Published online May 27, 2019. doi: 10.4254/wjh.v11.i5.450
Peer-review started: March 12, 2019
First decision: March 25, 2019
Revised: April 19, 2019
Accepted: April 26, 2019
Article in press: April 27, 2019
Published online: May 27, 2019
Processing time: 79 Days and 15.7 Hours
Progressive familial intrahepatic cholestasis (PFIC) refers to a disparate group of autosomal recessive disorders that are linked by the inability to appropriately form and excrete bile from hepatocytes, resulting in a hepatocellular form of cholestasis. While the diagnosis of such disorders had historically been based on pattern recognition of unremitting cholestasis without other identified molecular or anatomic cause, recent scientific advancements have uncovered multiple specific responsible proteins. The variety of identified defects has resulted in an ever-broadening phenotypic spectrum, ranging from traditional benign recurrent jaundice to progressive cholestasis and end-stage liver disease.
To review current data on defects in bile acid homeostasis, explore the expanding knowledge base of genetic based diseases in this field, and report disease characteristics and management.
We conducted a systemic review according to PRISMA guidelines. We performed a Medline/PubMed search in February-March 2019 for relevant articles relating to the understanding, diagnosis, and management of bile acid homeostasis with a focus on the family of diseases collectively known as PFIC. English only articles were accessed in full. The manual search included references of retrieved articles. We extracted data on disease characteristics, associations with other diseases, and treatment. Data was summarized and presented in text, figure, and table format.
Genetic-based liver disease resulting in the inability to properly form and secrete bile constitute an important cause of morbidity and mortality in children and increasingly in adults. A growing number of PFIC have been described based on an expanded understanding of biliary transport mechanism defects and the development of a common phenotype.
We present a summary of current advances made in a number of areas relevant to both the classically described FIC1 (ATP8B1), BSEP (ABCB11), and MDR3 (ABCB4) transporter deficiencies, as well as more recently described gene mutations -- TJP2 (TJP2), FXR (NR1H4), MYO5B (MYO5B), and others which expand the etiology and understanding of PFIC-related cholestatic diseases and bile transport.
Core tip: Progressive familial intrahepatic cholestasis is a heterogeneous cohort of diseases that present both diagnostic and treatment challenges for clinicians. Significant advancement in the knowledge base related to the genetic underpinnings regulating bile acid transport physiology has enabled new diseases to be identified with a breadth of phenotypes from neonates to adults.
- Citation: Henkel SA, Squires JH, Ayers M, Ganoza A, Mckiernan P, Squires JE. Expanding etiology of progressive familial intrahepatic cholestasis. World J Hepatol 2019; 11(5): 450-463
- URL: https://www.wjgnet.com/1948-5182/full/v11/i5/450.htm
- DOI: https://dx.doi.org/10.4254/wjh.v11.i5.450
Progressive familial intrahepatic cholestasis (PFIC) refers to a heterogeneous group of autosomal recessive disorders that are linked by the inability to appropriately form and excrete bile from hepatocytes, resulting in a hepatocellular form of cholestasis. While the diagnosis of such disorders had historically been based on pattern recognition of unremitting cholestasis without other identified molecular or anatomic cause, recent scientific advancements have uncovered multiple specific responsible proteins. The variety of identified defects has resulted in an ever-broadening phenotypic spectrum, ranging from traditional benign recurrent jaundice to progressive cholestasis and end-stage liver disease.
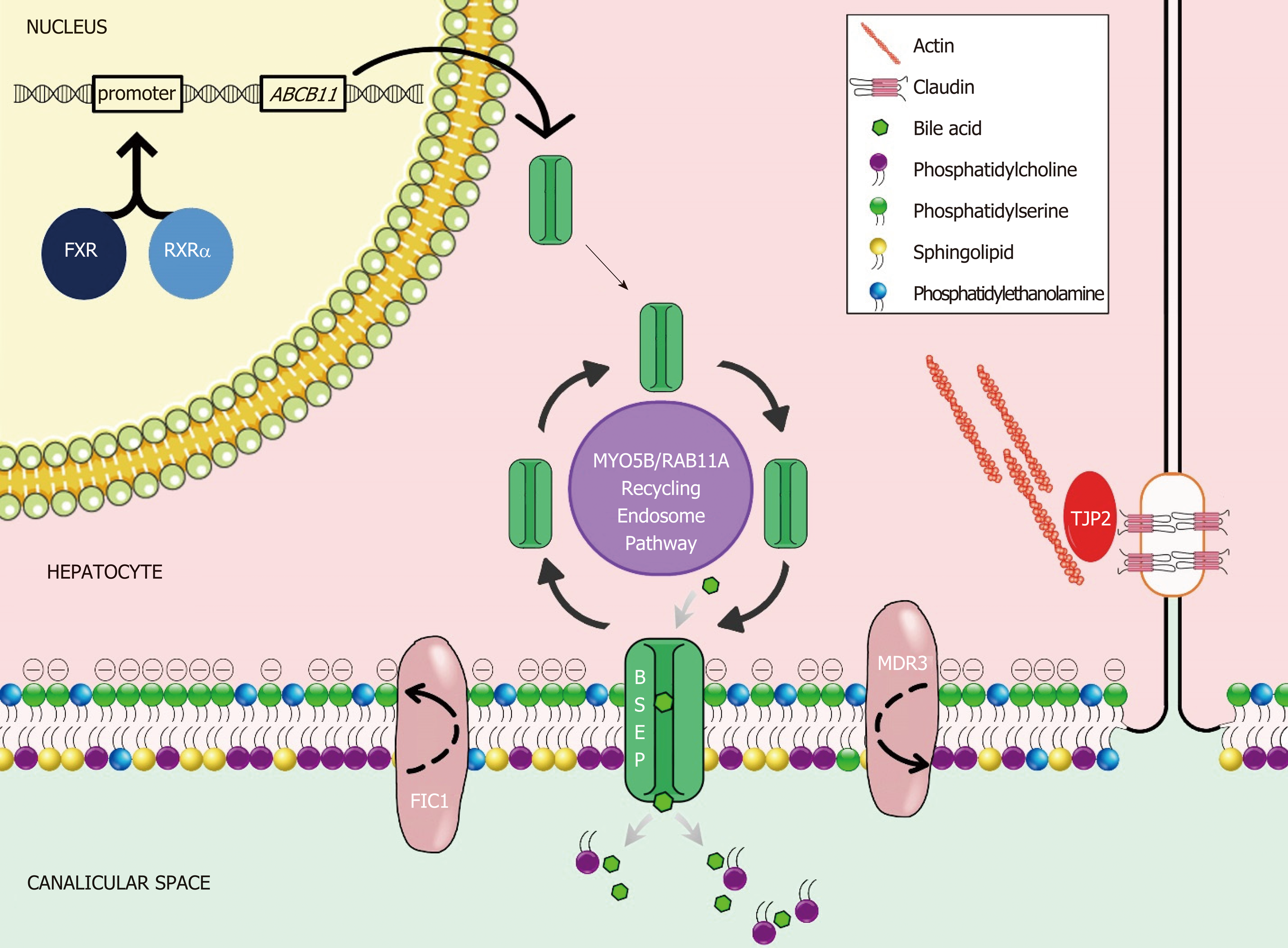
Bile is a unique aqueous secretion of the liver that is formed by the hepatocyte and modified downstream by absorptive and secretory properties of the bile duct epithelium. It is a combination of lipids (mainly phosphatidylcholine), bile acids, cholesterol, bilirubin, and other substances that serve to move toxins and waste metabolites out of the liver and into the gut for excretion[1]. Micellarized bile is then reabsorbed in the enterohepatic circulation in the distal small bowel via the apical sodium dependent bile transporter (ASBT; SLC10A)[2]. Bile salts are synthesized in hepatocytes and transported across the canalicular membrane via the bile salt export pump (BSEP); the expression and trafficking of which is regulated by the farnesoid X receptor (FXR) and dependent upon of MYO5B respectively[3,4]. The stability of the canalicular membrane, in which the BSEP transporter lies, is dependent on the FIC1 ATPase that regulates the phospholipid balance and the ABC translocase MDR3 which moves phosphatidylcholine across the canalicular membrane to inactivate bile acids. The integrity of the system is in part dependent upon hepatocyte connections, such as the TJP2-anchored tight junctions, which protect hepatocytes from bile salt reflux and subsequent damage[4] (Figure 1). Defects in these bile acid transport processes result in the accumulation of bile salts in the hepatic parenchyma, which are toxic due to their detergent nature, and the phenotypic manifestations collectively known as PFIC.
This systematic review was conducted according to the PRISMA guidelines. We searched Medline/PubMed in February–March 2019 for established cases of PFIC as well as reports of defects in PFIC-related genes contributing to morbidity in adult populations. English language only articles that were fully accessible were included in the review. Data was manually extracted on disease characteristics in established PFIC patients. Associated phenotypes with other diseases relating to specific genetic defects were also collected. Treatment strategies were summarized. Data was collated and presented in text, figure, and table format.
Descriptive statistics were utilized to present the data. The statistical methods of this study were reviewed by Suraj Nepal, lead data analyst from the UPMC Children’s Hospital of Pittsburgh department of surgery.
A summary of currently understood protein mechanisms, whose functions are critical to bile acid homeostasis, and whose dysfunction results in a phenotype of PFIC is presented in Figure 1. A gene-specific search identified 52 ATP8B1, 158 ABCB11, 250 ABCB4, 56 TJP2, 48 MYO5B, and 363 NR1H4 articles. Manual review to identify association with liver disease in humans revealed reports summarized in the current manuscript. The three “Historical” PFIC diseases, the expanded phenotypes, and emerging data on contributing morbidity in non-pediatric populations relating to defects in PFIC-related genes are summarized.
ATP8B1 (FIC1, PFIC1, Byler’s disease): The first reported PFIC, progressive familial intrahepatic cholestasis type 1, also called Byler’s disease, was described 1969 in seven Amish children (from the original Byler kindred in Western Pennsylvania) as a progressive cholestatic disease with associated extrahepatic symptoms[5]. The causative ATP8B1 gene and corresponding FIC1 protein was identified by Bull et al[6] in 1998 by analyzing the genetics of patients from the initial Amish cohort as well as patients from Northern Europe with benign recurrent intrahepatic cholestasis type 1 (BRIC1). Definitive FIC1 function remains ambiguous. Current understanding of its action as an aminophospholipid translocase which transports phospholipids from outside to inside the canalicular membrane is based on studies in Atp8b1-deficient mice[7]. Additional modifiers of disease phenotype, such as mutation-specific effects on FIC1 trafficking from the endoplasmic reticulum to the canalicular membrane, have been proposed[8]. Ultimately, without appropriate concentrations of intracellular phospholipids, bile acids accumulate intracellularly and are cytotoxic to the hepatocyte due to their detergent nature[9].
Deficient or defective FIC1 results in a low gamma glutamyl (GGT) cholestasis that often presents in the neonatal period, though milder forms with transient jaundice may present later in life[1,9,10]. Affected individuals have hyperbilirubinemia, mildly elevated transaminases, and elevated serum bile acids. Infants often present jaundiced, with pruritis and hepatosplenomegaly developing over the first months of life. Severe disease manifests with persistent, progressive cholestasis and the development of portal hypertension often in early childhood. Extrahepatic disease is also notable due to the broad distribution of FIC1, which can clinically distinguish FIC1 deficiency from other forms of intrahepatic cholestasis. Affected children frequently exhibit profound diarrhea, poor growth, short stature, pancreatic insufficiency, elevated sweat chloride, and sensorineural deafness[4,10]. Histopathology demonstrates canalicular cholestasis with biliary plugs, giant cell transformation, ductular paucity, and lobular disarray[11]. Visualized bile is termed as “bland” granular (Byler’s) bile[9,12].
Treatment for FIC1 deficiency, as with all PFIC diseases, is challenging with no definitive medical therapies available. Supportive measures are focused on improving nutritional deficiencies and managing complications of end stage liver disease. Patients should be treated with caloric, fat, and vitamin supplementation, with the majority of fat being medium chain triglycerides[9]. Ursodeoxycholic (UDCA), a hydrophilic bile acid which replaces hydrophobic bile salts and may also induce BSEP and MDR3 expression, can improve pruritis and biochemical markers of cholestasis[9]. Other antipruritic agents (Table 1) such as rifampin and cholestyramine may also be utilized but are often less helpful in FIC1 deficiency[9,10]. Certain CFTR folding correctors have been shown to improve defective trafficking of FIC1 in cell culture [13]; however, studies in human subjects are lacking.
| Medicine | Dose | Mechanism of action |
| Cholestyramine | Initial dose: 2 g BID | Ion exchange resin which acts as BA binder in the intestine |
| (max dose 24 g/d) | Decreased ileal BA absorption, Increased BA excretion (in feces) | |
| Naltrexone | Initial dose: 0.25-5 mg/kg per day | Opioid antagonist |
| (max dose 50 mg/d) | Block the permissive activity on pruritus neuronal signaling | |
| Rifampicin | Initial dose: 5 mg/kg | PXR agonist |
| Induces CYP3A4 | ||
| (max dose 20 mg/kg per day) | Increases metabolism and renal excretion of pruritogenic substances | |
| Antibacterial effect may modify intestinal metabolism of pruritogenic substances | ||
| Sertraline | Initial dose: 1 mg/kg per day | Serotonin reuptake inhibitor |
| (max dose: 4 mg/kg per day) | Proposed mechanism includes increase in central serotonergic tone, which regulates pruritus | |
| Ursodeoxycholic acid | 600 mg/m2 per day | Tertiary BA |
| Increases bile secretion | ||
| Reduces ileal absorption of hydrophilic BAs |
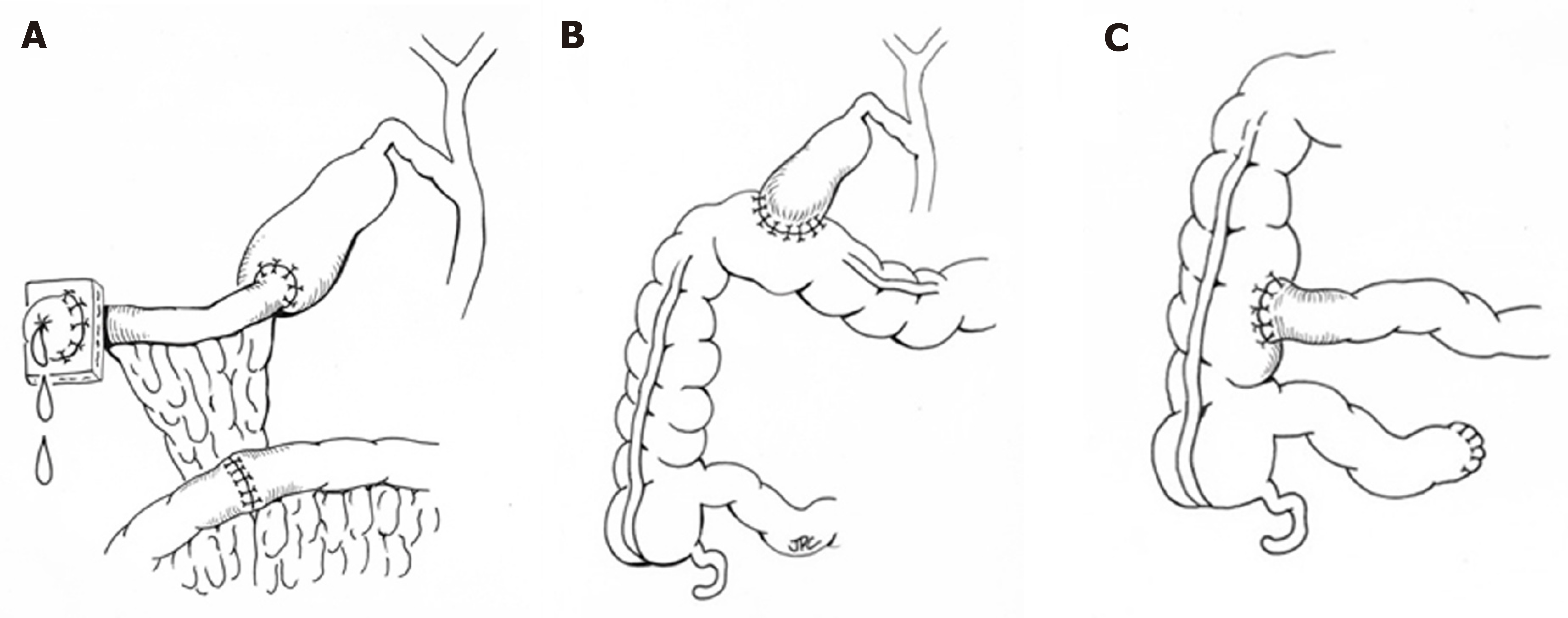
When medical therapy is insufficient, surgical intervention may be considered with the goal of bypassing the enterohepatic circulation and/or decreasing reabsorption of bile salts (Figure 2). Procedures including partial external biliary diversion (PEBD), partial internal biliary diversion, and ileal exclusion have generally, though not uniformly, resulted in sustained clinical improvement in PFIC patients[14-16]. A large surgical experience has been described in FIC1 deficiency[14-16].
Early procedures including PEBD were first reported more than 20 years ago[17]. PEBD utilizes an external stomal conduit (generally a cholecystojejunal cutaneous stoma) to enable partial, unregulated external bile flow, resulting in decreased bile acids in the enterohepatic circulation and reports of improved pruritis, growth, and possibly hepatic fibrosis[14,17]. Remarkably, PEBD has been recognized to provide an alternative to transplant, with many patients surviving with their native liver. However, complications can occur including recurrent episodes of pruritis, possible need for biliary diversion revision, continued need for aggressive vitamin supplementation, or progressive disease necessitating liver transplant[14,18,19].
An alternative to PEBD is the ileal bypass (IB, or ileal exclusion)[20]. This technique bypasses the distal 15% of the ileum to avoid the major site of bile acid reabsorption and is particularly useful in patients without an intact gall bladder[20]. Unfortunately, severe malabsorption can occur and refractory disease has been reported[18,21,22].
More recently, partial internal biliary diversions (PIBD) has been described. The procedure may involve the creation of a neo-conduit between the gall bladder and the colon to prevent reabsorption of bile acids in the terminal ileum. This procedure may utilize a cholecystojejunocolonic, cholecystoileocolonic, cholecystocolostomy, or cholecystoappendicocolonic anastomosis technique[22,23]. Reports in the literature suggest patients experience not only improvement of intractable pruritis and sleeping difficulties, but also significant biochemical decrease in both bilirubin and plasma bile acids[21-23]. Side effects described are most notable for diarrhea, which improved with cholestyramine[21,23]. Notably, no single procedure has demonstrated definitive superiority with center-experience likely driving center-specific approaches. Newer therapeutics including inhibitors of the ileal apical-sodium dependent bile acid transporter (ASBT) which effectively act as a ‘chemical’ biliary diversion are currently under investigation (NCT03566238)[24].
Liver transplant is indicated in those with a refractory course and in those who develop end stage disease. While hepatocellular carcinoma as an indication for transplant has been reported in other PFIC diseases, FIC1 deficiency is not known to associate with tumor development. However, mutations in ATP8B1 have been found while sequencing hepatocellular carcinoma in patients without cholestatic disease[25]. Importantly, patients should be counseled that the diarrhea associated with FIC1 deficiency may persist, or even worsen, following transplant. This phenomenon has been reported concomitant with the development of both allograft steatosis and fibrosis, which can progress requiring re-transplantation[9,26]. In order to prevent damaging steatosis in the graft, ileal diversion at the time of transplant has sometimes been utilized[27].
Notably, the recognition of variable disease courses and responses to therapy in individuals with identical ATP8B1 mutations would suggest the presence of disease modifiers[10,14]. While the majority of FIC1 deficiency presents in childhood, mutations in the ATP8B1 gene may also lead to more mild manifestations of disease including BRIC1 and intrahepatic cholestasis of pregnancy type 1 (ICP1)[28-30]. Dozens of mutations have been described, with missense mutations being more common in BRIC1 patients and nonsense or large deletions more common in severe FIC1 disease[31].
ABCB11 (BSEP, PFIC2): Historical PFIC2 results from defects or deficiency in the BSEP encoded by ABCB11. The location of the defect was initially mapped to chromosome 2q24 to be positional match to BSEP, which had been cloned previously in the mouse genome and was shown soon after to export bile acids[32,33]. This defect results in a severe hepatobiliary phenotype due to impairment of bile salt handling and subsequent damage to hepatocytes[9]. As of this writing, more than 200 causative mutations have been identified[34]. Affected infants initially present jaundiced, with pruritis developing around 4-5 mo of age and often progressing to the development of portal hypertension within the first year of life[9,35,36]. Scleral icterus, hepatomegaly, excoriation of skin, and poor growth due to fat malabsorption and fat-soluble vitamin deficiency may also be apparent due to cholestasis, though extrahepatic symptoms are less significant than in FIC1 deficiency[4,9]. Laboratory findings demonstrate a low GGT cholestasis with transaminases typically more than twice the upper limit of normal[36]. Similar to FIC1 deficiency, treatment is primarily supportive and focuses on nutritional supplementation and antipruritic agents. Zebrafish models of BSEP deficiency suggest a potential role for therapies aimed at promoting alternative transporters to excrete bile[37] while reports in human subjects using cell surface BSEP-enhancer molecules (i.e., 4-phenylbutyrate) alone[38] or as part of a cocktail of medications[39] have shown promise. Both approaches require more complete investigation, which may be facilitated through new disease models using patient-specific induced pluripotent stem cell-derived hepatocyte like cells[40]. Surgical interruption of the enterohepatic circulation may improve pruritis but may not change the course of disease[16]. Notably, the response to diversion has been shown to be dependent on the gene defect, with those who retain some residual protein function having better outcomes than those with mutations resulting in severely dysfunctional or absent protein[41,42]. Pathology typically demonstrates canalicular cholestasis, hepatocellular disarray, and lobular and portal fibrosis[9]. Importantly, there is up to 15% rate of malignancy (hepatocellular carcinoma and cholangio-carcinoma) that has been described in children as young as 13 mo[43]. Therefore, patients with PFIC2 should be screened for malignancy with an alpha-fetoprotein (AFP) level and abdominal ultrasound every 6-12 mo[9]. Liver transplant has been successfully used to treat severe BSEP disease and in those who develop tumor. While organ replacement has historically been considered a ‘’cure’’, patients can develop allo-reactive antibodies specific to the extracellular loop of the BSEP protein resulting in an immune mediated recurrence of their BSEP disease in the allograft[44-46]. Monitoring for disease recurrence is critical as most disease will respond to increased immunosuppression. However, with refractory disease recurrence, more intensive management such as B-cell depleting antibody therapy[47], allogenic hematopoietic stem cell transplant[48], and repeat solid organ transplant[49] may be required.
Similar to ATP8B1, a phenotypic continuum has been recognized with ABCB11 mutations. Transient neonatal cholestasis, benign recurrent intrahepatic cholestasis type 2 (BRIC2), intrahepatic cholestasis of pregnancy type 2 (ICP2), and drug induced cholestasis have all been associated with abnormalities in BSEP[50,51]. Two mutations have been found that prognosticate a modified disease course of BSEP disease: D482G leads to a more slowly progressive disease with the development of cirrhosis at a later age, and E297G results in PFIC2 or BRIC2 that may be more responsive to medical therapy[4,9]. Drug induced cholestasis is often associated with the V444A mutation, which leads to decreased BSEP expression, and specifically contraceptive induced cholestasis has been associated with the 1331T>C polymorphism[52,53].
ABCB4 (MDR3, PFIC3): Also described as a cholangiopathy, PFIC3 is secondary to defects in the multidrug resistance class 3 (MDR3) glycoprotein, encoded by ABCB4[54]. As a phospholipid translocator, MDR3 facilitates the incorporation of phosphatidylcholine into bile. Without phosphatidylcholine to neutralize bile acids, the imbalance of free bile acids damages cholangiocytes, and cholesterol crystallizes into liver-damaging stones[9]. As with other PFIC diseases, there is a spectrum of disease that can be explained by the extent to which MDR3 is impaired by a particular genetic mutation[55,56]. Those with a heterozygous mutation typically have a mild disease course, including forms of transient neonatal cholestasis[55,57]. Of the described defects in MDR3, the majority are missense mutations that result in defective processing or intracellular transport; while the minority have completely absent MDR3 expression secondary to early truncation or destruction of the protein[9,55,57,58]. While presentation in the first months of life are reported, MDR3 deficiency more often presents in late adolescence or even adulthood[59]. The phenotype of adults with ABCB4 mutations can be varied, ranging from slowly progressive disease, cholelithiasis, ICP, drug induced cholestasis, and benign recurrent intrahepatic cholestasis[58]. In children and adolescents, symptoms are typically few, and the first may be variceal bleeding secondary to portal hypertension[9]. A retrospective review of 38 patients found that those diagnosed in childhood presented with pruritis around 1 year of age and most had hepatosplenomegaly, portal hypertension, and jaundice at the time of presentation[58]. Pediatric disease has also been associated with growth restriction, reduced bone density, and learning disabilities[58]. GGT is typically elevated at presentation, with relatively milder elevation of transaminases and bilirubin[41,59]. Medical treatment should be initiated early in the disease course. Care is supportive including nutrition supplementation and antipruritic agents, though it is not clear if these therapies alter the disease course[4,55]. In vitro studies have suggested that disease-associated mutations resulting in impaired ABCB4 trafficking may be functionally rescued by chemical chaperones[56]. Temporizing surgical interventions as described above are rarely successful due to the severity of disease when diagnosed and liver transplant remains the only definitive therapy[4,59]. Histology typically demonstrates portal fibrosis and bile duct proliferation with mild giant cell hepatitis at disease onset with occasional intraductal cholelithiasis[9]. MDR3 immunohistochemical staining will be absent, decreased, or potentially normal if there are functional protein defects [9]. Carcinogenesis and the development of both cholangiocarcinoma and hepatocellular carcinoma have been reported[9,60,61].
TJP2 (TJP2): Recently, alternate proteins have been identified in whom mutations result in a phenotypic pattern that is similarly to ‘’classic’’ PFIC disease, mainly cholestasis presenting in the neonatal period. The first of these identified stems from loss of function mutations in TJP2 encoding the tight junction protein TJP2. TJP2 is one of the intracellular anchors for tight junctions that seal canaliculi and prevent damage from cytotoxic detergent bile salts[4,59]. To date the largest case series consists of 12 infants from 8 families (most consanguineous) who presented ≤ 3 mo of age with severe liver disease[62]. Though still exceedingly rare, advances in genetic understanding has enabled retrospective re-classification suggesting TJP2 deficiency may be more common than previously thought[63]. The disease results from biallelic mutations in TJP2 with extrahepatic manifestation in the respiratory and neurologic systems having been reported. The mechanism of injury is thought to relate to TJP2’s function maintaining junction integrity, the disturbance of which enables toxic molecules to reflux into the paracellular space; however, this is not clearly described[62]. Though few samples are available, pathology demonstrates intracellular cholestasis and giant cell transformation, with absence of TJP2 specific staining[4]. Several mutations have been noted specific to the families who manifested the disease, but it is not yet clear if some mutations pertain to less severe disease than others or if there is a milder form of disease that may be appreciated in adult patients. Hepatocellular carcinoma has been described at presentation in infants[64,65]. Due to the severity of presentation, 9 of the initial 12 patients described underwent liver transplant; 2 have survived with portal hypertension, and one passed away of their disease[62].
NR1H4 (FXR): PFIC phenotype can also result from mutations in NR1H4, which encodes the FXR, the nuclear receptor transcription factor which regulates BSEP expression via negative feedback loop and induces FGF19 to repress bile acid synthesis[4,66]. Patients reported with these defects are extremely rare, with only 5 patients reported in the literature[67,68]. Without appropriate regulation of BSEP, patients with this defect have presented in the neonatal period with normal GGT cholestasis, normal liver enzymes, elevated serum bile acids, extremely elevated AFP, and rapidly progressed to end stage liver disease with vitamin K independent coagulopathy and hyperammonemia[67,68]. On native liver pathology, the patients were found to have intralobular cholestasis with ductular reaction, hepatocellular ballooning, giant cell transformation, and fibrosis with progression to micronodular cirrhosis. Three patients underwent liver transplant with 2 of 3 showing steatosis in the graft organ on follow up[67].
MYO5B (MYO5B): Defects in MYO5B, on which BSEP depends to localize to the canalicular hepatocellular membrane, usually cause microvillus inclusion disease but also may result in isolated liver disease[4]. Without appropriate BSEP localization, secretion of bile acids is impaired and causes hepatocellular toxicity[69]. This results in a clinical picture of low GGT cholestasis, hepatomegaly, normal or mildly elevated transaminases. Patients have preserved synthetic function but struggle with pruritis and present around 1 year of age, similar to FIC1 and BSEP disease[69]. The hepatocellular damage results in a pathologic pattern of hepatocellular cholestasis with portal and lobular fibrosis and giant cell transformation. Present but abnormal BSEP and MDR3 staining suggest that these transporters are made but can’t appropriately migrate to the canalicular membrane[69].
Because MYO5B interacts with rab11 for appropriate functioning of polarized cells, extrahepatic manifestations can be present. MYO5B has previously been implicated in microvillous inclusion disease, thus some patients with genetic cholestasis have also had diarrheal manifestations of disease[69]. Similarly, some patients also suffer short stature, though others have normal growth. Finally, some patients with this disease have neurologic findings, though it is not clear if these are related to the gene defect[4,69]. In addition to supportive care for nutrition and diarrhea, patients have been treated with antipruritic and anticholestatic agents, including UDCA, rifampin, cholestyramine, traditional Chinese medicine[4,69]. If pruritis is refractory to medical therapy, some success has been seen with PEBD. Finally, liver transplant has been undertaken if pruritis is refractory, though it does not address extrahepatic symptoms[69]. At our institution, the association between MYO5B defects, intestinal failure, and isolated liver disease has made decisions regarding type of transplant (isolated bowel, liver bowel, multi-visceral, etc) challenging in patients with microvillus inclusion disease.
USP53 (USP53) and LSR (LSR): A recent report utilizing exome sequencing and positional mapping was able to identify 2 novel loci with defects associated with low-GGT cholestatic liver disease presenting in childhood[70]. In the first case, 3 members of a family (2 sisters and a cousin) presented with low-ggt cholestasis, liver enzyme elevations, and pruritus. Defects in the USP53 protein, thought to colocalize with TJP2 and be part of the tight junction complex[71], was identified. In the second case, a young boy who presented with hypocalcemic seizures, pruritus, liver enzyme elevation, and low-ggt cholestasis was found to have a mutation in lipolysis-stimulated lipoprotein receptor (LSR). Mechanisms by which LSR contributed to the liver disease were not reported, although LSRs role in animal models of liver development suggests an area for future research[70].
The traditional understanding of the PFIC-associated genes contributing to morbidity in adults mainly encompass the phenotypes of BRIC and ICP. The phenotype of BRIC is characterized by intermittent episodes of cholestasis with varying degrees of severity. Both ATP8B1 and ABCB11 mutations have been associated with the phenotype[12,51]. While classic descriptions of BRIC note complete symptom resolution without progression, several cases have been reported to transition to more persistent, progressive disease[72]. Treatment of cholestatic episodes with steroids, choleretic agents, and bile acid binders have generally been ineffective, although rifampicin has been shown to decrease pruritus and shorten exacerbations[73,74]. ICP is a common condition affecting about 1% of all pregnancies[75]. ICP manifests during pregnancy with pruritus, hepatic impairment, and cholestasis which usually resolves completely after delivery. While generally considered benign for the mother, adverse perinatal outcomes for the child, such as fetal distress, premature birth, and stillbirth, can occur[75]. While rare, stillbirth has been shown to be associated with bile acid concentrations of ≥ 100 μmol/L highlighting the importance of close monitoring[76]. The use of ursodiol has been shown to symptomatically improve pruritus and decrease the risk of premature birth[77,78]. An expanded understanding of the genetics associated with ICP has identified mutations in ABCB4, ABCB11, ATP8B1, ABCC2 (associated with Dubin-Johnson), and TJP2 contributing to disease[79]. Additionally, variations in NR1H4 may be implicated in ICP, possibly via downregulation of BSEP expression[80]. Beyond BRIC and ICP, drug-induced injury has been historically been linked to PFIC gene associated polymorphisms[52].
More recently, investigators have begun looking more broadly at the contributions that these genes may have on morbidity in adult populations(Table 2). Mutations in ATP8B1, ABCB11, ABCB4, and TJP2 have been reported in adults with cryptogenic cirrhosis[81] while ABCB4 defects have been linked to the development of sclerosing cholangitis, biliary cirrhosis, and low-phospholipid cholelithiasis[82,83]. Genetic sequencing of large cholestatic populations have revealed disease causing mutations in up to a third of patients, with common variants detected in a high number of those without known disease-causing defects suggesting that they still may have a contributing role to the development of cholestasis[83]. Importantly, several of the recently identified contributing genes, such as NR1H4, MYO5B, USP53 and LSR were not tested for in these studies, suggesting the burden may still be higher.
| Etiology | Genetic defect | Manifestations |
| FIC1 deficiency | ATP8B1 | BRIC1 |
| ICP1 and contraceptive-induced cholestasis | ||
| Cryptogenic cirrhosis | ||
| BSEP deficiency | ABCB11 | BRIC2 |
| ICP2 and contraceptive-induced cholestasis | ||
| DILI | ||
| Cryptogenic cirrhosis | ||
| MDR3 deficiency | ABCB4 | ICP3 and contraceptive-induced cholestasis |
| Drug induced cholestasis | ||
| Low phospholipid-associated cholestasis | ||
| Cholesterol gallstone disease | ||
| Biliary fibrosis or liver cirrhosis without cholestasis | ||
| Cryptogenic cirrhosis | ||
| TJP2 deficiency | TJP2 | Cryptogenic cirrhosis |
| FXR | NR1H4 | ICP |
| Drug induced cholestasis associated with propylthiouracil |
PFIC is a heterogeneous cohort of diseases that present both diagnostic and treatment challenges for clinicians. While significant advancement in bile transport physiology has been made by studying these diseases, the breadth of phenotypes from neonates to adults demonstrates that there remains much more to be understood. In the future, precise molecular diagnosis may allow individualized therapy through gene replacement or protein augmentation therapies.
Progressive familial intrahepatic cholestasis (PFIC) is an umbrella term originally used to describe 3 classic genetic-based cholestatic diseases in children. Recent advancements in how genetic defects in proteins affect bile acid homeostasis and caused disease has led to an expanded list of syndromes categorized as PFIC and a growing understanding of how adults can be affected. In this report, we review the literature to summarize the understanding of ‘classic’ PFIC diseases and present up-to-date information the expanding list of genetic defects that are now known to contribute to the PFIC phenotype.
Bile acid metabolism, homeostasis, and transport is a complex physiologic process, the importance of which is underscored when defects in the system cause disease. While recent advancements have identified critical genes and protein products that, when defective, contribute to disease, phenotypic variability persists, and treatment remains mainly supportive. Furthermore, it is clearly that additional genes and proteins are likely to be identified as the field continues to evolve. In the future, better diagnostics and precise molecular defect identification may identify individualized therapy options that will improve the care provided to these patients.
The objectives of this work were to thoroughly review the current published literature and present an up-to-date summarization of both the ‘’Classic’’ and ‘’Expanded’’ PFIC diseases.
A Medline/PubMed search was performed to identify established articles relating to PFIC as well as reports of defects in PFIC-related genes contributing to morbidity in adult and pediatric populations. Data was manually extracted on disease characteristics. Associated phenotypes with other diseases relating to specific genetic defects were also collected. Treatment strategies were summarized. Data was collated and presented in text, figure, and table format.
We present a comprehensive summary of the ‘’Classic’’ PFIC disorders resulting from defects in ATP8B1 (FIC1 protein), ABCB11 (BSEP protein), and ABCB4 (MDR3 protein). We further explore and summarize the “Expanded” PFIC disorders including those related to TJP1 (TJP2 protein), NR1H4 (FXR protein), MYO5B (MYO5B protein, USP53 (USP53 protein), and LSR (LSR protein) defects. While many of these disorders have historically affected children, we also looked to present the growing literature related to the significant morbidity that these diseases cause in adults.
In this review, we present a comprehensive summary of the current understanding and management of PFIC-related disorders. The recent identification of the “Expanded” disorders underscores the importance of continued exploration of the genetic basis of bile acid homeostasis. However, idiopathic disease remains a considerable challenge to patients and healthcare professionals suggesting opportunities for further investigation. Future strategies to improve the treatment provided to patients affected by these devastating diseases are also critically needed.
Since their first description in 1969, the last 50 years has brought dramatic advancements in both the understanding and management of PFIC-related diseases. Still, challenges remain. Continued idiopathic disease suggest improvement in diagnostic strategies are needed and treatment options remain frustratingly small. Variability in both phenotype and response to therapy opens the possibility that specific gene defects or modifiers can identify sub-populations where more personalized approaches can be more affective. Improved disease models, both in vitro and in vivo, are needed to better understand mechanisms and identify therapeutic strategies. Finally, the growing morbidity linked to defects in PFIC-related genes identified in adults highlights the urgency, but also the opportunity, for future investigation.
Manuscript source: Invited Manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Erginel B, Tan YW S-Editor: Cui LJ L-Editor: A E-Editor: Zhang YL
| 1. | Linton KJ. Lipid flopping in the liver. Biochem Soc Trans. 2015;43:1003-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Dawson PA, Haywood J, Craddock AL, Wilson M, Tietjen M, Kluckman K, Maeda N, Parks JS. Targeted deletion of the ileal bile acid transporter eliminates enterohepatic cycling of bile acids in mice. J Biol Chem. 2003;278:33920-33927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 259] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 3. | Boyer JL. Bile formation and secretion. Compr Physiol. 2013;3:1035-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 514] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 4. | Bull LN, Thompson RJ. Progressive Familial Intrahepatic Cholestasis. Clin Liver Dis. 2018;22:657-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 111] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 5. | Clayton RJ, Iber FL, Ruebner BH, McKusick VA. Byler disease. Fatal familial intrahepatic cholestasis in an Amish kindred. Am J Dis Child. 1969;117:112-124. [PubMed] |
| 6. | Bull LN, van Eijk MJ, Pawlikowska L, DeYoung JA, Juijn JA, Liao M, Klomp LW, Lomri N, Berger R, Scharschmidt BF, Knisely AS, Houwen RH, Freimer NB. A gene encoding a P-type ATPase mutated in two forms of hereditary cholestasis. Nat Genet. 1998;18:219-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 505] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 7. | Paulusma CC, Groen A, Kunne C, Ho-Mok KS, Spijkerboer AL, Rudi de Waart D, Hoek FJ, Vreeling H, Hoeben KA, van Marle J, Pawlikowska L, Bull LN, Hofmann AF, Knisely AS, Oude Elferink RP. Atp8b1 deficiency in mice reduces resistance of the canalicular membrane to hydrophobic bile salts and impairs bile salt transport. Hepatology. 2006;44:195-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 161] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 8. | Folmer DE, van der Mark VA, Ho-Mok KS, Oude Elferink RP, Paulusma CC. Differential effects of progressive familial intrahepatic cholestasis type 1 and benign recurrent intrahepatic cholestasis type 1 mutations on canalicular localization of ATP8B1. Hepatology. 2009;50:1597-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Srivastava A. Progressive familial intrahepatic cholestasis. J Clin Exp Hepatol. 2014;4:25-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 181] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 10. | Davit-Spraul A, Fabre M, Branchereau S, Baussan C, Gonzales E, Stieger B, Bernard O, Jacquemin E. ATP8B1 and ABCB11 analysis in 62 children with normal gamma-glutamyl transferase progressive familial intrahepatic cholestasis (PFIC): phenotypic differences between PFIC1 and PFIC2 and natural history. Hepatology. 2010;51:1645-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 196] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 11. | Alonso EM, Snover DC, Montag A, Freese DK, Whitington PF. Histologic pathology of the liver in progressive familial intrahepatic cholestasis. J Pediatr Gastroenterol Nutr. 1994;18:128-133. [PubMed] |
| 12. | Bull LN, Carlton VE, Stricker NL, Baharloo S, DeYoung JA, Freimer NB, Magid MS, Kahn E, Markowitz J, DiCarlo FJ, McLoughlin L, Boyle JT, Dahms BB, Faught PR, Fitzgerald JF, Piccoli DA, Witzleben CL, O'Connell NC, Setchell KD, Agostini RM, Kocoshis SA, Reyes J, Knisely AS. Genetic and morphological findings in progressive familial intrahepatic cholestasis (Byler disease [PFIC-1] and Byler syndrome): evidence for heterogeneity. Hepatology. 1997;26:155-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 160] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | van der Woerd WL, Wichers CG, Vestergaard AL, Andersen JP, Paulusma CC, Houwen RH, van de Graaf SF. Rescue of defective ATP8B1 trafficking by CFTR correctors as a therapeutic strategy for familial intrahepatic cholestasis. J Hepatol. 2016;64:1339-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Squires JE, Celik N, Morris A, Soltys K, Mazariegos G, Shneider B, Squires RH. Clinical Variability After Partial External Biliary Diversion in Familial Intrahepatic Cholestasis 1 Deficiency. J Pediatr Gastroenterol Nutr. 2017;64:425-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Emond JC, Whitington PF. Selective surgical management of progressive familial intrahepatic cholestasis (Byler's disease). J Pediatr Surg. 1995;30:1635-1641. [PubMed] |
| 16. | Wang KS, Tiao G, Bass LM, Hertel PM, Mogul D, Kerkar N, Clifton M, Azen C, Bull L, Rosenthal P, Stewart D, Superina R, Arnon R, Bozic M, Brandt ML, Dillon PA, Fecteau A, Iyer K, Kamath B, Karpen S, Karrer F, Loomes KM, Mack C, Mattei P, Miethke A, Soltys K, Turmelle YP, West K, Zagory J, Goodhue C, Shneider BL; Childhood Liver Disease Research Network (ChiLDReN). Analysis of surgical interruption of the enterohepatic circulation as a treatment for pediatric cholestasis. Hepatology. 2017;65:1645-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 17. | Whitington PF, Whitington GL. Partial external diversion of bile for the treatment of intractable pruritus associated with intrahepatic cholestasis. Gastroenterology. 1988;95:130-136. [PubMed] |
| 18. | Kaliciński PJ, Ismail H, Jankowska I, Kamiński A, Pawłowska J, Drewniak T, Markiewicz M, Szymczak M. Surgical treatment of progressive familial intrahepatic cholestasis: comparison of partial external biliary diversion and ileal bypass. Eur J Pediatr Surg. 2003;13:307-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Ismail H, Kaliciński P, Markiewicz M, Jankowska I, Pawłowska J, Kluge P, Eliadou E, Kamiński A, Szymczak M, Drewniak T, Revillon Y. Treatment of progressive familial intrahepatic cholestasis: liver transplantation or partial external biliary diversion. Pediatr Transplant. 1999;3:219-224. [PubMed] |
| 20. | Hollands CM, Rivera-Pedrogo FJ, Gonzalez-Vallina R, Loret-de-Mola O, Nahmad M, Burnweit CA. Ileal exclusion for Byler's disease: an alternative surgical approach with promising early results for pruritus. J Pediatr Surg. 1998;33:220-224. [PubMed] |
| 21. | Gün F, Erginel B, Durmaz O, Sökücü S, Salman T, Celik A. An outstanding non-transplant surgical intervention in progressive familial intrahepatic cholestasis: partial internal biliary diversion. Pediatr Surg Int. 2010;26:831-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Gunaydin M, Tander B, Demirel D, Caltepe G, Kalayci AG, Eren E, Bicakcı U, Rizalar R, Ariturk E, Bernay F. Different techniques for biliary diversion in progressive familial intrahepatic cholestasis. J Pediatr Surg. 2016;51:386-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Bustorff-Silva J, Sbraggia Neto L, Olímpio H, de Alcantara RV, Matsushima E, De Tommaso AM, Brandão MA, Hessel G. Partial internal biliary diversion through a cholecystojejunocolonic anastomosis--a novel surgical approach for patients with progressive familial intrahepatic cholestasis: a preliminary report. J Pediatr Surg. 2007;42:1337-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Thébaut A, Debray D, Gonzales E. An update on the physiopathology and therapeutic management of cholestatic pruritus in children. Clin Res Hepatol Gastroenterol. 2018;42:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Meerzaman DM, Yan C, Chen QR, Edmonson MN, Schaefer CF, Clifford RJ, Dunn BK, Dong L, Finney RP, Cultraro CM, Hu Y, Yang Z, Nguyen CV, Kelley JM, Cai S, Zhang H, Zhang J, Wilson R, Messmer L, Chung YH, Kim JA, Park NH, Lyu MS, Song IH, Komatsoulis G, Buetow KH. Genome-wide transcriptional sequencing identifies novel mutations in metabolic genes in human hepatocellular carcinoma. Cancer Genomics Proteomics. 2014;11:1-12. [PubMed] |
| 26. | Squires JE. Protecting the allograft following liver transplantation for PFIC1. Pediatr Transplant. 2016;20:882-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Mali VP, Fukuda A, Shigeta T, Uchida H, Hirata Y, Rahayatri TH, Kanazawa H, Sasaki K, de Ville de Goyet J, Kasahara M. Total internal biliary diversion during liver transplantation for type 1 progressive familial intrahepatic cholestasis: a novel approach. Pediatr Transplant. 2016;20:981-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Hsu YC, Chen HL, Wu MZ, Liu YJ, Lee PH, Sheu JC, Chen CH. Adult progressive intrahepatic cholestasis associated with genetic variations in ATP8B1 and ABCB11. Hepatol Res. 2009;39:625-631. [PubMed] |
| 29. | Dixon PH, Wadsworth CA, Chambers J, Donnelly J, Cooley S, Buckley R, Mannino R, Jarvis S, Syngelaki A, Geenes V, Paul P, Sothinathan M, Kubitz R, Lammert F, Tribe RM, Ch'ng CL, Marschall HU, Glantz A, Khan SA, Nicolaides K, Whittaker J, Geary M, Williamson C. A comprehensive analysis of common genetic variation around six candidate loci for intrahepatic cholestasis of pregnancy. Am J Gastroenterol. 2014;109:76-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 30. | Müllenbach R, Bennett A, Tetlow N, Patel N, Hamilton G, Cheng F, Chambers J, Howard R, Taylor-Robinson SD, Williamson C. ATP8B1 mutations in British cases with intrahepatic cholestasis of pregnancy. Gut. 2005;54:829-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 105] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 31. | Klomp LW, Vargas JC, van Mil SW, Pawlikowska L, Strautnieks SS, van Eijk MJ, Juijn JA, Pabón-Peña C, Smith LB, DeYoung JA, Byrne JA, Gombert J, van der Brugge G, Berger R, Jankowska I, Pawlowska J, Villa E, Knisely AS, Thompson RJ, Freimer NB, Houwen RH, Bull LN. Characterization of mutations in ATP8B1 associated with hereditary cholestasis. Hepatology. 2004;40:27-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 188] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 32. | Strautnieks SS, Bull LN, Knisely AS, Kocoshis SA, Dahl N, Arnell H, Sokal E, Dahan K, Childs S, Ling V, Tanner MS, Kagalwalla AF, Németh A, Pawlowska J, Baker A, Mieli-Vergani G, Freimer NB, Gardiner RM, Thompson RJ. A gene encoding a liver-specific ABC transporter is mutated in progressive familial intrahepatic cholestasis. Nat Genet. 1998;20:233-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 766] [Cited by in RCA: 674] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 33. | Gerloff T, Stieger B, Hagenbuch B, Madon J, Landmann L, Roth J, Hofmann AF, Meier PJ. The sister of P-glycoprotein represents the canalicular bile salt export pump of mammalian liver. J Biol Chem. 1998;273:10046-10050. [PubMed] |
| 34. | Imagawa K, Hayashi H, Sabu Y, Tanikawa K, Fujishiro J, Kajikawa D, Wada H, Kudo T, Kage M, Kusuhara H, Sumazaki R. Clinical phenotype and molecular analysis of a homozygous ABCB11 mutation responsible for progressive infantile cholestasis. J Hum Genet. 2018;63:569-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 35. | Thompson R, Strautnieks S. BSEP: function and role in progressive familial intrahepatic cholestasis. Semin Liver Dis. 2001;21:545-550. [PubMed] |
| 36. | Chen HL, Chang PS, Hsu HC, Ni YH, Hsu HY, Lee JH, Jeng YM, Shau WY, Chang MH. FIC1 and BSEP defects in Taiwanese patients with chronic intrahepatic cholestasis with low gamma-glutamyltranspeptidase levels. J Pediatr. 2002;140:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Ellis JL, Bove KE, Schuetz EG, Leino D, Valencia CA, Schuetz JD, Miethke A, Yin C. Zebrafish abcb11b mutant reveals strategies to restore bile excretion impaired by bile salt export pump deficiency. Hepatology. 2018;67:1531-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 38. | Naoi S, Hayashi H, Inoue T, Tanikawa K, Igarashi K, Nagasaka H, Kage M, Takikawa H, Sugiyama Y, Inui A, Nagai T, Kusuhara H. Improved liver function and relieved pruritus after 4-phenylbutyrate therapy in a patient with progressive familial intrahepatic cholestasis type 2. J Pediatr. 2014;164:1219-1227.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 39. | Malatack JJ, Doyle D. A Drug Regimen for Progressive Familial Cholestasis Type 2. Pediatrics. 2018;141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | Imagawa K, Takayama K, Isoyama S, Tanikawa K, Shinkai M, Harada K, Tachibana M, Sakurai F, Noguchi E, Hirata K, Kage M, Kawabata K, Sumazaki R, Mizuguchi H. Generation of a bile salt export pump deficiency model using patient-specific induced pluripotent stem cell-derived hepatocyte-like cells. Sci Rep. 2017;7:41806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 41. | Bull LN, Pawlikowska L, Strautnieks S, Jankowska I, Czubkowski P, Dodge JL, Emerick K, Wanty C, Wali S, Blanchard S, Lacaille F, Byrne JA, van Eerde AM, Kolho KL, Houwen R, Lobritto S, Hupertz V, McClean P, Mieli-Vergani G, Sokal E, Rosenthal P, Whitington PF, Pawlowska J, Thompson RJ. Outcomes of surgical management of familial intrahepatic cholestasis 1 and bile salt export protein deficiencies. Hepatol Commun. 2018;2:515-528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 42. | Ellinger P, Stindt J, Dröge C, Sattler K, Stross C, Kluge S, Herebian D, Smits SHJ, Burdelski M, Schulz-Jürgensen S, Ballauff A, Schulte Am Esch J, Mayatepek E, Häussinger D, Kubitz R, Schmitt L. Partial external biliary diversion in bile salt export pump deficiency: Association between outcome and mutation. World J Gastroenterol. 2017;23:5295-5303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 43. | Knisely AS, Strautnieks SS, Meier Y, Stieger B, Byrne JA, Portmann BC, Bull LN, Pawlikowska L, Bilezikçi B, Ozçay F, László A, Tiszlavicz L, Moore L, Raftos J, Arnell H, Fischler B, Németh A, Papadogiannakis N, Cielecka-Kuszyk J, Jankowska I, Pawłowska J, Melín-Aldana H, Emerick KM, Whitington PF, Mieli-Vergani G, Thompson RJ. Hepatocellular carcinoma in ten children under five years of age with bile salt export pump deficiency. Hepatology. 2006;44:478-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 253] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 44. | Jara P, Hierro L, Martínez-Fernández P, Alvarez-Doforno R, Yánez F, Diaz MC, Camarena C, De la Vega A, Frauca E, Muñoz-Bartolo G, López-Santamaría M, Larrauri J, Alvarez L. Recurrence of bile salt export pump deficiency after liver transplantation. N Engl J Med. 2009;361:1359-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 45. | Siebold L, Dick AA, Thompson R, Maggiore G, Jacquemin E, Jaffe R, Strautnieks S, Grammatikopoulos T, Horslen S, Whitington PF, Shneider BL. Recurrent low gamma-glutamyl transpeptidase cholestasis following liver transplantation for bile salt export pump (BSEP) disease (posttransplant recurrent BSEP disease). Liver Transpl. 2010;16:856-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 46. | Stindt J, Kluge S, Dröge C, Keitel V, Stross C, Baumann U, Brinkert F, Dhawan A, Engelmann G, Ganschow R, Gerner P, Grabhorn E, Knisely AS, Noli KA, Pukite I, Shepherd RW, Ueno T, Schmitt L, Wiek C, Hanenberg H, Häussinger D, Kubitz R. Bile salt export pump-reactive antibodies form a polyclonal, multi-inhibitory response in antibody-induced bile salt export pump deficiency. Hepatology. 2016;63:524-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 47. | Lin HC, Alvarez L, Laroche G, Melin-Aldana H, Pfeifer K, Schwarz K, Whitington PF, Alonso EM, Ekong UD. Rituximab as therapy for the recurrence of bile salt export pump deficiency after liver transplantation. Liver Transpl. 2013;19:1403-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 48. | Brinkert F, Pukite I, Krebs-Schmitt D, Briem-Richter A, Stindt J, Häussinger D, Keitel V, Müller I, Grabhorn E. Allogeneic haematopoietic stem cell transplantation eliminates alloreactive inhibitory antibodies after liver transplantation for bile salt export pump deficiency. J Hepatol. 2018;69:961-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 49. | Krebs-Schmitt D, Briem-Richter A, Brinkert F, Keitel V, Pukite I, Lenhartz H, Fischer L, Grabhorn E. Alloimmunity and Cholestasis After Liver Transplantation in Children With Progressive Familial Intrahepatic Cholestasis. J Pediatr Gastroenterol Nutr. 2019;68:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 50. | Kubitz R, Dröge C, Stindt J, Weissenberger K, Häussinger D. The bile salt export pump (BSEP) in health and disease. Clin Res Hepatol Gastroenterol. 2012;36:536-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 141] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 51. | van Mil SW, van der Woerd WL, van der Brugge G, Sturm E, Jansen PL, Bull LN, van den Berg IE, Berger R, Houwen RH, Klomp LW. Benign recurrent intrahepatic cholestasis type 2 is caused by mutations in ABCB11. Gastroenterology. 2004;127:379-384. [PubMed] |
| 52. | Lang C, Meier Y, Stieger B, Beuers U, Lang T, Kerb R, Kullak-Ublick GA, Meier PJ, Pauli-Magnus C. Mutations and polymorphisms in the bile salt export pump and the multidrug resistance protein 3 associated with drug-induced liver injury. Pharmacogenet Genomics. 2007;17:47-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 222] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 53. | Meier Y, Zodan T, Lang C, Zimmermann R, Kullak-Ublick GA, Meier PJ, Stieger B, Pauli-Magnus C. Increased susceptibility for intrahepatic cholestasis of pregnancy and contraceptive-induced cholestasis in carriers of the 1331T>C polymorphism in the bile salt export pump. World J Gastroenterol. 2008;14:38-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 126] [Cited by in RCA: 118] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 54. | Mauad TH, van Nieuwkerk CM, Dingemans KP, Smit JJ, Schinkel AH, Notenboom RG, van den Bergh Weerman MA, Verkruisen RP, Groen AK, Oude Elferink RP. Mice with homozygous disruption of the mdr2 P-glycoprotein gene. A novel animal model for studies of nonsuppurative inflammatory cholangitis and hepatocarcinogenesis. Am J Pathol. 1994;145:1237-1245. [PubMed] |
| 55. | Davit-Spraul A, Gonzales E, Baussan C, Jacquemin E. The spectrum of liver diseases related to ABCB4 gene mutations: pathophysiology and clinical aspects. Semin Liver Dis. 2010;30:134-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 162] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 56. | Gordo-Gilart R, Andueza S, Hierro L, Jara P, Alvarez L. Functional Rescue of Trafficking-Impaired ABCB4 Mutants by Chemical Chaperones. PLoS One. 2016;11:e0150098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 57. | Gonzales E, Davit-Spraul A, Baussan C, Buffet C, Maurice M, Jacquemin E. Liver diseases related to MDR3 (ABCB4) gene deficiency. Front Biosci (Landmark Ed. ). 2009;14:4242-4256. [PubMed] |
| 58. | Schatz SB, Jüngst C, Keitel-Anselmo V, Kubitz R, Becker C, Gerner P, Pfister ED, Goldschmidt I, Junge N, Wenning D, Gehring S, Arens S, Bretschneider D, Grothues D, Engelmann G, Lammert F, Baumann U. Phenotypic spectrum and diagnostic pitfalls of ABCB4 deficiency depending on age of onset. Hepatol Commun. 2018;2:504-514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 59. | Squires JE, McKiernan P. Molecular Mechanisms in Pediatric Cholestasis. Gastroenterol Clin North Am. 2018;47:921-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 60. | Vij M, Shanmugam NP, Reddy MS, Govil S, Rela M. Hepatocarcinogenesis in multidrug-resistant P-glycoprotein 3 deficiency. Pediatr Transplant. 2017;21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 61. | Tougeron D, Fotsing G, Barbu V, Beauchant M. ABCB4/MDR3 gene mutations and cholangiocarcinomas. J Hepatol. 2012;57:467-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 62. | Sambrotta M, Strautnieks S, Papouli E, Rushton P, Clark BE, Parry DA, Logan CV, Newbury LJ, Kamath BM, Ling S, Grammatikopoulos T, Wagner BE, Magee JC, Sokol RJ, Mieli-Vergani G; University of Washington Center for Mendelian Genomics, Smith JD, Johnson CA, McClean P, Simpson MA, Knisely AS, Bull LN, Thompson RJ. Mutations in TJP2 cause progressive cholestatic liver disease. Nat Genet. 2014;46:326-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 203] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 63. | Shagrani M, Burkholder J, Broering D, Abouelhoda M, Faquih T, El-Kalioby M, Subhani SN, Goljan E, Albar R, Monies D, Mazhar N, AlAbdulaziz BS, Abdelrahman KA, Altassan N, Alkuraya FS. Genetic profiling of children with advanced cholestatic liver disease. Clin Genet. 2017;92:52-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 64. | Vij M, Shanmugam NP, Reddy MS, Sankaranarayanan S, Rela M. Paediatric hepatocellular carcinoma in tight junction protein 2 (TJP2) deficiency. Virchows Arch. 2017;471:679-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 65. | Zhou S, Hertel PM, Finegold MJ, Wang L, Kerkar N, Wang J, Wong LJ, Plon SE, Sambrotta M, Foskett P, Niu Z, Thompson RJ, Knisely AS. Hepatocellular carcinoma associated with tight-junction protein 2 deficiency. Hepatology. 2015;62:1914-1916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 66. | Davit-Spraul A, Gonzales E, Jacquemin E. NR1H4 analysis in patients with progressive familial intrahepatic cholestasis, drug-induced cholestasis or intrahepatic cholestasis of pregnancy unrelated to ATP8B1, ABCB11 and ABCB4 mutations. Clin Res Hepatol Gastroenterol. 2012;36:569-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 67. | Gomez-Ospina N, Potter CJ, Xiao R, Manickam K, Kim MS, Kim KH, Shneider BL, Picarsic JL, Jacobson TA, Zhang J, He W, Liu P, Knisely AS, Finegold MJ, Muzny DM, Boerwinkle E, Lupski JR, Plon SE, Gibbs RA, Eng CM, Yang Y, Washington GC, Porteus MH, Berquist WE, Kambham N, Singh RJ, Xia F, Enns GM, Moore DD. Mutations in the nuclear bile acid receptor FXR cause progressive familial intrahepatic cholestasis. Nat Commun. 2016;7:10713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 201] [Article Influence: 22.3] [Reference Citation Analysis (2)] |
| 68. | Chen HL, Li HY, Wu JF, Wu SH, Chen HL, Yang YH, Hsu YH, Liou BY, Chang MH, Ni YH. Panel-Based Next-Generation Sequencing for the Diagnosis of Cholestatic Genetic Liver Diseases: Clinical Utility and Challenges. J Pediatr. 2019;205:153-159.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 69. | Gonzales E, Taylor SA, Davit-Spraul A, Thébaut A, Thomassin N, Guettier C, Whitington PF, Jacquemin E. MYO5B mutations cause cholestasis with normal serum gamma-glutamyl transferase activity in children without microvillous inclusion disease. Hepatology. 2017;65:164-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 103] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 70. | Maddirevula S, Alhebbi H, Alqahtani A, Algoufi T, Alsaif HS, Ibrahim N, Abdulwahab F, Barr M, Alzaidan H, Almehaideb A, AlSasi O, Alhashem A, Hussaini HA, Wali S, Alkuraya FS. Identification of novel loci for pediatric cholestatic liver disease defined by KIF12, PPM1F, USP53, LSR, and WDR83OS pathogenic variants. Genet Med. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 71. | Kazmierczak M, Harris SL, Kazmierczak P, Shah P, Starovoytov V, Ohlemiller KK, Schwander M. Progressive Hearing Loss in Mice Carrying a Mutation in Usp53. J Neurosci. 2015;35:15582-15598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 72. | van Ooteghem NA, Klomp LW, van Berge-Henegouwen GP, Houwen RH. Benign recurrent intrahepatic cholestasis progressing to progressive familial intrahepatic cholestasis: low GGT cholestasis is a clinical continuum. J Hepatol. 2002;36:439-443. [PubMed] |
| 73. | Beuers U, Trauner M, Jansen P, Poupon R. New paradigms in the treatment of hepatic cholestasis: from UDCA to FXR, PXR and beyond. J Hepatol. 2015;62:S25-S37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 384] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 74. | Sticova E, Jirsa M, Pawłowska J. New Insights in Genetic Cholestasis: From Molecular Mechanisms to Clinical Implications. Can J Gastroenterol Hepatol. 2018;2018:2313675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 75. | Dixon PH, Williamson C. The pathophysiology of intrahepatic cholestasis of pregnancy. Clin Res Hepatol Gastroenterol. 2016;40:141-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 123] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 76. | Ovadia C, Seed PT, Sklavounos A, Geenes V, Di Ilio C, Chambers J, Kohari K, Bacq Y, Bozkurt N, Brun-Furrer R, Bull L, Estiú MC, Grymowicz M, Gunaydin B, Hague WM, Haslinger C, Hu Y, Kawakita T, Kebapcilar AG, Kebapcilar L, Kondrackienė J, Koster MPH, Kowalska-Kańka A, Kupčinskas L, Lee RH, Locatelli A, Macias RIR, Marschall HU, Oudijk MA, Raz Y, Rimon E, Shan D, Shao Y, Tribe R, Tripodi V, Yayla Abide C, Yenidede I, Thornton JG, Chappell LC, Williamson C. Association of adverse perinatal outcomes of intrahepatic cholestasis of pregnancy with biochemical markers: results of aggregate and individual patient data meta-analyses. Lancet. 2019;393:899-909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 271] [Cited by in RCA: 322] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 77. | Binder T, Salaj P, Zima T, Vítek L. Randomized prospective comparative study of ursodeoxycholic acid and S-adenosyl-L-methionine in the treatment of intrahepatic cholestasis of pregnancy. J Perinat Med. 2006;34:383-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 78. | Glantz A, Marschall HU, Lammert F, Mattsson LA. Intrahepatic cholestasis of pregnancy: a randomized controlled trial comparing dexamethasone and ursodeoxycholic acid. Hepatology. 2005;42:1399-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 156] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 79. | Dixon PH, Sambrotta M, Chambers J, Taylor-Harris P, Syngelaki A, Nicolaides K, Knisely AS, Thompson RJ, Williamson C. An expanded role for heterozygous mutations of ABCB4, ABCB11, ATP8B1, ABCC2 and TJP2 in intrahepatic cholestasis of pregnancy. Sci Rep. 2017;7:11823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 80. | Van Mil SW, Milona A, Dixon PH, Mullenbach R, Geenes VL, Chambers J, Shevchuk V, Moore GE, Lammert F, Glantz AG, Mattsson LA, Whittaker J, Parker MG, White R, Williamson C. Functional variants of the central bile acid sensor FXR identified in intrahepatic cholestasis of pregnancy. Gastroenterology. 2007;133:507-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 181] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 81. | Vitale G, Gitto S, Raimondi F, Mattiaccio A, Mantovani V, Vukotic R, D'Errico A, Seri M, Russell RB, Andreone P. Cryptogenic cholestasis in young and adults: ATP8B1, ABCB11, ABCB4, and TJP2 gene variants analysis by high-throughput sequencing. J Gastroenterol. 2018;53:945-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 82. | Andress EJ, Nicolaou M, McGeoghan F, Linton KJ. ABCB4 missense mutations D243A, K435T, G535D, I490T, R545C, and S978P significantly impair the lipid floppase and likely predispose to secondary pathologies in the human population. Cell Mol Life Sci. 2017;74:2513-2524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 83. | Dröge C, Bonus M, Baumann U, Klindt C, Lainka E, Kathemann S, Brinkert F, Grabhorn E, Pfister ED, Wenning D, Fichtner A, Gotthardt DN, Weiss KH, Mckiernan P, Puri RD, Verma IC, Kluge S, Gohlke H, Schmitt L, Kubitz R, Häussinger D, Keitel V. Sequencing of FIC1, BSEP and MDR3 in a large cohort of patients with cholestasis revealed a high number of different genetic variants. J Hepatol. 2017;67:1253-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |