Published online Feb 27, 2019. doi: 10.4254/wjh.v11.i2.199
Peer-review started: September 10, 2018
First decision: October 5, 2018
Revised: November 5, 2018
Accepted: January 28, 2019
Article in press: January 28, 2019
Published online: February 27, 2019
Processing time: 171 Days and 6.7 Hours
Major hepatectomies are routinely performed because they are often the only curative treatment for metastatic liver disease. There has been a trend to concentrate major hepatectomies in referral hospitals that perform these operations at high volumes. These high volume referral centers are usually located in developed countries, but many patients in developing nations are not able to access these centers because of financial limitations, lack of social support and/or travel restrictions. Therefore, local hospitals are often the only options many of these patients have for surgical treatment of metastatic liver disease. This is the situation in many Caribbean countries.
To determine the clinical outcomes after major liver resections in a low-resource hepatobiliary center in the Caribbean.
We prospectively studied all patients who underwent major liver resections over five years. The following data were extracted: patient demographics, diagnoses, ECOG status, operation performed, post-operative morbidity and mortality. Statistical analyses were performed using SPSS ver 16.0
There were 69 major liver resections performed by two teams at a mean case volume of 13.8 major resections/year. Sixty-nine major hepatic resections were performed for: colorectal liver metastases 40 (58%), non-colorectal metastases 9 (13%), hepatocellular carcinoma 8 (11.6%), ruptured adenomas 4 (5.8%), hilar cholangiocarcinomas 4 (5.8%), hemangiomata 2 (2.9%), trauma 1 (1.5%) and hepatoblastoma 1 (1.5%). Twenty-one patients had at least one complication, for an overall morbidity rate of 30.4%. There were minor complications in 17 (24.6%) patients, major complications in 11 (15.9%) patients and 4 (5.8%) deaths.
There are unique geographic, political and financial limitations to healthcare delivery in the Caribbean. Nevertheless, clinical outcomes are acceptable in the established, low-volume hepatobiliary centers in the Eastern Caribbean.
Core tip: Although there has been a global trend to concentrate major liver resections in tertiary referral centers, it is not practical in the Caribbean region. However, the hepatobiliary centers in the Caribbean do not meet the criteria to be defined as high-volume centers. This study prospectively evaluated outcomes after 69 consecutive major liver resections in a Caribbean center that only performed 13.8 resections per year. With a major morbidity rate of 15.9% and mortality rate of 5.8%, we have shown that the clinical outcomes after major liver resections are acceptable in the established, low-volume hepatobiliary centers in the Eastern Caribbean.
- Citation: Cawich SO, Maharaj R, Naraynsingh V, Pearce N, Francis W, Bonadie KO, Thomas DA. Clinical outcomes after major hepatectomy are acceptable in low-volume centers in the Caribbean. World J Hepatol 2019; 11(2): 199-207
- URL: https://www.wjgnet.com/1948-5182/full/v11/i2/199.htm
- DOI: https://dx.doi.org/10.4254/wjh.v11.i2.199
Major hepatectomies are routinely performed because they are often the only curative treatment for metastatic liver disease[1]. They are accepted to be safe procedures when performed by trained hepatobiliary teams in specialized, high-volume centers[1-3].
There has been a trend to concentrate major hepatectomies in referral hospitals that perform these operations at high volumes[3,4,5]. These high volume referral centers are usually located in developed countries, but many patients in developing nations are not able to access these centers because of financial limitations, lack of social support and/or travel restrictions. Therefore, local hospitals are often the only options many of these patients have for surgical treatment of metastatic liver disease. This is the situation in many Caribbean countries.
While there are hepatobiliary units in the Caribbean, none meet the criteria to be defined as high-volume[3,4,5,6,7]. Additionally, hepatobiliary units in the Caribbean operate in challenging, resource-poor environments. In this study, we sought to determine whether the clinical outcomes were acceptable when major hepatectomies were performed in a low-volume, resource-poor hepatobiliary unit in the Eastern Caribbean.
In 2011, an attempt was made to achieve service centralization in the Caribbean with the establishment of three hepatobiliary units in the Bahamas, Jamaica and Trinidad and Tobago. They were intended to serve as regional referral centers for patients requiring major hepatectomies across the English-speaking Caribbean[8]. This was supported by the Americas Hepatopancreatobiliary Association (AHPBA), culminating with the formation of a Caribbean Chapter of the AHPBA in 2015.
The hepatobiliary unit in Trinidad and Tobago is the largest referral unit in the English-speaking Caribbean[8-9]. This unit is comprised of two hepatobiliary teams each headed by fellowship-trained hepatobiliary surgeons. All cases are discussed in a multidisciplinary team meeting where decisions are made for treatment of patients with hepatobiliary diseases.
The local institutional review board granted permission to collect and examine data from all patients who underwent major hepatectomies in this setting.
We prospectively recorded data from all patients who underwent major hepatectomies with the hepatobiliary unit in Trinidad and Tobago over a five-year period from January 1, 2012 to December 30, 2016. We used the standardized definition of major hepatectomies as defined by Reddy et al[10]: resection of four or more liver segments.
The following data were recorded for all patients who underwent major hepatectomies during the study period: patient demographics, diagnoses, ECOG status, operation performed, operative details, therapeutic outcomes, post-operative morbidity and mortality. Complications were classified according to the modified Clavien-Dindo system[11]. Statistical analyses were performed using SPSS ver 16.0.
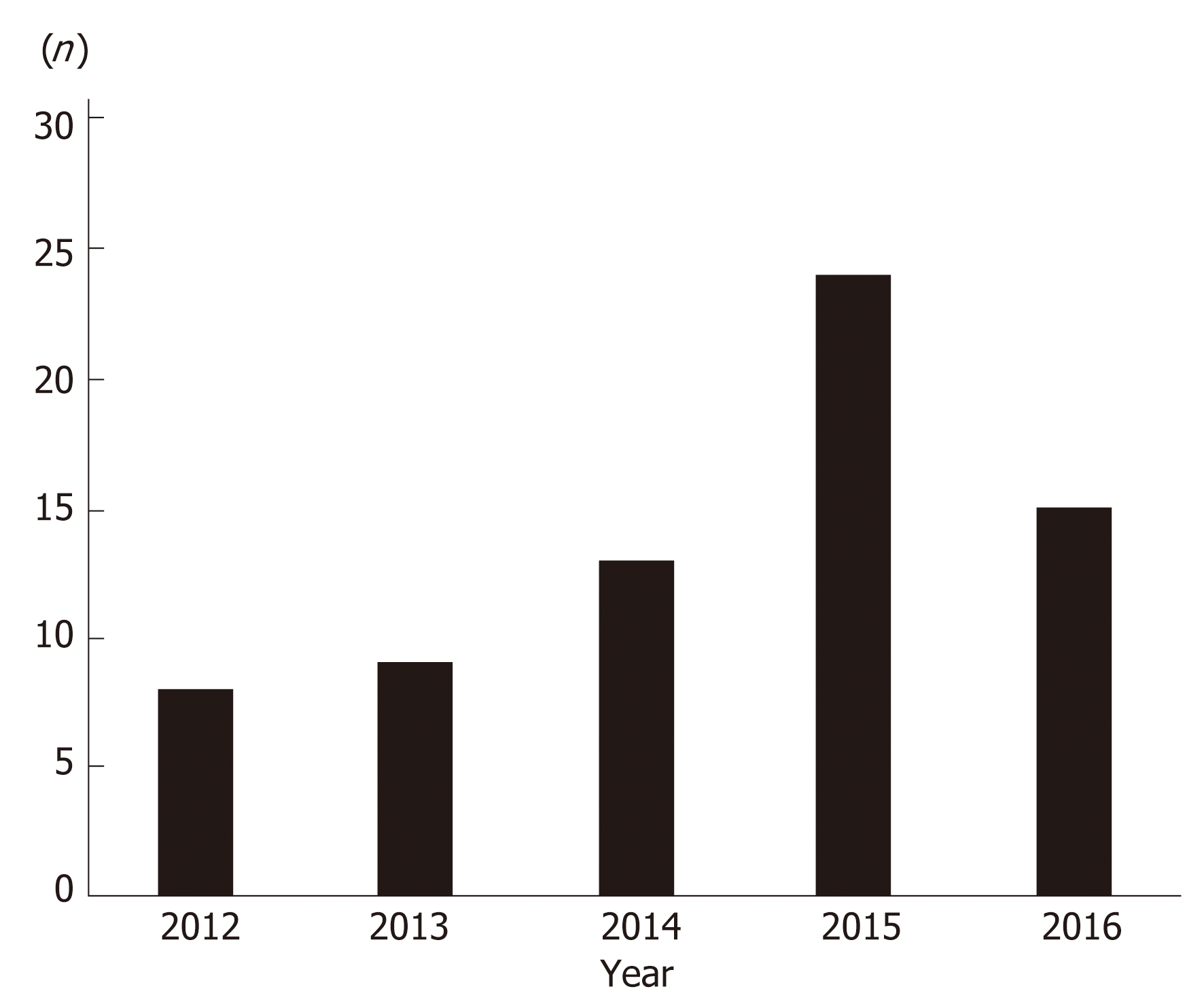
There were 69 major hepatectomies performed over the five-year study period. Therefore, the mean annual case volume was 13.8 major hepatectomies per annum. When examined chronologically, there was a steady increase in the number of hepatectomies performed each year, except in the year 2016 (Figure 1). During this time, the nation experienced an economic recession.
All major hepatectomies were performed by one of two trained hepatobiliary surgeons for the following indications: colorectal liver metastases 40 (58%), non-colorectal metastases 9 (13%), hepatocellular carcinoma 8 (11.6%), ruptured adenomas 4 (5.8%), hilar cholangiocarcinomas 4 (5.8%), hemangiomata 2 (2.9%), trauma 1 (1.5%) and hepatoblastoma 1 (1.5%).
The patients in this series consisted of 40 men and 29 women, with a mean age of 63 years (Range 34-80; SD +/- 10.3; Median 65). Sixty-four (93%) patients had at least one co-morbidity. Overall, there were 40 (58%) patients with ASA scores ≥ III, as detailed in Table 1, and 39 (56.5%) patients with ECOG scores ≥ 2, as detailed in Table 2.
| Score | ASA descriptor | n (%) |
| I | Completely healthy | 5 (7.3) |
| II | Mild systemic disease | 24 (34.8) |
| III | Severe systemic disease that is not incapacitating | 31 (44.9) |
| IV | Incapacitating disease that is a threat to life | 9 (13) |
| V | Moribund and not expected to survive > 24 h | 0 |
| Grade | ECOG Performance status | n (%) |
| 0 | Fully active, able to carry out all activities without restriction | 10 (14.5) |
| 1 | Restricted in physically strenuous activity, but ambulatory and able to carry out light work | 20 (29) |
| 2 | Ambulatory and capable of self care, but unable to carry out work activities. Up and about > 50% of waking hours | 33 (47.8) |
| 3 | Capable of limited self care and confined to bed or chair for more than 50% of waking hours | 5 (7.3) |
| 4 | Completely disabled and cannot carry on self care. Confined to bed or chair | 1 (1.5) |
| 5 | Dead | 0 |
After pre-operative multidisciplinary review, we anticipated that the hepatectomy procedure would be technically complex in 26 (37.7%) patients for: emergency hepatectomy for ruptured tumours or trauma (6), multiple intra-hepatic hepatico-jejunostomies for hilar cholangiocarcinomas (4), IVC resection and reconstruction (4), borderline future liver remnants (4), synchronous colorectal operations (3), synchronous gastric resections (2), prior open hepatectomy scheduled for repeat laparoscopic resections (2) and synchronous nephrectomy (1).
Fourteen (20.3%) hepatectomies were attempted using the laparoscopic approach, with 3 (21.4%) conversions for unclear anatomy (1), bleeding (1) and repair of IVC injury (1). The remaining 55 (79.7%) operations were planned using an open approach. No patients in this series underwent veno-venous bypass during major hepatectomies. The hanging maneuver with anterior parenchymal transection technique was used to complete hepatectomy in 18 (26.1%) patients and the conventional technique was used in the remaining 51 (73.9%) cases.
Excluding patients who had synchronous resections performed, the mean operating time for a major hepatectomy alone was 380 min (Range 260-600; SD +/-75.8; Median 350). The operations in these patients were accompanied by a mean blood loss of 1405 mL (Range 600-4000; SD+/- 729; Median 1200) and mean transfusion requirements of 1.8 units of packed cells (Range 0-5; SD +/- 1.43; Median 2).
When we evaluated the subset of 26 patients in whom technically complex operations were anticipated, the mean operating time was 461.5 min (Range 300-650; SD+/-95.6; Median 455), mean estimated blood loss was 2009 mL (Range 800-3500; SD+/-667.4; Median 2000) and the mean transfusion requirement was 3.2 units of packed cells (Range 1-5; SD+/-1.05; Median 3).
In the 43 cases where technical difficulty was not anticipated pre-operatively, the mean operating time was 367 min (Range 260-600; SD+/-69.4; Median 350), mean EBL of 1236.7 mL (Range 600-4000; SD+/-679.5; Median 1000) and mean transfusion requirements of 1.37 units (Range 0-4; SD+/-1.3; Median 1).
In this setting, we maintained a policy of mandatory ICU admission after major hepatectomy because institutional limitations generally did not allow the expected level of supportive care outside of the ICU setting. Therefore, all patients were admitted to the ICU post-hepatectomy, with a mean ICU stay of 5.3 d (Range 1-40; SD+/-7.37; Median 3). Fifteen (21.7%) patients required a prolonged ICU stay beyond 72 h for invasive treatment, ventilator and/or inotropic support. Overall, the mean duration of hospitalization after major hepatectomy was 16 d (Range 9-103; SD+/-13.35; Median 12).
There were 58 patients with no complications or minor morbidity. These patients had a mean ICU stay of 3.2 d (Range 1-8; SD+/-1.55; Median 3) and mean hospital stay of 13.2 d (Range 9-35; SD+/-6.85; Median 10). In comparison, the 11 patients with major morbidity had a mean ICU stay of 16.3 d (Range 5-40; SD+/-14.1; median 6) and mean overall hospital stay of 31.1 d (Range 13-103; SD+/-25.4; Median 23).
Twenty-one patients experienced at least one complication in this series. Minor complications were recorded in 17 (24.6%) patients and major complications in 11 (15.9%) patients. The individual complications are outlined in Table 3.
| Morbidity | Description | n | Percent |
| Overall | Number of patients with any complication | 21/69 | 30.4% |
| Minor | Clavien-Dindo I or II | 17/69 | 24.6% |
| Pneumonia | 2 | 2.9% | |
| Deep Vein Thrombosis | 2 | 2.9% | |
| Surgical site infections | 2 | 2.9% | |
| Bile leaks | 4 | 5.8% | |
| ISGLS Grade-B post-hepatic liver failure | 7 | 10.1% | |
| Major | Clavien-Dindo III or IV | 11/69 | 15.9% |
| Anastomotic dehiscence | 1 | 1.5% | |
| Intra-abdominal collection | 4 | 5.8% | |
| Right hepatic artery injury | 1 | 1.5% | |
| Strangulated internal hernia | 1 | 1.5% | |
| ISGLS Grade-C post-hepatic liver failure | 4 | 5.8% | |
| Mortality | 30-d mortality: All causes (1) 69 yr-old man: Sepsis after bladder leak after abdomino-perineal resection for synchronous colorectal liver metastases (2) 80 yr-old man: bile leak after extended right hepatectomy for colorectal liver metastases (3) 69 yr-old woman: Post-Hepatic Liver Failure after extended right hepatectomy for hepatocellular carcinoma (4) 79 yr-old man: Anastomotic leak after extended right hepatectomy for hilar cholangiocarcinoma | 4/69 | 5.8% |
There were 4 (5.8%) reported deaths within 30 d of operation in this series. These included: (1) a 69 year-old man who underwent an abdomino-perineal resection and synchronous major hepatectomy. He developed intra-abdominal sepsis after a leak from a bladder injury; (2) an 80-year old man who underwent an extended right hepatectomy for colorectal liver metastases. He developed a significant bile leak, with resultant collections and eventually succumbed to intra-abdominal sepsis; (3) a 69-year old woman who had extended right hepatectomy for hepatocellular carcinoma and developed post-hepatectomy liver failure despite a 40% functional liver remnant; and (4) a 79-year old man who had an extended right hepatectomy for hilar cholangiocarcinoma. He developed a small bowel anastomotic leak and eventually succumbed to intra-abdominal sepsis.
At the turn of the 21st century, we witnessed the era of service centralization where surgical treatment for complex diseases was concentrated in specific centers in order to support sub-specialty teams performing these operations at high volumes[7,12,13]. This trend was supported by accumulating data to suggest that there were better peri-operative outcomes in high-volume referral hospitals[3,4,5,6,14,15,16,17].
Specifically for major hepatectomies, the data demonstrated that high-volume centers achieved significant reductions in overall morbidity[3,5,6,14,16], 30-day mortality[3,5,14,15,16,17], readmission rates[16], cost[14] and the duration of hospital stay[6,14,16]. Lu et al[14] also reported that high-volume centers achieved longer 5-year survival rates. These data seem to lend strong support to the principle of centralization.
However, a closer look at the existing data revealed that there is no standardized definition of “high volumes”, with researchers applying ad-hoc definitions that range from as low as 10 as cases per annum[16] to as high as 150 cases per annum[18]. Most papers in the literature quote numbers in excess of 20 cases per annum[3,19,20,21,22,23]. Using these definitions, the hepatobiliary unit in Trinidad and Tobago does not qualify as high volume, with a mean case volume of 13.8 major hepatectomies per year.
Furthermore, the high volume centers are often tertiary referral hospitals that serve large catchment populations and attract significant funding. They are usually located in major cities within developed countries. Unfortunately, many patients in less-developed Caribbean countries cannot access care in these high volume centers because of travel restrictions, financial limitations, lack of health insurance coverage and/or a paucity of social support structures. Even within the United States, Eppsteiner et al[17] noted that there was socio-economic inequity for access to care at high volume centers.
We observed that most current reports, even those supporting centralization, documented that the majority of major hepatectomies are being performed in low-volume centers - even in developed countries in the 21st century. Fong et al[3] reported in 2005 that only 1% of the hospitals that offered major hepatectomies in the United States of America actually qualified as high-volume centers. In fact, Fong et al[3] reported that the 1272 low-volume centers in the United States performed an average of 1 major hepatectomy per annum - substantially below the “high-volume mark”.
Similar findings were reported by other researchers: Choti et al[5] reported that only 2.7% (1) of the 37 facilities performing major liver resections in the state of Maryland qualified as high-volume. The low-volume facilities performed an average of 1.5 cases annually[5]. Similarly, Glasgow et al[6] reported that only 3% of 138 hospitals performing liver resections in the state of California qualified as high volume. The low-volume hospitals performed ≤ 3 hepatectomies per annum[6].
It seems that there is still not universal buy in to the concept of centralization for major hepatectomies. One reason for this may be the lack of practicality. This is especially true in developing countries and it can be appreciated by examining the health care environment in the Anglophone Caribbean. Narayansingh et al[24] outlined the unique challenges to healthcare delivery in this setting: (1) many countries are island states that are geographically separated by the Caribbean Sea; (2) there are political barriers since each country is independent and separately governed; (3) each island has distinctly different cultures; and (4) many surgeons, even those with subspecialty training, are required to perform a wide repertoire of general surgical procedures at low volumes. In addition, most of these countries have underfunded health care systems[8], leadership deficiencies[25,26], cultural resistance to multidisciplinary collaboration[8] and limited access to specialists and subspecialists[9,24]. These factors were all obstacles to service centralization in the Anglophone Caribbean.
Despite the obstacles, surgical leaders recognized the need and established a hepatobiliary unit in Trinidad and Tobago to serve patients in the Eastern Caribbean[8]. There has been some success in this regard, as measured by the consistent increase in the annual number of major liver resections performed by this unit (Figure 1). The reduction in case volumes in the year 2016 correlated with the country experiencing a recession. This led to a lack of consumables in Government-funded hospitals and it highlights our point that these demanding operations require significant resources. Coupled with the fact that these hospitals face unique challenges (scarcity of specialized equipment, blood products, ICU space and operating lists), one can realize that the environment is not always conducive to observing best practice recommendations.
Nevertheless, the clinical outcomes in this established low-volume hepatobiliary unit were acceptable. The perioperative mortality rate was 5.8% in our setting. Although reported 30-d mortality in high-volume centers ranged widely from 1.5%[5] to 9.4%[6], most high volume centers reported 30-d mortality between 4% and 6.5%[3,5,16,19]. Our results compared favourably to these high-volume centers. In contrast, the reported 30-day mortality in low volume centers range from 5%-22.7%[3,5,6,17,22].
Major complications occurred in 15.9% of our patients. This was comparable to reports in the existing medical literature, where major hepatectomies in high-volume centers resulted in major morbidity in 13.2%[22] to 27%[27] of cases. Similarly, minor morbidity (24.6%) rates were comparable to existing reports from high-volume centers, ranging from 9.3%[18] to 26.9%[22].
Potential critics may suggest that therapeutic outcomes may appear reasonable because of “case selection”, where high-risk patients are referred onward to high-volume referral centers. Obviously, it could have skewed the results toward improved outcomes if only low-risk patients were selected for major hepatectomies in our setting. However, more than half of the major hepatectomies at our facility were performed in high-risk patients, with ASA scores ≥ III (58%), ECOG scores ≥ 2 (57%) and at least one co-morbidity (93%). Moreover, after pre-operative MDT assessment a further 38% of the major hepatectomies performed in this setting were technically difficult operations.
We do acknowledge that high volume referral centers treat more patients, including high-risk cases with multiple co-morbidities and complicated surgical histories. However, in our setting in the Caribbean, we did not have the luxury of “case selection” because the patients treated at our facilities had no other options for care, for reasons already discussed. We believe, therefore, that referral practices/case selection could not account for the clinical outcomes in this setting. Furthermore, this was a resource-poor environment with limited support services and numerous institutional limitations. These results demonstrated that, despite multiple challenges, the outcomes are not solely dependent on numbers.
We agree with Gasper et al[28] that modern hospitals are complex adaptive systems whose outputs are determined by interactions between internal agents. We also agree with Hashimoto et al[29] that annual volume only contributes a partial assessment and that there is also a substantial contribution by surgeon training and experience. In this regard, we attribute our outcomes to the unit staff (1) having appropriate training; (2) developing an intimate knowledge of the health care system in which they work; (3) fostering a spirit of collective teamwork; (4) maintaining due diligence in care administration; (5) continued audit; and (6) knowledge of population-based data[30,31,32].
In conclusion, Caribbean hospitals do not, and possibly never will, qualify as high-volume centers due to unique geographic, political and financial limitations to healthcare delivery in the region. Nevertheless, there can be good short-term outcomes when major hepatectomies are performed in low-volume hepatobiliary units in the Eastern Caribbean, despite a high proportion of high-risk patients requiring technically complex operations. This demonstrates that case volume is not the only determinant of good outcomes after major hepatectomy. To achieve good outcomes, there is also the need for teamwork, appropriately trained staff, due diligence in care administration, continued audit and knowledge of population-based data.
In the past two decades, there was a trend to concentrate major hepatectomies in specific centers in order to support sub-specialty teams performing these operations at high volumes. This trend was supported by accumulating data to suggest that there were better peri-operative outcomes in high-volume referral hospitals. However, this is not practical in the Caribbean and other resource-poor countries.
Clinicians in the Caribbean do not have the luxury of “case selection” because most patients treated at our facilities have no other options for care. Therefore, these patients must receive treatment at low-volume, resource-poor centers with limited support services and numerous institutional limitations. The motivation for our research was to determine if the clinical outcomes are acceptable despite the numerous limitations.
To determine the clinical outcomes after major hepatectomies in a low-volume, resource-poor center in the Caribbean.
We prospectively studied post-operative morbidity and mortality in all patients undergoing major hepatectomies in a low-volume Caribbean hepatobiliary center over a five-year study period. Statistical analyses were performed using SPSS ver 16.0.
There were 69 major hepatectomies performed over the study period (mean case volume of 13.8 major resections/year). More than half of the major hepatectomies were performed in high-risk patients, with ASA scores ≥ III (58%), ECOG scores ≥ 2 (57%) or at least one co-morbidity (93%). A further 38% of the major hepatectomies performed in this setting were technically difficult operations. Twenty-one patients experienced at least 1 complication, for an overall morbidity rate of 30.4%. There were minor complications in 17 (24.6%) patients, major complications in 11 (15.9%) patients and 4 (5.8%) deaths.
Although Caribbean hospitals do not qualify as high-volume centers, there can be good short-term outcomes after major hepatectomies are performed in established hepatobiliary units. This demonstrates that case volume is not the only determinant of good outcomes after major hepatectomy.
To achieve good outcomes, there is the need for teamwork, appropriately trained staff, due diligence in care administration, continued audit and knowledge of population-based data. Case volume is not the only determinant of good outcomes after major hepatectomy.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Trinidad and Tobago
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Hilmi I, Nah YW, Mastoraki A S- Editor: Dou Y L- Editor: A E- Editor: Zhang YL
| 1. | Fong Y, Blumgart LH, Fortner JG, Brennan MF. Pancreatic or liver resection for malignancy is safe and effective for the elderly. Ann Surg. 1995;222:426-34; discussion 434-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 195] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 2. | Nagorney DM, van Heerden JA, Ilstrup DM, Adson MA. Primary hepatic malignancy: surgical management and determinants of survival. Surgery. 1989;106:740-8; discussion 748-9. [PubMed] |
| 3. | Fong Y, Gonen M, Rubin D, Radzyner M, Brennan MF. Long-term survival is superior after resection for cancer in high-volume centers. Ann Surg. 2005;242:540-4; discussion 544-7. [PubMed] |
| 4. | Begg CB, Cramer LD, Hoskins WJ, Brennan MF. Impact of hospital volume on operative mortality for major cancer surgery. JAMA. 1998;280:1747-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1241] [Cited by in RCA: 1275] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 5. | Choti MA, Bowman HM, Pitt HA, Sosa JA, Sitzmann JV, Cameron JL, Gordon TA. Should hepatic resections be performed at high-volume referral centers? J Gastrointest Surg. 1998;2:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 142] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Glasgow RE, Showstack JA, Katz PP, Corvera CU, Warren RS, Mulvihill SJ. The relationship between hospital volume and outcomes of hepatic resection for hepatocellular carcinoma. Arch Surg. 1999;134:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 131] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med. 1979;301:1364-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1231] [Cited by in RCA: 1230] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 8. | Cawich SO, Johnson PB, Shah S, Roberts P, Arthurs M, Murphy T, Bonadie KO, Crandon IW, Harding HE, Abu Hilal M, Pearce NW. Overcoming obstacles to establish a multidisciplinary team approach to hepatobiliary diseases: a working model in a Caribbean setting. J Multidiscip Healthc. 2014;7:227-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Cawich SO, Thomas DA, Ramjit C, Bhagan R, Naraynsingh V. Complex liver resections for colorectal metastases: are they safe in the low-volume, resource-poor Caribbean setting? Case Rep Surg. 2015;2015:570968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Reddy SK, Barbas AS, Turley RS, Steel JL, Tsung A, Marsh JW, Geller DA, Clary BM. A standard definition of major hepatectomy: resection of four or more liver segments. HPB (Oxford). 2011;13:494-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 153] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 11. | Téoule P, Bartel F, Birgin E, Rückert F, Wilhelm TJ. The Clavien-Dindo Classification in Pancreatic Surgery: A Clinical and Economic Validation. J Invest Surg. 2018;1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Farber BF, Kaiser DL, Wenzel RP. Relation between surgical volume and incidence of postoperative wound infection. N Engl J Med. 1981;305:200-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 53] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Finlayson EV, Goodney PP, Birkmeyer JD. Hospital volume and operative mortality in cancer surgery: a national study. Arch Surg. 2003;138:721-5; discussion 726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 395] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 14. | Lu CC, Chiu CC, Wang JJ, Chiu YH, Shi HY. Volume-outcome associations after major hepatectomy for hepatocellular carcinoma: a nationwide Taiwan study. J Gastrointest Surg. 2014;18:1138-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Richardson AJ, Pang TC, Johnston E, Hollands MJ, Lam VW, Pleass HC. The volume effect in liver surgery--a systematic review and meta-analysis. J Gastrointest Surg. 2013;17:1984-1996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Schneider EB, Ejaz A, Spolverato G, Hirose K, Makary MA, Wolfgang CL, Ahuja N, Weiss M, Pawlik TM. Hospital volume and patient outcomes in hepato-pancreatico-biliary surgery: is assessing differences in mortality enough? J Gastrointest Surg. 2014;18:2105-2115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Eppsteiner RW, Csikesz NG, Simons JP, Tseng JF, Shah SA. High volume and outcome after liver resection: surgeon or center? J Gastrointest Surg. 2008;12:1709-16; discussion 1716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Idrees JJ, Johnston FM, Canner JK, Dillhoff M, Schmidt C, Haut ER, Pawlik TM. Cost of Major Complications After Liver Resection in the United States: Are High-volume Centers Cost-effective? Ann Surg. 2019;269:503-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Nygård IE, Lassen K, Kjæve J, Revhaug A. Mortality and survival rates after elective hepatic surgery in a low-volume centre are comparable to those of high-volume centres. ISRN Surg. 2012;2012:783932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Asiyanbola B, Chang D, Gleisner AL, Nathan H, Choti MA, Schulick RD, Pawlik TM. Operative mortality after hepatic resection: are literature-based rates broadly applicable? J Gastrointest Surg. 2008;12:842-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 21. | Csikesz NG, Simons JP, Tseng JF, Shah SA. Surgical specialization and operative mortality in hepato-pancreatico-biliary (HPB) surgery. J Gastrointest Surg. 2008;12:1534-1539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Botea F, Ionescu M, Braşoveanu V, Hrehoreţ D, Alexandrescu S, Grigorie M, Stanciulea O, Nicolaescu D, Tomescu D, Droc G, Ungureanu D, Fota R, Croitoru A, Gheorghe L, Gheorghe C, Lupescu I, Grasu M, Boroş M, Dumitru R, Toma M, Herlea V, Popescu I. Liver Resections in a High-Volume Center: Form Standard Procedures to Extreme Surgery and Ultrasound-guided Resections. Chirurgia (Bucur). 2017;112:259-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Shaw JJ, Santry HP, Shah SA. Specialization and utilization after hepatectomy in academic medical centers. J Surg Res. 2013;185:433-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Naraynsingh V, Bahadursingh S, Maharaj R, Harnarayan P, Cawich SO. Surgery in the West Indies: A perspective from Trinidad. J Curr Med Res Pract. 2014;4:1126-1129 [DOI 10.1016/j.cmrp.2014.06.001]. |
| 25. | Cawich SO, Johnson PB, Dan D, Naraynsingh V. Surgical leadership in the time of significant generational diversity. Surgeon. 2014;12:235-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Cawich SO, Harding HE, Crandon IW, McGaw CD, Barnett AT, Tennant I, Evans NR, Martin AC, Simpson LK, Johnson P. Leadership in surgery for public sector hospitals in Jamaica: strategies for the operating room. Perm J. 2013;17:e121-e125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Ubink I, Jongen JMJ, Nijkamp MW, Meijer EFJ, Vellinga TT, van Hillegersberg R, Molenaar IQ, Borel Rinkes IHM, Hagendoorn J. Surgical and Oncologic Outcomes After Major Liver Surgery and Extended Hemihepatectomy for Colorectal Liver Metastases. Clin Colorectal Cancer. 2016;15:e193-e198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Gasper WJ, Glidden DV, Jin C, Way LW, Patti MG. Has recognition of the relationship between mortality rates and hospital volume for major cancer surgery in California made a difference?: A follow-up analysis of another decade. Ann Surg. 2009;250:472-483. [PubMed] |
| 29. | Hashimoto DA, Bababekov YJ, Mehtsun WT, Stapleton SM, Warshaw AL, Lillemoe KD, Chang DC, Vagefi PA. Is Annual Volume Enough? The Role of Experience and Specialization on Inpatient Mortality After Hepatectomy. Ann Surg. 2017;266:603-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Cawich SO, Thomas D, Ragoonanan V, Naraynsingh V. The hanging manoeuver to complete liver resection for a locally advanced angiosarcoma: A case report. Int J Surg Case Rep. 2015;16:52-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Gardner MT, Cawich SO, Shetty R, Pearce NW, Naraynsingh V. Hepatic surface grooves in an Afro-Caribbean population: a cadaver study. Ital J Anat Embryol. 2015;120:117-126. [PubMed] |
| 32. | Johnson PB, Cawich SO, Shah S, Gardner MT, Roberts P, Stedman B, Pearce NW. Vascular supply to the liver: a report of a rare arterial variant. Case Rep Radiol. 2013;2013:969327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |