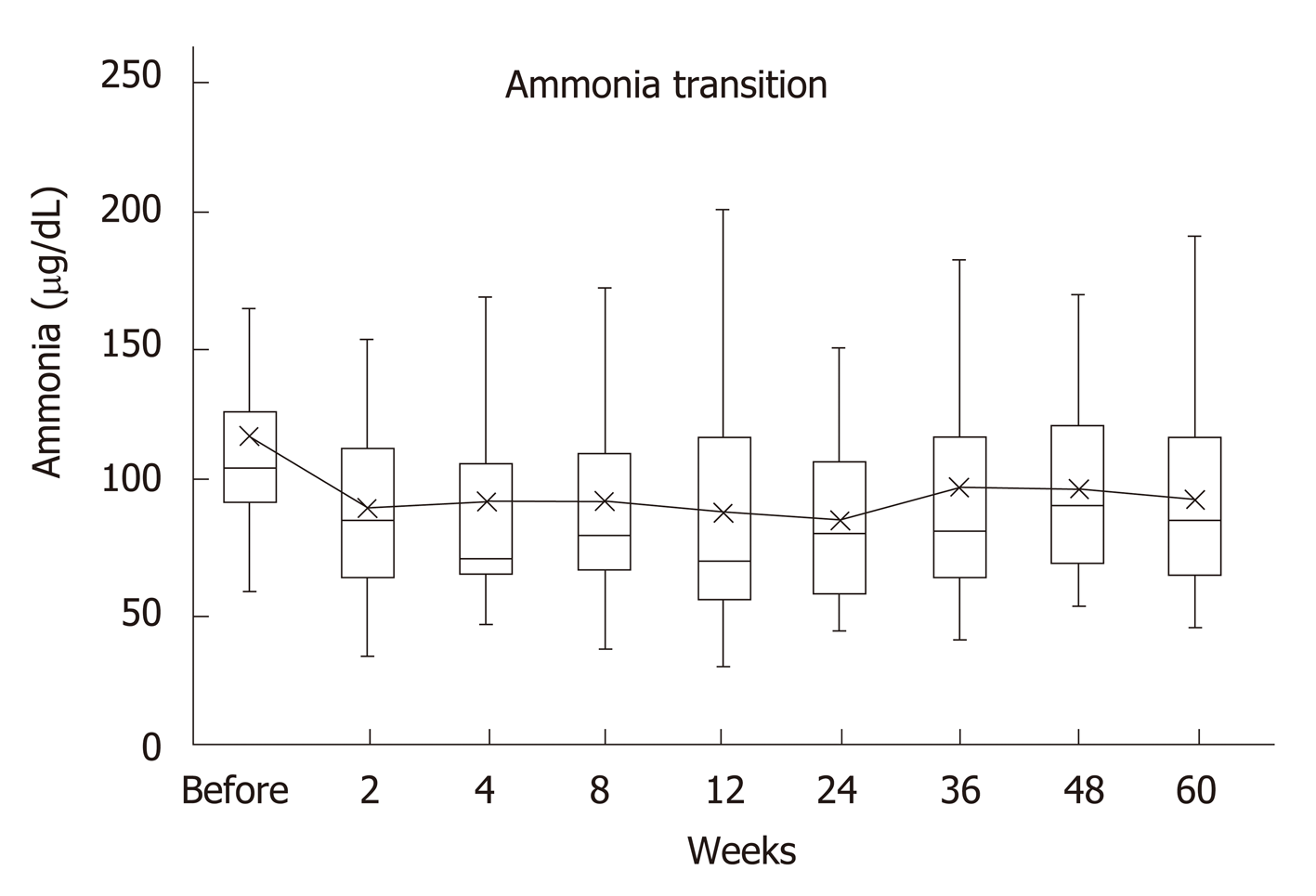
Serum ammonia levels were significantly decreased 2 wk after the beginning of rifaximin compared to the pretreatment levels (P = 0.002), and it remained significantly lower for up to 60 wk. Decrease was noted at all the points after the start of the treatment when compared with the ones before it.
There were 3 recurrence cases of overt encephalopathy: 2 occurred during drainage of the hepatic hydrothorax and 1 followed the self-interruption of rifaximin therapy.
There were no cases of spontaneous bacterial peritonitis (SBP) during the process (Figure 1).
There were no significant differences in the blood albumin level, total bilirubin, prothrombin time, and platelet count 24 wk after the treatment. Rifaximin did not worsen the functionality of the liver. Adverse events included 2 cases of diarrhea (5.3%), which improved following the intake of probiotics. There were no cases of treatment interruption due to adverse events.
There were 2 cases without any shunts (5.3%), 8 cases with one systemic shunt (21.1%), 15 cases (39.5%) with two, 12 cases (31.6%) with three, and 1 case (2.6%) with four systemic shunts.
There were 17 (44.7%) cases with shunts over 8 mm who had a median serum ammonia value of 112 (93-297) μg/dL before rifaximin treatment, which decreased to 86 (54-150) μg/dL after 24 wk, with a significant difference (P = 0.002). On the other hand, there were 19 cases (50%) with shunts below 8 mm whose median serum ammonia value was 99 (59-184) μg/dL before the treatment and 67 (44-148) μg/dL after the treatment, with a significant difference (P = 0.037).
There were 28 cases (73.7%) with multiple shunts with a median serum ammonia value of 104 (69-297) μg/dL before treatment, which decreased to 80.5 (44-150) μg/dL after 24 wk, with a significant difference (P < 0.001).
There were 10 cases (26.3%) with no or only one shunt with a median serum ammonia value of 111.5 (59-184) μg/dL before treatment, which decreased to 81 (45-148) μg/dL after 24 wk (P = 0.375).
Univariate analyses revealed that the insufficient improvement of serum ammonia level (below 80 μg/dL at 24 wk) was associated with two variables: maximum shunt diameter and the albumin-bilirubin (ALBI) score. These factors were evaluated in the multivariate analyses, which revealed that the insufficient improvement in the serum ammonia level was independently associated with the maximum shunt diameter of ≥ 8.0 mm (risk ratio = 5.52, P = 0.040) (Table 2).
| Category | HR (95%CI) | P value |
| Univariate analysis | ||
| Age (yr at MRE) | ||
| < 70 | 1 | |
| ≥ 70 | 2.2 (0.56-8.7) | 0.26 |
| Sex | ||
| female | 1 | |
| male | 0.491 (0.128-1.88) | 0.30 |
| BMI (kg/m2) | ||
| < 28 | 1 | |
| ≥ 28 | 0.42 (0.094-1.85) | 0.25 |
| AST (IU/L) | ||
| < 36 | 1 | |
| ≥ 36 | 0.372 (0.094-1.47) | 0.16 |
| ALT (IU/L) | ||
| < 30 | 1 | |
| ≥ 30 | 0.43 (0.11-1.7) | 0.23 |
| GGTP (IU/L)a | ||
| < 42 | 1 | |
| ≥ 42 | 0.71 (0.18-2.87) | 0.64 |
| Albumin (g/dL) | ||
| ≥ 3.0 | 1 | |
| < 3.0 | 3.3 (0.83-13) | 0.091 |
| Total bilirubin (mg/dL) | ||
| < 1.4 | 1 | |
| ≥ 1.4 | 3.0 (0.76-12) | 0.12 |
| Platelet count (×103/μL) | ||
| ≥ 6.8 | 1 | |
| < 6.8 | 4.1 (0.67-25) | 0.13 |
| Prothrombin activity (%) | ||
| < 58 | 1 | 0.13 |
| ≥ 58 | 3.0 (0.72-13) | |
| AFP (ng/mL) | ||
| < 6.5 | 1 | |
| ≥ 6.5 | 3.25 (0.55-19) | 0.20 |
| DCP (mAU/mL) | ||
| < 18 | 1 | |
| ≥ 18 | 3.6 (0.57-23) | 0.17 |
| Ascites | ||
| Absent | 1 | 0.16 |
| Present | 2.5 (0.70-8.8) | |
| Esophageal varices | ||
| Absent | 1 | 0.42 |
| Present | 1.8 (0.43-7.5) | |
| History of hepatic encephalopathy | ||
| Absent | 1 | 0.11 |
| Present | 3.6 (0.77-16) | |
| HCC1 | ||
| Absent | 1 | 0.72 |
| Present | 0.75 (0.16-3.6) | |
| Sarcopenia | ||
| Absent | 1 | 0.49 |
| Present | 1.64 (0.41-6.6) | |
| Child-Pugh Score | 0.061 | |
| < 7 | 1 | |
| ≥ 7 | 3.9 (0.94-16) | |
| ALBI Score | ||
| < -1.6 | 1 | |
| ≥ -1.6 | 5.3 (1.1-25) | 0.033 |
| MELD Score | ||
| < 12 | 1 | 0.13 |
| ≥ 12 | 3.0 (0.72-13) | |
| Fibrosis 4 index | ||
| < 8.3 | 1 | |
| ≥ 8.3 | 0.42 (0.094-1.9) | 0.25 |
| MRE (kPa) | ||
| < 5.0 | 1 | 0.43 |
| ≥ 5.0 | 1.63 (0.40-8.3) | |
| SWE (kPa) | ||
| < 17.7 | 1 | |
| ≥ 17.7 | 3.0 (0.65-14) | 0.16 |
| Max diameter of portosystemic shunt | ||
| < 8.0 | 1 | |
| ≥ 8.0 | 6.8 (1.4-33) | 0.017 |
| Number of portosystemic shunt systems | ||
| 0,1 | 1 | 0.68 |
| 2-4 | 1.36 (0.32-5.9) | |
| Multivariate analysis | ||
| Max diameter of portosystemic shunt | ||
| < 8.0 | 1 | |
| ≥ 8.0 | 5.5 (1.1-28) | 0.039 |
| ALBI Score | 0.16 | |
| < -1.6 | 1 | |
| ≥ -1.6 | 3.3 (0.62-18) |
The cutoff values for predicting the insufficient improvement in ammonia levels after rifaximin treatment were determined by receiver operator characteristics analysis.
The median follow-up period was 62 wk for the Rifaximin group and 91 wk for the control group.
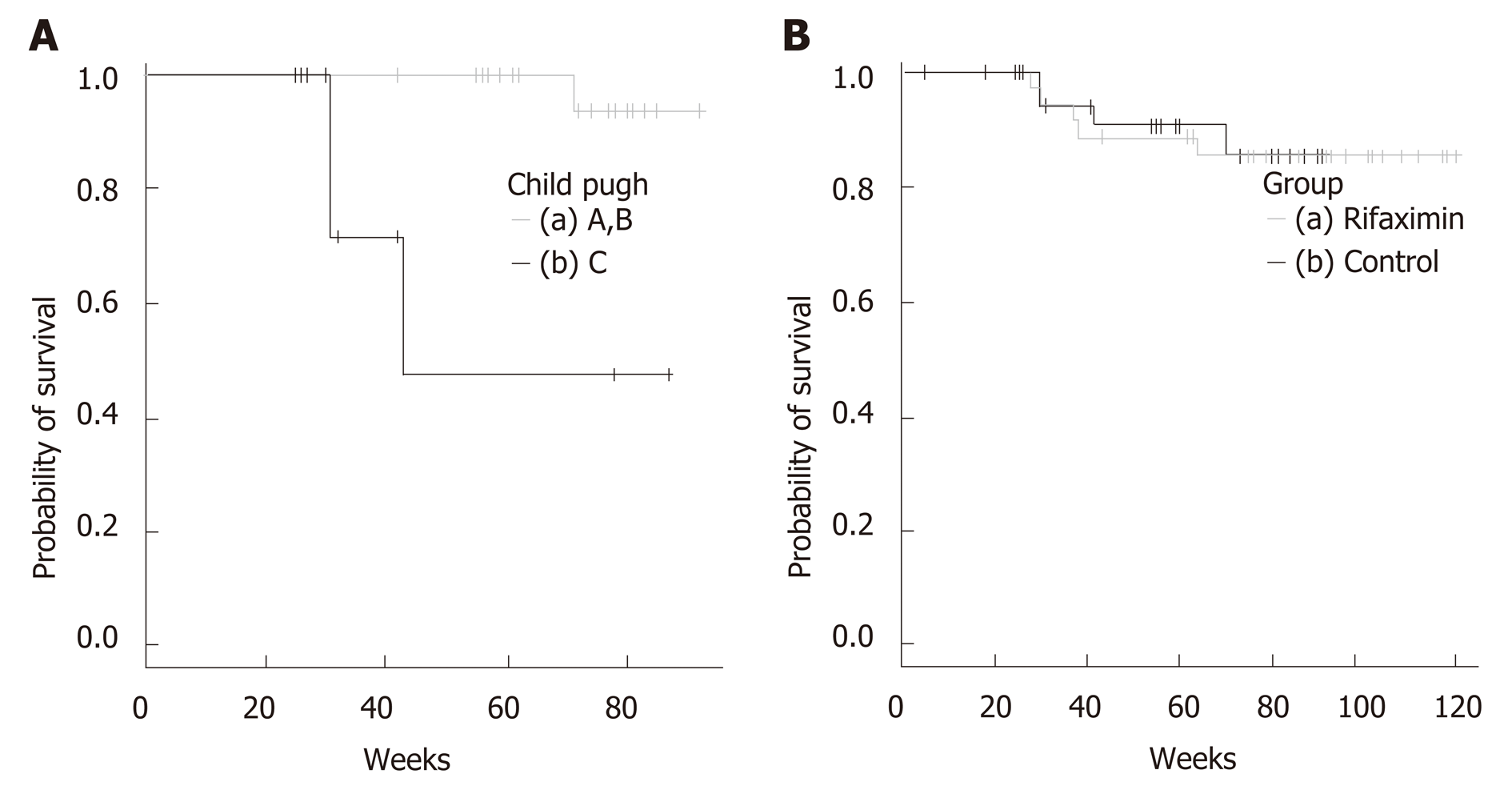
There were 4 deaths in the observation period: 2 cases of liver failure caused by pleurodesis for pleural effusion and 2 cases of liver failure brought about by worsened cirrhosis. When classifying according to Child-Pugh, the survival time span of Child C was significantly shortened. (Figure 2A)
There were 5 deaths in the control group. Hepatic insufficiency due to worsening of the HCC was the cause of death in 2 patients. Disseminated intravascular coagulation due to thoracic empyema by intestinal bacteria was the cause of death in 1 patient and cardiovascular disease was the cause of death in 2 patients.
Altogether, there were no significant differences when we compared the rifaximin group with the control group (P = 0.897) (Figure 2B).
HE is one of the major complications of liver cirrhosis, and in Japan, rifaximin has been available for treating HE since November 2016. This study assessed the efficacy and safety of long-term rifaximin use in the Japanese population. In addition, we also investigated the differences in the kind of portosystemic shunts and the infections related to E. coli. The serum ammonia level significantly decreased 2 wk after initiating treatment compared to the pretreatment level, and the decrease was maintained for as long as 60 wk. Moreover, there was recurrence of overt HE in cases of drainage of hepatic pleural effusion and self-cessation of rifaximin therapy, but not in other cases. This suggests that the long-term use of rifaximin has good efficacy and rifaximin can be used continuously for a long time without discontinuation.
As to adverse events, 2 cases (5.3%) had diarrhea which improved with probiotics, and there were no cases of cessation of rifaximin therapy due to side effects. The efficacy and safety of long-term use of rifaximin has been proved in the West, but there has been no reported evidence in Japan thus far. Therefore, the result of this study, that the long-term use of rifaximin is effective and safe, is important.
HE is said to be precipitated by events such as infections and enteric bleeding. In this study, there was recurrence of overt HE in 3 cases, 2 of which occurred on draining the hepatic pleural effusion; it was considered that the recurrences occurred due to dehydration and poor general health conditions, including infections and intestinal bleeding. West Haven criteria is used to assess HE[19]. However the West Haven test is not sufficient to identify subclinical encephalopathy and the Trail Making Test was more useful to detect minimal encephalopathy[20]. In this retrospective study, we could not evaluate the Trail Making Test.
It was reported that the prevalence of large portosystemic shunts was 70% among patients suffering from chronic HE and 14% among the control group. In other words, there is a strong association between HE and the development of portosystemic shunts. One previous study reported that the grade of portosystemic collateral veins was concordant with ammonia levels[21]. This finding was consistent with our results. There have been no studies to date investigating the relationship between portosystemic shunts and rifaximin therapy. This study revealed that rifaximin could improve the elevated serum ammonia levels and HE of patients who had large portosystemic shunts as well as that of those who had not developed shunts. It is expected that rifaximin can be a useful treatment for HE. In this study, some patients did not show a decrease in their serum ammonia levels to below 80 μg/dL and multivariate analyses revealed that this was independently associated with a maximum shunt diameter of ≥ 8.0 mm. This result suggests that although rifaximin improved overt HE, minimal HE may remain in cases with large shunts and that the grade of portosystemic shunts affected the serum level of ammonia as stated in the previous report[21]. Interventional radiology or surgical treatment should be considered for those patients who do not show adequate improvement of HE on rifaximin therapy.
It has been suggested that in liver cirrhosis patients, the function of the gut microbiome is altered, and advanced liver disease has a significantly decreased the cirrhosis dysbiosis ratio (CDR); altered gut microbiota was observed in patients with severe liver cirrhosis. It was reported that the mortality was highest in these patients[22].
There were no SBP cases reported in both rifaximin and control groups in this study, but one patient died of thoracic empyema caused by intestinal bacteria.
This suggests that rifaximin suppressed E. coli-related infections as previously reported[23].
Also, no significant difference was recognized between the rifaximin group and the control group in the survival rates. Among the rifaximin group, the survival rate was significantly different based on the Child-Pugh classification, and especially cases of Child-Pugh C group had the shortest survival. We can therefore infer that rifaximin cannot treat liver cirrhosis itself, and that is the limitation of this treatment. Previous report indicated that spleno-renal shunts are burdened by an increased incidence of HCC[24]. We also need to be aware of the existence of HCC.
One of the limitations of this study was that we did not examine each test to diagnose minimal HE, and the improvement of minimal HE cannot be proved. Also, there was no set standard for initiating rifaximin treatment; it was started based on the judgement of the attending physicians. This was a retrospective study of a few cases in a single institution, and it is necessary to evaluate a larger number of cases in a multiple-center study to assess survival. It is also necessary to consider HCC, infections, malnutrition, and cardiovascular disease, etc. as causes of death in patients with liver cirrhosis.
In conclusion, the long-term use of rifaximin was found to be safe and effective in the Japanese population.
Hepatic encephalopathy (HE) is a complication of liver cirrhosis. Rifaximin, an antibiotic, has been reported to decrease the occurrence of overt HE and improve cognitive function in studies from Europe and the United States of America. There is not enough evidence of the relationship between the long-term use of rifaximin and its clinical effects in the Japanese.
To determine the clinical effects of long-term rifaximin therapy in decompensated liver cirrhosis patients, with overt HE or hyperammonemia. We evaluated the relationship between the long-term use of rifaximin and its clinical effects and the size of the shunts.
In this single-center retrospective observational cohort study, we reviewed the data of 38 patients who had taken rifaximin at the dose of 1200 mg/d for more than 24 wk. The primary outcome measured was the efficacy of long-term rifaximin use, and the secondary outcome measured was the safety of its long-term use as determined by its influence on portosystemic shunts.
Rifaximin did not worsen the functionality of the liver 24 wk after the treatment. Adverse events included 2 cases of diarrhea (5.3%), which improved following the intake of probiotics. There were no cases of treatment interruption due to adverse events. Serum ammonia levels were significantly decreased 2 wk after the beginning of rifaximin compared to the pretreatment levels (P = 0.002), and it remained significantly lower for up to 60 wk. Multivariate analyses revealed that the insufficient improvement in the serum ammonia level was independently associated with the maximum shunt diameter of ≥ 8.0 mm (risk ratio = 5.52, P = 0.040).
The long-term use of rifaximin was found to be safe and effective in the Japanese population.
In this retrospective study, we did not examine each test to diagnose minimal HE, and the improvement of minimal HE cannot be proved. Tests as the Trail Making Test should be evaluated to minimal HE in the future investigation.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and Hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tarantino G, Tzamaloukas AH S-Editor: Cui LJ L-Editor: A E-Editor: Zhang YL
| 1. | Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, Wong P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 1393] [Article Influence: 126.6] [Reference Citation Analysis (1)] |
| 2. | Hadjihambi A, Arias N, Sheikh M, Jalan R. Hepatic encephalopathy: a critical current review. Hepatol Int. 2018;12:135-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 125] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 3. | Patidar KR, Thacker LR, Wade JB, Sterling RK, Sanyal AJ, Siddiqui MS, Matherly SC, Stravitz RT, Puri P, Luketic VA, Fuchs M, White MB, Noble NA, Unser AB, Gilles H, Heuman DM, Bajaj JS. Covert hepatic encephalopathy is independently associated with poor survival and increased risk of hospitalization. Am J Gastroenterol. 2014;109:1757-1763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 4. | Scarpignato C, Pelosini I. Rifaximin, a poorly absorbed antibiotic: pharmacology and clinical potential. Chemotherapy. 2005;51 Suppl 1:36-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 164] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 5. | Phongsamran PV, Kim JW, Cupo Abbott J, Rosenblatt A. Pharmacotherapy for hepatic encephalopathy. Drugs. 2010;70:1131-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Mullen KD, Sanyal AJ, Bass NM, Poordad FF, Sheikh MY, Frederick RT, Bortey E, Forbes WP. Rifaximin is safe and well tolerated for long-term maintenance of remission from overt hepatic encephalopathy. Clin Gastroenterol Hepatol. 2014;12:1390-1397.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 150] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 7. | Bass NM, Mullen KD, Sanyal A, Poordad F, Neff G, Leevy CB, Sigal S, Sheikh MY, Beavers K, Frederick T, Teperman L, Hillebrand D, Huang S, Merchant K, Shaw A, Bortey E, Forbes WP. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 862] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 8. | Ahluwalia V, Wade JB, Heuman DM, Hammeke TA, Sanyal AJ, Sterling RK, Stravitz RT, Luketic V, Siddiqui MS, Puri P, Fuchs M, Lennon MJ, Kraft KA, Gilles H, White MB, Noble NA, Bajaj JS. Enhancement of functional connectivity, working memory and inhibitory control on multi-modal brain MR imaging with Rifaximin in Cirrhosis: implications for the gut-liver-brain axis. Metab Brain Dis. 2014;29:1017-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 9. | Jiang Q, Jiang XH, Zheng MH, Jiang LM, Chen YP, Wang L. Rifaximin versus nonabsorbable disaccharides in the management of hepatic encephalopathy: a meta-analysis. Eur J Gastroenterol Hepatol. 2008;20:1064-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 10. | Sharma BC, Sharma P, Lunia MK, Srivastava S, Goyal R, Sarin SK. A randomized, double-blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. Am J Gastroenterol. 2013;108:1458-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 229] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 11. | Bhanji RA, Moctezuma-Velazquez C, Duarte-Rojo A, Ebadi M, Ghosh S, Rose C, Montano-Loza AJ. Myosteatosis and sarcopenia are associated with hepatic encephalopathy in patients with cirrhosis. Hepatol Int. 2018;12:377-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 148] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 12. | Riggio O, Efrati C, Catalano C, Pediconi F, Mecarelli O, Accornero N, Nicolao F, Angeloni S, Masini A, Ridola L, Attili AF, Merli M. High prevalence of spontaneous portal-systemic shunts in persistent hepatic encephalopathy: a case-control study. Hepatology. 2005;42:1158-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 131] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 13. | Suzuki K, Endo R, Takikawa Y, Moriyasu F, Aoyagi Y, Moriwaki H, Terai S, Sakaida I, Sakai Y, Nishiguchi S, Ishikawa T, Takagi H, Naganuma A, Genda T, Ichida T, Takaguchi K, Miyazawa K, Okita K. Efficacy and safety of rifaximin in Japanese patients with hepatic encephalopathy: A phase II/III, multicenter, randomized, evaluator-blinded, active-controlled trial and a phase III, multicenter, open trial. Hepatol Res. 2018;48:411-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Simón-Talero M, Roccarina D, Martínez J, Lampichler K, Baiges A, Low G, Llop E, Praktiknjo M, Maurer MH, Zipprich A, Triolo M, Vangrinsven G, Garcia-Martinez R, Dam A, Majumdar A, Picón C, Toth D, Darnell A, Abraldes JG, Lopez M, Kukuk G, Krag A, Bañares R, Laleman W, La Mura V, Ripoll C, Berzigotti A, Trebicka J, Calleja JL, Tandon P, Hernandez-Gea V, Reiberger T, Albillos A, Tsochatzis EA, Augustin S, Genescà J; Baveno VI-SPSS group from the Baveno Cooperation. Association Between Portosystemic Shunts and Increased Complications and Mortality in Patients With Cirrhosis. Gastroenterology. 2018;154:1694-1705.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 160] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 15. | Sakurabayashi S, Sezai S, Yamamoto Y, Hirano M, Oka H. Embolization of portal-systemic shunts in cirrhotic patients with chronic recurrent hepatic encephalopathy. Cardiovasc Intervent Radiol. 1997;20:120-124. [PubMed] |
| 16. | Loomba R, Wolfson T, Ang B, Hooker J, Behling C, Peterson M, Valasek M, Lin G, Brenner D, Gamst A, Ehman R, Sirlin C. Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: a prospective study. Hepatology. 2014;60:1920-1928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 313] [Cited by in RCA: 384] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 17. | Kim D, Kim WR, Talwalkar JA, Kim HJ, Ehman RL. Advanced fibrosis in nonalcoholic fatty liver disease: noninvasive assessment with MR elastography. Radiology. 2013;268:411-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 183] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 18. | Venkatesh SK, Yin M, Ehman RL. Magnetic resonance elastography of liver: technique, analysis, and clinical applications. J Magn Reson Imaging. 2013;37:544-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 497] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 19. | Conn HO, Liberathal MM. The hepatic coma syndromes and lactulose. Williams and Wilkins. 1979;1-121. [DOI] [Full Text] |
| 20. | Citro V, Milan G, Tripodi FS, Gennari A, Sorrentino P, Gallotta G, Postiglione A, Tarantino G. Mental status impairment in patients with West Haven grade zero hepatic encephalopathy: the role of HCV infection. J Gastroenterol. 2007;42:79-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Tarantino G, Citro V, Esposito P, Giaquinto S, de Leone A, Milan G, Tripodi FS, Cirillo M, Lobello R. Blood ammonia levels in liver cirrhosis: a clue for the presence of portosystemic collateral veins. BMC Gastroenterol. 2009;9:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, White MB, Monteith P, Noble NA, Unser AB, Daita K, Fisher AR, Sikaroodi M, Gillevet PM. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. 2014;60:940-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 829] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 23. | Goel A, Rahim U, Nguyen LH, Stave C, Nguyen MH. Systematic review with meta-analysis: rifaximin for the prophylaxis of spontaneous bacterial peritonitis. Aliment Pharmacol Ther. 2017;46:1029-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 24. | Tarantino G, Citro V, Conca P, Riccio A, Tarantino M, Capone D, Cirillo M, Lobello R, Iaccarino V. What are the implications of the spontaneous spleno-renal shunts in liver cirrhosis? BMC Gastroenterol. 2009;9:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |