Published online Mar 27, 2019. doi: 10.4254/wjh.v11.i3.250
Peer-review started: December 3, 2018
First decision: January 8, 2019
Revised: February 20, 2019
Accepted: February 26, 2019
Article in press: February 27, 2019
Published online: March 27, 2019
Processing time: 119 Days and 22.4 Hours
Gastric varices (GV) have different physiology and clinical characteristics compared to oesophageal varices (OV). There is little information about the management of GV. Most part of the recommendations is extrapolated from studies where the majority of participants had OV. Thus, most recommendations lack of strong evidence. This is a comprehensive review on all aspects of management of GV, i.e., primary, secondary prophylaxis and management of acute bleeding. The papers on which international societies’ recommendations are based are scrutinised in this review and areas of research are identified.
Core tip: This review focuses on an area in Hepatology which needs updating due to the recent contradictory recommendations from different international societies, i.e., American Association for the Study of Liver Disease, Baveno-VI and United Kingdom guidelines. Contradiction arises from lack of strong evidence. This comprehensive review analyses critically the key papers on which recommendations are based, and it also detects areas needing urgent research. There are also graphs and information which would help clinicians in their decision-making process.
- Citation: Vine LJ, Subhani M, Acevedo JG. Update on management of gastric varices. World J Hepatol 2019; 11(3): 250-260
- URL: https://www.wjgnet.com/1948-5182/full/v11/i3/250.htm
- DOI: https://dx.doi.org/10.4254/wjh.v11.i3.250
Gastric varices (GV) are present in around 20% of patients with cirrhosis, portal hypertension and varices detected in the endoscopy[1]. GV are quite different from oesophageal varices (OV), they are supplied by the short gastric, left gastric and polar renal veins and thus, they have different venous afferents compared to OV. GV bleed less frequently, but they bleed more significantly than OV. Bleeding from GV is less directly related to the degree of portal hypertension and more related to the size of the varix and wall tension.
In one large study, including 568 patients, GV were present in 20% at initial endoscopy with 9% of patients developing GV over a medium follow up period of 24.6 ± 5.3 mo, however this was after eradication of OV’s the authors reported a mortality rate of 45%[1]. In comparison, a more recent study reported the six-week mortality of bleeding GV as being only 16.7%[2].
Another study reviewing 117 patients with fundal GV who had never bled showed that the cumulative risk for GV bleeding at 1, 3, and 5 years was 16%, 36%, and 44%, respectively with a total of 34/117 patients bleeding[3]; this was higher than a later study of 604 patients which showed a cumulative incidence of GV bleeding at 4.8%, 19.9%, and 23.2% at 1, 3, and 5 years respectively[4].
Currently, there is much more information on management of OV than on GV. Hence, most recommendations are based on expert opinions and not on evidence-based medicine.
Primary GV are present at the time of the initial endoscopy and before any endoscopic/surgical intervention, and secondary GV are those which appear after endoscopic/surgical intervention.
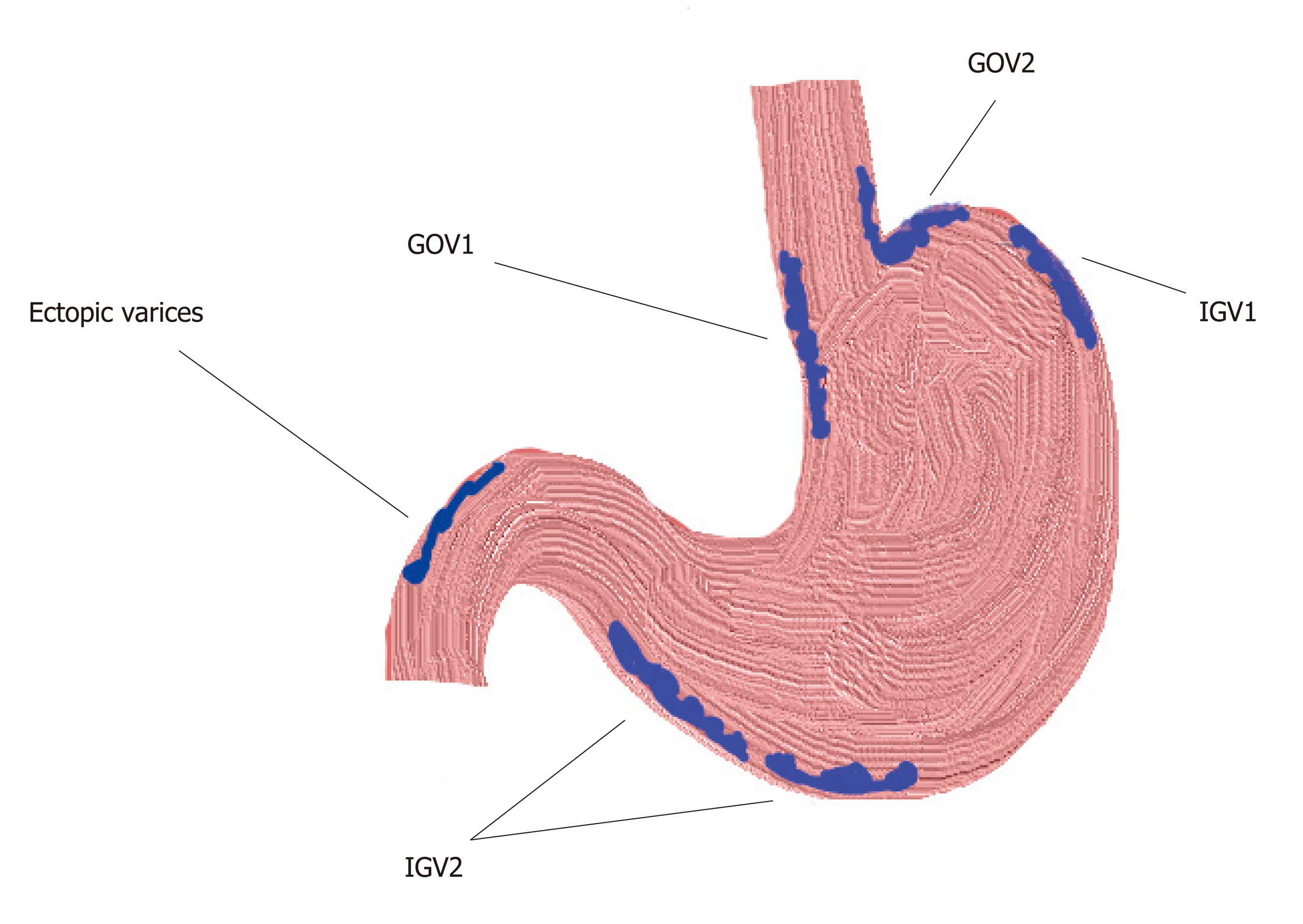
There are four types[1](Figure 1). Gastroesophageal varices (GOV)-1; gastro-OV type-1 are OV extending beneath the cardia through the lesser curvature. They are the most common type of GV, comprising around 75% of all GV. They are almost always associated with large OV (92%).
GOV-2; gastro-OV type-2 are OV extending beneath the cardias through the fundus. They comprise around 21% of all GV and are associated with the presence of large OV in 50% of cases.
Isolated gastric varices (IGV)-1; isolated GV type 1 are not connected with OV and they are located on the fundus. They are seen in only 1.6% of patients with GV.
IGV-2; isolated GV type 2 are not connected with OV and are present in the stomach but not in the fundus. They are seen in 4.2% of patients with GV. This type of varices usually develops during or after endoscopic obliteration of oesophageal or GV, around 85% of the cases. In the other 15% of the cases portal vein thrombosis with or without liver cirrhosis is found. Most of these cases (59%) have other types of GV associated. Only 6% of these patients bleed during a mean follow up of 3 years[5].
A new type has been recently proposed by Singh, around 11% of patients with oesophageal and GV who cannot be classified in any of the other types, i.e., having OV and GV in the body, pylorus or antrum[6]. Clinical characteristic of this new type of GV has not been investigated yet.
They are classified in small (< 5 mm), medium (5 to 10 mm) and large (> 10 mm). GV are more common in segmental portal hypertension caused by portal/splenic vein thrombosis, than in generalized portal hypertension due to cirrhosis[7]. This is probably due to a more direct transmission of increased portal pressure to the short and posterior GV. Fundal varices, i.e., IVG-1 and GOV-2 type varices, are developed by dilation of short and posterior gastric veins and large gastro-renal shunts are usually present.
Bleeding from GV is diagnosed when (1) active bleeding from a gastric varix is seen in endoscopy, (2) a clot or ulcer is seen over the gastric varix, (3) in the presence of large GV, absence of OV, and no other cause for upper gastrointestinal bleeding detected.
GOV2s bleed more frequently than GOV1s. Secondary GOV2s carry the worst prognosis, 38% of patients with this type of varices died of bleeding. IGV-1s bleed in a similar frequency as GOV2s, but are less frequently present[1].
The main risk factors for bleeding are the degree of liver dysfunction, location, size, and presence of red spots on the varix[3,8,9].
GV bleed less frequently than OV, but it seems GV bleed more severely than OV. The transfusion requirement is higher in gastric than in oesophageal variceal bleeding, 4.8 ± 0.6 vs 2.9 ± 0.3 transfusion unit/person, P < 0.01[3].
In view of the absence of data GOV1 should follow same guidelines as OV[10] and thus, we are going to discuss the management of fundal varices only in this section.
There is only one trial assessing primary prophylaxis in patients with GV and it was a prospective study. Eighty-nine cirrhotic patients with high risk GV, i.e., large (> 10 mm) and located in the fundus (GOV2/IGV1) were included. They had no history of gastrointestinal bleeding and no OV were present at diagnostic endoscopy. They were randomized to receive either cyanoacrylate injection until complete obliteration (Group I), or propranolol with a target heart rate of 55/min or maximal dose of 360 mg/d (Group II), or no treatment (Group III). The median follow-up period was 26 mo[11,12] (Table 1).
| Characteristics | Cyanoacrylate Group I(n = 30) | PropranololGroup II(n = 29) | No treatmentGroup III(n = 30) | P value |
| GV bleed | 10% | 38% | 53% | 0.003 |
| Bleed-related mortality | 0 | 10% | 24% | 0.034 |
| Overall mortality | 7% | 17% | 26% | 0.113 |
| Complications | 3% | 3% | 7% | 1 |
This study showed that cyanoacrylate injection was superior to propranolol and to no therapy in preventing bleeding. Some experts recommend using non-selective beta-blockers (NSBB) as primary prophylaxis and avoiding cyanoacrylate injections because they consider this study very particular since it was conducted in a single expert centre and thus, it is considered not enough evidence to generalise its findings[13,14]. Glue injection requires expertise which is not always available and the low complication rate in the study reflects the high skills of endoscopists who conducted the study, which is not widely reproducible. Moreover, data suggesting that carvedilol is more effective in reducing the hepatic venous pressure gradient (HVPG) may reduce the gap in efficacy between NSBB and cyanoacrylate injection. On the other hand, it is well known that bleeding from GV does not depend only on the HVPG, but also on the wall tension and size of the varix. Some experts refrain from issuing any recommendation in view of the lack of strong evidence[10]. There is a clear need for research in this area.
In view of the absence of data GOV1 should follow same guidelines as OV[10] and thus, we are going to discuss management of fundal varices only in this section as well.
There is less evidence supporting prevention of re-bleeding in GV compared to OV. There have been trials testing NSBB, endoscopic injection of tissue adhesives, endoscopic band ligation (EBL), Transjugular Intrahepatic Portosystemic Shunt (TIPS), and Balloon-occluded Retrograde Transvenous Obliteration (BRTO). It is important to note that these studies included patients at the time of the index bleeding which could be confusing in terms of overlap with treatment of acute bleeding, but patients with uncontrolled bleeding were excluded. Therefore, all of participants had successful treatment of the acute bleeding.
There are two prospective trials comparing NSBB versus glue injection alone and versus glue injection plus NSBB[15,16].
The first study compared endoscopic injection of cyanoacrylate versus propranolol. Thirty-two patients were allocated in each group. All patients had portal hypertension secondary to liver cirrhosis and all had fundal varices, i.e., GOV-2 with eradicated OV or patients with IGV-1, all of them were large, i.e., at least 10mm in width. These types of GV have the highest risk of bleeding. Re-bleeding from GV was managed with TIPS or surgery in the cyanoacrylate group and with cyanoacrylate injection in the propranolol group. The fact that the two groups received different rescue therapies may be a bias affecting survival. Patients on the propranolol branch were monitored daily until the target dose was achieved. Afterwards, they were monitored every three months. Results showed that rate of re-bleeding and mortality were significantly lower in the cyanoacrylate group, 10% vs 44%, P = 0.004 and 3% vs 23%, P = 0.023[15]. We must interpret these results with caution because all patients were enrolled shortly after GV bleeding but 77% of them did not receive endoscopic treatment to control the acute bleeding. Thus, an important part of the patients allocated to Beta-blocker group did not receive adequate endoscopic treatment for the episode of index bleeding while the patients allocated to the glue injection group did. This difference would be a disadvantage in the Beta-blocker group regarding re-bleeding rate.
The second study compared glue injection alone versus glue injection plus propranolol. Forty-eight and 47 patients were included in each group, respectively. The study showed similar re-bleeding rates between both groups, 54% vs 47%, P = NS. Mortality rate was also similar, 42% vs 47%, P= NS[16]. Authors stated that these findings could be explained because portal hypertension may not be as critical as in OV[17], and because most patients have segmental portal hypertension or gastro-renal portosystemic shunting. Patient in the beta-blocker group experienced more asthenia, 60% vs 23%, P < 0.01, but overall side effects were similar in both groups. Experts recommend eradication with cyanoacrylate injections as first line therapy[14].
Histoacryl® is monomeric n-butyl-2-cyanoacrylate. One prospective study compared both treatments in patients with cirrhosis and gastric variceal bleeding. Participants were selected at the time of index bleeding from GV. Patients bleeding from OV were excluded. Patients with severe decompensation of cirrhosis were also excluded. Acute bleeding was treated with somatostastin and glue injection initially. Thirty-five patients were allocated to TIPS and 37 to cyanoacrylate injections. Re-bleeding from GV was lower in the TIPS group, 11% vs 38%, P = 0.014. Nevertheless, upper GI bleeding and 2-year survival were similar between both groups: 43% vs 59% and 70% vs 83%, respectively[18]. The lack of impact on mortality may be attributed to the increased rate of hepatic encephalopathy and to liver dysfunction in the TIPS group, two of the patients in the TIPS group developed liver failure. According to the authors, there was some delay in elective treatment. In fact, two episodes of gastric variceal bleeding occurred between randomization and elective treatment. Moreover, the high rate of re-bleeding in the Histoacryl® group could be related to the non-compliance of some patients and to the low dose injected at each session. Furthermore, some of the bleeding episodes attributed to GV were ulcers post glue injection and not to portal hypertension. Those patients were started on proton pump inhibitors only when an ulcer was diagnosed during endoscopic follow-up and not as standard prophylaxis. Finally, half of the patients included in this study had GOV1 which clinical characteristics are similar to OV and not to fundal varices (GOV2 and IGV1). There is a clear need of research in this area.
Thrombin injection and BRTO have been evaluated only in the acute bleeding setting, but no as prophylactic treatment.
Multiple guidelines are available that discuss the management of active variceal bleeding, these include the American Association for the Study of Liver Disease 2016 guidelines[13], the British Society of Gastroenterology (BSG) 2015 guidelines[14], and the 2015 International consensus statement (Baveno VI)[10].
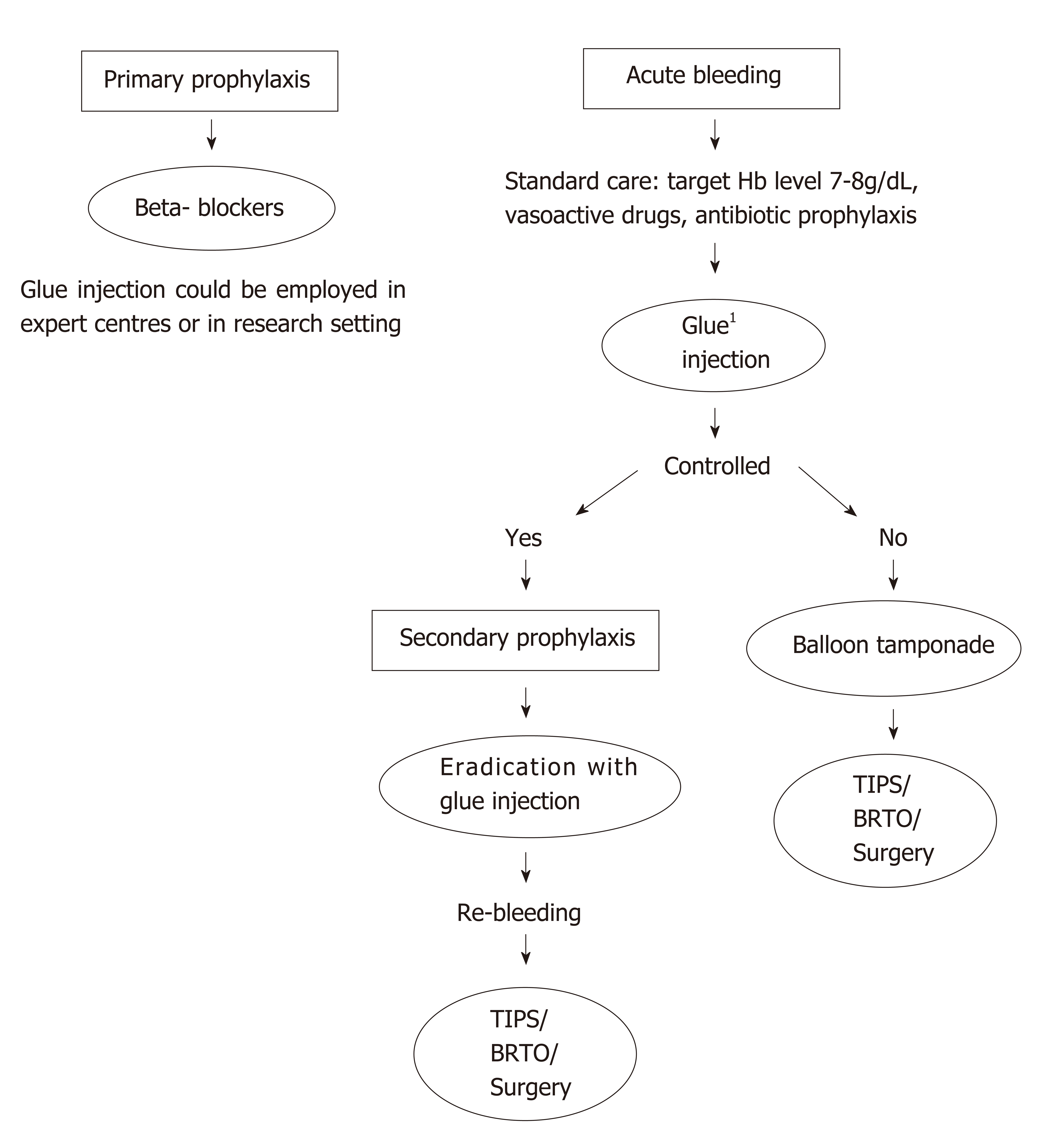
The main stay of treatment for gastric variceal bleeding is initially similar to that of oesophageal variceal bleeding and is based on good fluid resuscitation, correction of coagulopathies, early pharmacological treatments with antibiotics and vasoactive medications and early endoscopic intervention. Radiological management, balloon tamponade and surgical management are typically reserved for those who fail to achieve haemostasis with endoscopic and pharmacological therapy; although the early use of interventional radiological procedures is likely to play a greater role in the management of gastric variceal bleeding[10].
Restoration of circulating volume should be achieved whilst avoiding over transfusion to prevent a rebound of portal hypertension and precipitate re-bleeding. A recent study showed that a restrictive transfusion policy to a haemoglobin of 7-8 g/dL resulted in better outcomes and less complications[19]. With regards to coagulopathies the BSG guidelines suggest a platelet transfusion if the count is below < 50 × 109/L and fresh frozen plasma to be used for patients with a fibrinogen level of < 1 g/L or clotting derangement > 1.5 times greater than normal[14]. Although, in contrast, the Baveno Consensus statement feel that recommendations regarding coagulopathy and thrombocytopenia could not be made on the basis of currently available data[10]. We would suggest that correction of coagulopathies be based on local guidelines and patient factors such as the severity of the bleeding and their co-morbidities.
Initial management of GV bleed should include early use of pharmacological agents in the form of prophylactic antibiotics and vasoactive drugs and these should be initiated at an early stage[10].
Antibiotics: Prophylactic antibiotics should be given to all patients with variceal bleeding to decrease the risk of bacterial infections and improve survival and is recommended in all of the prior mention guidelines and consensus. Antibiotics of choice are those that are active against enteric bacteria of which Cephalosporins are the most widely used and studied. Data was first published in 1985 showing prophylactic antibiotics reduced the rate of infections in patients with cirrhosis and variceal bleeding[20] and multiple studies and meta-analysis since have confirmed this[21]. Moreover, other studies showed that re-bleeding rates are lower after the use of prophylactic antibiotics[22]. No studies have been published looking specifically at antibiotic therapy solely in GV haemorrhage but many studies discussed variceal bleeding without differentiating between OV and GV bleeding.
A 2011 meta-analysis of 12 trials including 1241 patients looked at antibiotic prophylaxis against placebo/no antibiotic prophylaxis in patients with cirrhosis and upper gastro intestinal bleeding and reported that antibiotic prophylaxis was associated with reduced mortality (RR 0.79, 95%CI: 0.63-0.98), reduced bacterial infections (RR 0.35, 95%CI: 0.26-0.47), reduced re-bleeding (RR 0.53, 95%CI: 0.38-0.74) and shorter length of stay (MD -1.91, 95%CI: -3.80-0.02)[23]. No note was made of whether any of these trials looked purely at GV bleeding and in fact, some trials included non-variceal haemorrhage.
A current Cochrane review protocol has been published in November 2018 stating the aim of reviewing the role of antibiotics in patients with cirrhosis and variceal bleeding[24].
The choice of antibiotics should be guided by local microbiology advice and guidelines and take into account the prevalence of local resistance, the prior use of prophylactic antibiotics and other external facts, for example norfloxacin is no longer available in the United States nor in the United Kingdom.
Vasoactive medications: Vasoactive drugs decrease the portal venous blood flow. They include vasopressin and its analogue terlipressin, and somatostatin and its analogue octreotide. Their use is recommended in all major guidelines although again, it must be noted that no studies have looked purely at the use of vasoactive medication in GV bleeding[10,13,14].
In 2012, a metanalysis of 30 trials including 3111 patients showed that the use of vasoactive agents was associated with a significantly lower risk of 7-d mortality (RR 0.74; 95%CI: 0.57-0.95), an improvement in haemostasis (RR 1.21, 95%CI: 1.13-1.30), less transfusions requirement (pooled mean difference -0.70 units of blood transfused, 95%CI: -1.01 to -0.38;) and a shorter duration of hospitalisation (pooled mean difference -0.71 d; 95%CI: -1.23 to -0.19). They reported that studies comparing different vasoactive agents did not show a difference in efficacy, although the quality of evidence was very low[25].
Terlipressin is a synthetic analogue of vasopressin that is administered as intermittent injections. In a meta-analysis it showed lower risk of complications compared to vasopressin[26]. It is currently not licensed for use in the United States but is the preferred medication of choice outside of the United States. A Cochrane review in 2003 showed that terlipressin was the only medication to reduce mortality[27].
Studies reviewing the endoscopic management of GV bleeding are limited compared to those related to OV bleeding interventions; however, endoscopy intervention is still the main stay of treatment and should be offered to all patients with suspected severe variceal bleed immediately after resuscitation or for more stable patients within 24 h[14].
Tissue adhesives: Endoscopic therapies that have been studied include tissues adhesives mainly cyanoacrylate glues but also fibrin and thrombin therapy, EBL and sclerosants including alcohol; with the use of tissue adhesive being consider as first-choice treatment in most parts of the world.
Sclerosing agents including alcohol have been used with varying success in GV bleeding, often with high re-bleeding rates[28] and the most current ASGE guidelines on sclerosing agents, whilst focusing mainly on their use in OV’s, suggest their use should be limited but may be considered in some circumstances such as treating OGV combined with EBL[29].
Only a small number of studies could be found looking at EBL for active GV bleeding and often with low numbers of participants. One study reported a series of 27 patients with GV, with active bleeding in 18 patients, EBL achieved haemostasis in 16 of the 18 patients (88.8%). However, recurrent bleeding was noted in five of the 27 patients (18.5%)[30]. Another study reported 22 patients with active bleeding from GV treated with EBL; all patients achieved initial haemostasis and there was no immediate complication; however, four patients (18.2%) developed early re-bleeding[31].
One randomised study looked at the use of tissue adhesive versus EBL for acute GV bleeding and whilst both interventions were as effective at controlling the initial bleed, the use of glue was associated with lower re-bleeding rates[32].
A meta-analysis of tissue adhesive versus EBL for active GV bleeding, only including three suitable trials with 194 patients, reported that control of bleeding was achieved in 93.9% of patients treated with tissue adhesive versus 79.5% in the EBL groups (P = 0.032). Re-bleeding rate was comparable in GOV2 between the 2 interventions (35.7% vs 34.8%, P = 0.895), but cyanoacrylate use was superior at reducing re-bleeding rates in GOV1 and IGV1[33]. They concluded that tissue adhesives were superior but that in places where it was not available EBL could still be a useful treatment option.
With regards to tissue adhesives cyanoacrylates glues are the commonest used and are a group of synthetic glues that solidify rapidly on contact with weak bases i.e., water and blood. They are often mixed with lipiodol, any oily emulsion, to slow their rate of solidification thus reducing the chance of inadvertent adherence to the endoscope or catheter and also allowing imaging visualisation of the glue after injection in the case of complications and distal embolization.
ASGE reports that cyanoacrylates use has an initial haemostasis rates in the reign of 80%-90% and that tissue adhesive is superior to sclerotherapy or EBL for control of GV haemorrhage[34]. Higher rates of haemostasis have been reported in many studies ranging from 91%-100% with re-bleeding rates ranging from 7% to 28%[35,36]. The Baveno consensus and the BSG guidelines recommend the use of tissue adhesives such as N-butyl cyanoacrylate in the use of GV bleeding[10,14].
Other tissues adhesives include thrombin which is a human or bovine protein that affects haemostasis by converting fibrinogen to a fibrin clot. There are no controlled trials looking at its use but one case series looked at 37 patients and reported that human thrombin was an effective treatment for active GV bleeding but re-bleeding occurred in 4 patients[37]. Other case series would suggest its use is safe and effective with low side effect profile but with repeated interventions sometimes needed[38,39].
Hemospray: TC-325 (Hemospray, Cook Medical, Winstom-Salem, North Carolina, United States) is a haemostatic powder which becomes cohesive and adhesive when gets in contact with blood or tissue in the GI tract, forming an effective mechanical barrier covering the bleeding site and thus, achieving quick haemostasis. Its effect lasts approximately 24 hours, because the haemostatic layer sloughs off. Currently, it is only licensed for the treatment of non-variceal Upper GI bleed. However, two recent studies by Ibrahim et al have shown that Hemospray could be employed in active variceal bleeding as a bridge to a definitive treatment[40,41].
The first study[40] was a single arm prospective study on 38 patients admitted with acute variceal bleed from oesophageal or GV (GV were present in 10% and IGV2 were present in 6.6%). Gastroscopy was performed within 6 hours of admission after hemodynamic stabilization to confirm acute variceal bleeding and Hemospray was applied as primary measure. Clinical haemostasis was achieved in 29 of 30 (96.7%). Only 13 of 30 patients (43.4%) had active bleeding at the time of endoscopy. A follow up endoscopy was performed within 24 h for definitive treatment with either banding or cyanoacrylate injection.
The second study[41] was a recent prospective, randomized study including 86 patients with active variceal bleeding from oesophageal or GV who were randomly allocated to early endoscopy (within 2 h) with application of hemospray plus pharmacological therapy or to a group who received pharmacological therapy alone. The authors showed higher haemostasis rate at the time of definitive endoscopy (within 12-24 h) and lower mortality rate in the intervention group. The authors concluded that hemospray could be employed as a bridge to definitive intervention in remote centres where the expertise to apply banding/glue injection is not easily available but the one to apply hemospray is. Probably, this recommendation is applicable to a minor proportion of centres. Moreover, their findings may suggest that earlier endoscopic intervention is better, regardless of the type of intervention, especially if the medical treatment is not the recommended first line option, they used octreotide instead of terlipressin.
There was also an anecdotic report employing Hemospray as a rescue therapy of actively bleeding gastric varix after failure of cyanoacrylate injection[42]. Currently, there is still little evidence to support the routine use of Hemospray in the management of active variceal bleeding.
Balloon tamponade: Balloon tamponade is an effective short-term measure to achieve control of bleeding; however, due to the observed complication rates and the high risk of re-bleeding once the balloon is deflated this measure should be considered a temporary measure until definitive control of the bleeding can be achieved.
The use of balloon tamponade was first described as early as 1930 by Westphal but was named as Sengstaken-Blakemore (SB) tube in after the Sengstaken and Blakemore paper in 1950. Three tubes are available, the SB tube, which has two balloons, gastric and oesophageal, and a gastric suction port, the Minnesota tube (a modified SB tube with the addition of an oesophageal suction port to try and prevent aspiration), and the Linton- Nachlas tube, which has a single gastric balloon, but of larger volume and a gastric suction port only. Their use has decreased over time as endoscopic and pharmacological measures improve outcomes and remove the need for balloon tamponade[43].
Most studies regarding balloon tamponade relate to OV bleeding[44] or do not differentiate between OV and GV bleeding, however one study found initial success rates of 88% with the use of balloon tamponade in GV (vs 91.5% in OV bleeding) whilst their reported complication rate was 10% and mainly related to aspiration[45].
Complications of balloon tamponade relate normally to misplacement of the tube or pressure effects from over inflation or the balloon being inflated for too long. Complications include oesophageal ulceration, necrosis and rupture and aspiration pneumonia[46] and consequently their use is recommended to be limited to temporary control until more definitive management can be put in place[14].
TIPS: It is widely used as a salvage option for GV bleeding and is increasingly used as first line treatment, especially in the United States and Europe whilst BRTO remains more commonly used in the Eastern countries.
TIPS was first shown to be successful for GV bleeding in a 1998 study that showed outcomes for TIPS in acute GV versus OV bleeding was equal with haemostasis being achieved in all but one patient. Re-bleeding occurred in 4/28 patients due to shunt thrombosis or occlusion, which the authors report was easily diagnosed and managed. The study therefore established the role of TIPS as a rescue procedure in management of uncontrolled GV[47].
Investigating the role of TIPS in GV remains difficult as many studies do not distinguish OV from GV bleeding and some pivotal studies in TIPS exclude patients with isolated GV bleeding[48]. Due to severe hepatic dysfunction often patients are not TIPS candidates and TIPS is not available in all centres thus the use of cyanoacrylate glue may be the only viable option for control of the haemorrhage.
A single centre study that showed in cyanoacrylate glue vs TIPS there was no difference in re-bleeding rates but noted that patients treated with glue therapy had significantly less long-term morbidity then the TIPS patients[49] and this was again reported in a retrospective review in 2015 comparing cyanoacrylate glue with TIPS which noted no difference in re-bleeding rates and mortality[50].
BRTO: BRTO is an interventional radiology technique which accessed the GV via a gastrorenal shunt, which is occluded with a balloon while the sclerosant agent is injected in the GV. There has been a series of retrospective reports, but no prospective trial has been conducted so far. Early reports showed it was a feasible procedure[51]. Technical success is high and it is an effective method when employed as prophylaxis[52] and also in the acute setting[53]. The main side effects include the development of new or progression of pre-existing OV, vascular damage or migration of the sclerosant agent when the balloon is inadvertently displaced. When BRTO is compared with TIPS it seems that BRTO is equally effective[54] or even superior than TIPS[55], but the evidence is based only on retrospective series of patients and thus, these results cannot be generalized. There has been a recent meta-analysis based on six studies comparing TIPS vs BRTO which showed a decrease in mortality rate in the BRTO compared to the TIPS group (RR 0.44, 95%CI: 0.35-0.56, P < 0.01). It also showed BRTO group had lower re-bleeding risk (RR0.38, 95%CI: 0.24-0.59, P < 0.01) and lower encephalopathy risk (RR: 0.07, 95%CI: 0.03-0.16, P < 0.01)[56].
The current recommendation for BRTO is to be applied as a rescue therapy when TIPS is contraindicated, such as in cases of advanced liver failure or hepatic encephalopathy. Nevertheless, a gastrorenal shunt must be present to use BRTO.
Surgery: It is applicable only in highly specialised centres and consists of selective shunts in carefully selected patients, with well-preserved synthetic function, otherwise risk of complications is unacceptably high[57]. In cases of segmental portal hypertension, splenectomy could be considered[7](Figure 2).
There is little literature regarding management of GV compared with the abundant quantity published on OV. Most recommendations for the management of acute bleeding are extrapolated from trials including mainly patients with OV bleeding and only a small proportion with GV. In fundal varices (i.e., GOV2 and IGV1) management with cyanoacrylate injections is the preferred option leaving TIPS or BRTO as a rescue therapy. With regards to prophylaxis, there is not enough evidence on secondary prophylaxis and even less on primary prophylaxis to make strong recommendations. Probably, cyanoacrylate injection has a role in both primary and secondary prophylaxis, but most experts prefer to suggest NSBB in primary prophylaxis as they are less invasive and easily accessible. There is still a wide area for research in GV therapy.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Hernanda PY, Kao JT, Konishi H, Lo GH, Pallav K, Rodríguez-Perálvarez M S-Editor: Cui LJ L-Editor: A E-Editor: Zhang YL
| 1. | Sarin SK, Lahoti D, Saxena SP, Murthy NS, Makwana UK. Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology. 1992;16:1343-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 848] [Article Influence: 25.7] [Reference Citation Analysis (42)] |
| 2. | Teng W, Chen WT, Ho YP, Jeng WJ, Huang CH, Chen YC, Lin SM, Chiu CT, Lin CY, Sheen IS. Predictors of mortality within 6 weeks after treatment of gastric variceal bleeding in cirrhotic patients. Medicine (Baltimore). 2014;93:e321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Kim T, Shijo H, Kokawa H, Tokumitsu H, Kubara K, Ota K, Akiyoshi N, Iida T, Yokoyama M, Okumura M. Risk factors for hemorrhage from gastric fundal varices. Hepatology. 1997;25:307-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 4. | Lee CH, Lee JH, Choi YS, Paik SW, Sinn DH, Lee CY, Koh KC, Gwak GY, Choi MS, Yoo BC. Natural history of gastric varices and risk factors for bleeding. Korean J Hepatol. 2008;14:331-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Sarin SK, Jain AK, Lamba GS, Gupta R, Chowdhary A. Isolated gastric varices: prevalence, clinical relevance and natural history. Dig Surg. 2003;20:42-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Verma N, Kumari S, Kumari P, De A, Singh V. New classification of gastric varices: a twenty-year experience. J Hepatol. 2017;66:S543-S750. [DOI] [Full Text] |
| 7. | Madsen MS, Petersen TH, Sommer H. Segmental portal hypertension. Ann Surg. 1986;204:72-77. [PubMed] |
| 8. | Komori K, Kubokawa M, Ihara E, Akahoshi K, Nakamura K, Motomura K, Masumoto A. Prognostic factors associated with mortality in patients with gastric fundal variceal bleeding. World J Gastroenterol. 2017;23:496-504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Triantafyllou M, Stanley AJ. Update on gastric varices. World J Gastrointest Endosc. 2014;6:168-175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2011] [Cited by in RCA: 2294] [Article Influence: 229.4] [Reference Citation Analysis (3)] |
| 11. | Mishra SR, Sharma BC, Kumar A, Sarin SK. Primary prophylaxis of gastric variceal bleeding comparing cyanoacrylate injection and beta-blockers: a randomized controlled trial. J Hepatol. 2011;54:1161-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 142] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 12. | Tripathi D. Primary prophylaxis against gastric variceal bleeding: is there a sticky solution at last? Hepatology. 2011;54:1094-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1441] [Article Influence: 180.1] [Reference Citation Analysis (3)] |
| 14. | Tripathi D, Stanley AJ, Hayes PC, Patch D, Millson C, Mehrzad H, Austin A, Ferguson JW, Olliff SP, Hudson M, Christie JM; Clinical Services and Standards Committee of the British Society of Gastroenterology. U.K. guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut. 2015;64:1680-1704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 454] [Cited by in RCA: 416] [Article Influence: 41.6] [Reference Citation Analysis (2)] |
| 15. | Mishra SR, Chander Sharma B, Kumar A, Sarin SK. Endoscopic cyanoacrylate injection versus beta-blocker for secondary prophylaxis of gastric variceal bleed: a randomised controlled trial. Gut. 2010;59:729-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Hung HH, Chang CJ, Hou MC, Liao WC, Chan CC, Huang HC, Lin HC, Lee FY, Lee SD. Efficacy of non-selective β-blockers as adjunct to endoscopic prophylactic treatment for gastric variceal bleeding: a randomized controlled trial. J Hepatol. 2012;56:1025-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 17. | Tripathi D, Therapondos G, Jackson E, Redhead DN, Hayes PC. The role of the transjugular intrahepatic portosystemic stent shunt (TIPSS) in the management of bleeding gastric varices: clinical and haemodynamic correlations. Gut. 2002;51:270-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 163] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 18. | Lo GH, Liang HL, Chen WC, Chen MH, Lai KH, Hsu PI, Lin CK, Chan HH, Pan HB. A prospective, randomized controlled trial of transjugular intrahepatic portosystemic shunt versus cyanoacrylate injection in the prevention of gastric variceal rebleeding. Endoscopy. 2007;39:679-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 222] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 19. | Villanueva C, Colomo A, Bosch A. Transfusion for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:1362-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1186] [Cited by in RCA: 1069] [Article Influence: 89.1] [Reference Citation Analysis (0)] |
| 20. | Rimola A, Bory F, Teres J, Perez-Ayuso RM, Arroyo V, Rodes J. Oral, nonabsorbable antibiotics prevent infection in cirrhotics with gastrointestinal hemorrhage. Hepatology. 1985;5:463-467. [PubMed] |
| 21. | Fernández J, Acevedo J. New antibiotic strategies in patients with cirrhosis and bacterial infection. Expert Rev Gastroenterol Hepatol. 2015;9:1495-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Hou MC, Lin HC, Liu TT, Kuo BI, Lee FY, Chang FY, Lee SD. Antibiotic prophylaxis after endoscopic therapy prevents rebleeding in acute variceal hemorrhage: a randomized trial. Hepatology. 2004;39:746-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 246] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 23. | Chavez-Tapia NC, Barrientos-Gutierrez T, Tellez-Avila FI, Soares-Weiser K, Uribe M. Antibiotic prophylaxis for cirrhotic patients with upper gastrointestinal bleeding. Cochrane Database Syst Rev. 2010;CD002907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 24. | Sanchez-Jimenez B, Chavez-Tapia NC, Jakobsen JC. Cochrane Database of Systematic Reviews. Antibiotic prophylaxis versus placebo or no intervention for people with cirrhosis and variceal bleeding Cochrane Systematic Review. [Accessed 08 November 2018] Intervention. Protocol Version. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Wells M, Chande N, Adams P, Beaton M, Levstik M, Boyce E, Mrkobrada M. Meta-analysis: vasoactive medications for the management of acute variceal bleeds. Aliment Pharmacol Ther. 2012;35:1267-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 147] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 26. | Zhou X, Tripathi D, Song T, Shao L, Han B, Zhu J, Han D, Liu F, Qi X. Terlipressin for the treatment of acute variceal bleeding: A systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2018;97:e13437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 27. | Ioannou G, Doust J, Rockey DC. Terlipressin for acute esophageal variceal hemorrhage. Cochrane Database Syst Rev. 2003;CD002147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 28. | Trudeau W, Prindiville T. Endoscopic injection sclerosis in bleeding gastric varices. Gastrointest Endosc. 1986;32:264-268. [PubMed] |
| 29. | Hwang JH, Shergill AK, Acosta RD, Chandrasekhara V, Chathadi KV, Decker GA, Early DS, Evans JA, Fanelli RD, Fisher DA, Foley KQ, Fonkalsrud L, Jue T, Khashab MA, Lightdale JR, Muthusamy VR, Pasha SF, Saltzman JR, Sharaf R, Cash BD; American Society for Gastrointestinal Endoscopy. The role of endoscopy in the management of variceal hemorrhage. Gastrointest Endosc. 2014;80:221-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 149] [Article Influence: 13.5] [Reference Citation Analysis (1)] |
| 30. | Shiha G, El-Sayed SS. Gastric variceal ligation: a new technique. Gastrointest Endosc. 1999;49:437-441. [PubMed] |
| 31. | Lee TH, Shih LN. Clinical experience of endoscopic banding ligation for bleeding gastric varices. Hepatogastroenterology. 2008;55:766-769. [PubMed] |
| 32. | Tan PC, Hou MC, Lin HC, Liu TT, Lee FY, Chang FY, Lee SD. A randomized trial of endoscopic treatment of acute gastric variceal hemorrhage: N-butyl-2-cyanoacrylate injection versus band ligation. Hepatology. 2006;43:690-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 227] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 33. | Qiao W, Ren Y, Bai Y, Liu S, Zhang Q, Zhi F. Cyanoacrylate Injection Versus Band Ligation in the Endoscopic Management of Acute Gastric Variceal Bleeding: Meta-Analysis of Randomized, Controlled Studies Based on the PRISMA Statement. Medicine (Baltimore). 2015;94:e1725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 34. | ASGE Technology Committee. Bhat YM, Banerjee S, Barth BA, Chauhan SS, Gottlieb KT, Konda V, Maple JT, Murad FM, Pfau PR, Pleskow DK, Siddiqui UD, Tokar JL, Wang A, Rodriguez SA. Tissue adhesives: cyanoacrylate glue and fibrin sealant. Gastrointest Endosc. 2013;78:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 35. | Franco MC, Gomes GF, Nakao FS, de Paulo GA, Ferrari AP, Libera ED. Efficacy and safety of endoscopic prophylactic treatment with undiluted cyanoacrylate for gastric varices. World J Gastrointest Endosc. 2014;6:254-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | Kang EJ, Jeong SW, Jang JY, Cho JY, Lee SH, Kim HG, Kim SG, Kim YS, Cheon YK, Cho YD, Kim HS, Kim BS. Long-term result of endoscopic Histoacryl (N-butyl-2-cyanoacrylate) injection for treatment of gastric varices. World J Gastroenterol. 2011;17:1494-1500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 37. | McAvoy NC, Plevris JN, Hayes PC. Human thrombin for the treatment of gastric and ectopic varices. World J Gastroenterol. 2012;18:5912-5917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 38. | Ramesh J, Limdi JK, Sharma V, Makin AJ. The use of thrombin injections in the management of bleeding gastric varices: a single-center experience. Gastrointest Endosc. 2008;68:877-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Jhajharia A, Wanjari SJ, Ashdhir P, Pokharna R, Nijhawan S. Role and safety of human thrombin injection for the treatment of bleeding gastric varices. Indian J Gastroenterol. 2018;37:321-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | Ibrahim M, El-Mikkawy A, Abdalla H, Mostafa I, Devière J. Management of acute variceal bleeding using hemostatic powder. United European Gastroenterol J. 2015;3:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 41. | Ibrahim M, El-Mikkawy A, Abdel Hamid M, Abdalla H, Lemmers A, Mostafa I, Devière J. Early application of haemostatic powder added to standard management for oesophagogastric variceal bleeding: a randomised trial. Gut. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 42. | Holster IL, Poley JW, Kuipers EJ, Tjwa ET. Controlling gastric variceal bleeding with endoscopically applied hemostatic powder (Hemospray™). J Hepatol. 2012;57:1397-1398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 43. | Carbonell N, Pauwels A, Serfaty L, Fourdan O, Lévy VG, Poupon R. Improved survival after variceal bleeding in patients with cirrhosis over the past two decades. Hepatology. 2004;40:652-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 527] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 44. | Sarin SK, Nundy S. Balloon tamponade in the management of bleeding oesophageal varices. Ann R Coll Surg Engl. 1984;66:30-32. [PubMed] |
| 45. | Panés J, Terés J, Bosch J, Rodés J. Efficacy of balloon tamponade in treatment of bleeding gastric and esophageal varices. Results in 151 consecutive episodes. Dig Dis Sci. 1988;33:454-459. [PubMed] |
| 46. | Cook D, Laine L. Indications, technique, and complications of balloon tamponade for variceal gastrointestinal bleeding. J Intensive Care Med. 1992;7:212-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 47. | Chau TN, Patch D, Chan YW, Nagral A, Dick R, Burroughs AK. "Salvage" transjugular intrahepatic portosystemic shunts: gastric fundal compared with esophageal variceal bleeding. Gastroenterology. 1998;114:981-987. [PubMed] |
| 48. | García-Pagán JC, Caca K, Bureau C, Laleman W, Appenrodt B, Luca A, Abraldes JG, Nevens F, Vinel JP, Mössner J, Bosch J; Early TIPS (Transjugular Intrahepatic Portosystemic Shunt) Cooperative Study Group. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362:2370-2379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 842] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 49. | Procaccini NJ, Al-Osaimi AM, Northup P, Argo C, Caldwell SH. Endoscopic cyanoacrylate versus transjugular intrahepatic portosystemic shunt for gastric variceal bleeding: a single-center U.S. analysis. Gastrointest Endosc. 2009;70:881-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 50. | Kochhar GS, Navaneethan U, Hartman J, Mari Parungao J, Lopez R, Gupta R, Kapoor B, Mehta P, Sanaka M. Comparative study of endoscopy vs. transjugular intrahepatic portosystemic shunt in the management of gastric variceal bleeding. Gastroenterol Rep (Oxf). 2015;3:75-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 51. | Choi YH, Yoon CJ, Park JH, Chung JW, Kwon JW, Choi GM. Balloon-occluded retrograde transvenous obliteration for gastric variceal bleeding: its feasibility compared with transjugular intrahepatic portosystemic shunt. Korean J Radiol. 2003;4:109-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 114] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 52. | Katoh K, Sone M, Hirose A, Inoue Y, Fujino Y, Onodera M. Balloon-occluded retrograde transvenous obliteration for gastric varices: the relationship between the clinical outcome and gastrorenal shunt occlusion. BMC Med Imaging. 2010;10:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 53. | Jang SY, Kim GH, Park SY, Cho CM, Tak WY, Kim JH, Choe WH, Kwon SY, Lee JM, Kim SG, Kim DY, Kim YS, Lee SO, Min YW, Lee JH, Paik SW, Yoo BC, Lim JW, Kim HJ, Cho YK, Sohn JH, Jeong JY, Lee YH, Kim TY, Kweon YO. Clinical outcomes of balloon-occluded retrograde transvenous obliteration for the treatment of gastric variceal hemorrhage in Korean patients with liver cirrhosis: a retrospective multicenter study. Clin Mol Hepatol. 2012;18:368-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 54. | Kim SK, Lee KA, Sauk S, Korenblat K. Comparison of Transjugular Intrahepatic Portosystemic Shunt with Covered Stent and Balloon-Occluded Retrograde Transvenous Obliteration in Managing Isolated Gastric Varices. Korean J Radiol. 2017;18:345-354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 55. | Lee SJ, Kim SU, Kim MD, Kim YH, Kim GM, Park SI, Won JY, Lee DY, Lee KH. Comparison of treatment outcomes between balloon-occluded retrograde transvenous obliteration and transjugular intrahepatic portosystemic shunt for gastric variceal bleeding hemostasis. J Gastroenterol Hepatol. 2017;32:1487-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 56. | Hamdeh S, Altayar O, Aziz M. Balloon-Occluded Retrograde Transvenous Obliteration (BRTO) versus Transjugular Intrahepatic Portosystemic Shunt (TIPS) in the management of gastric variceal bleeding: A systematic review and meta-analysis. Hepatology. 2018;68S475A. |
| 57. | Orozco H, Mercado MA. The evolution of portal hypertension surgery: lessons from 1000 operations and 50 Years' experience. Arch Surg. 2000;135:1389-93; discussion 1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |