Published online Dec 27, 2019. doi: 10.4254/wjh.v11.i12.773
Peer-review started: August 22, 2019
First decision: November 2, 2019
Revised: November 11, 2019
Accepted: November 25, 2019
Article in press: November 25, 2019
Published online: December 27, 2019
Processing time: 126 Days and 4.8 Hours
Splenosis is defined as the process by which tissue from the spleen disseminates through the body and grows in an ectopic location following trauma or a splenectomy. Visceral sites of splenosis are rare.
We report a case of intrahepatic splenosis in a 57-year-old man with a history of trauma over 40 years ago who initially presented with chest pain. Findings initially mimicked malignancy but a diagnosis of intrahepatic splenosis was confirmed using computed tomography and scintigraphy with technetium-99m heat-denatured red blood cells (Tc-99 DRBC).
Scintigraphy with Tc-99 DRBC is a reliable technique to diagnose splenosis and should be performed before using more invasive procedures are carried out. Splenosis should be considered as a possible differential diagnosis for a hepatic nodule in any patient with a history of abdominal trauma, previous splenectomy or atypical radiological features on imaging.
Core tip: Intrahepatic splenosis is rare. On imaging it is difficult to distinguish splenosis from hepatic malignancy, particularly hepatocellular carcinoma. We report a case of a patient with intrahepatic and intra-abdominal splenosis diagnosed using scintigraphy with technetium-99m heat-denatured red blood cells. To the author’s knowledge, this is the first case where hepatic splenosis was confirmed without using invasive procedures such as biopsy or surgery. Splenosis should be considered as an important differential for a hepatic lesion in a patient with a history of trauma or splenectomy, particularly if the lesion is located near the capsule and associated with multiple abdominal deposits.
- Citation: Ananthan K, Yusuf GT, Kumar M. Intrahepatic and intra-abdominal splenosis: A case report and review of literature. World J Hepatol 2019; 11(12): 773-779
- URL: https://www.wjgnet.com/1948-5182/full/v11/i12/773.htm
- DOI: https://dx.doi.org/10.4254/wjh.v11.i12.773
Splenosis is a benign acquired condition. Following trauma or a splenectomy, splenic tissue may autotransplant in an ectopic location. Common sites include the serosal surface of the small or large intestine, greater omentum or the peritoneum[1]. Less frequently, splenic nodules may be found in the liver[2], stomach[3], pancreas[4] and following rupture of the diaphragm in the thorax[5]. The kidneys[6], ovaries[7] and subcutaneous tissue[8] are even rarer sites of splenosis. Splenosis is usually asymptomatic and when incidentally discovered can be difficult to distinguish from malignancy using computed tomography (CT) or magnetic resonance imaging (MRI).
A 57-year-old male presented to the Emergency Department with severe right-sided pleuritic chest pain radiating to his back. There was no associated breathlessness, fever, cough, haemoptysis, dizziness, syncope, numbness, paraesthesia or weakness.
The patient reported that the symptoms began abruptly two days ago and progressively worsened, without any triggers. The pain settled following the administration of morphine after admission to hospital.
In 2015 he was diagnosed with benign prostatic hypertrophy and had suffered traumatic injury following a road traffic accident over 40 years ago. Of note, he had no history of hepatic disease.
There was marked tenderness on inspiration on the right side of the chest, but otherwise physical examination was unremarkable. The patient’s vital signs were normal with a temperature of 37.0 °C, heart rate of 74 bpm, blood pressure of 125/75 mmHg, respiratory rate of 15 breaths/min and oxygen saturations of 98% in room air.
Routine blood tests were within normal ranges including liver function tests, alpha-fetoprotein, prothrombin time and a normal D-Dimer.
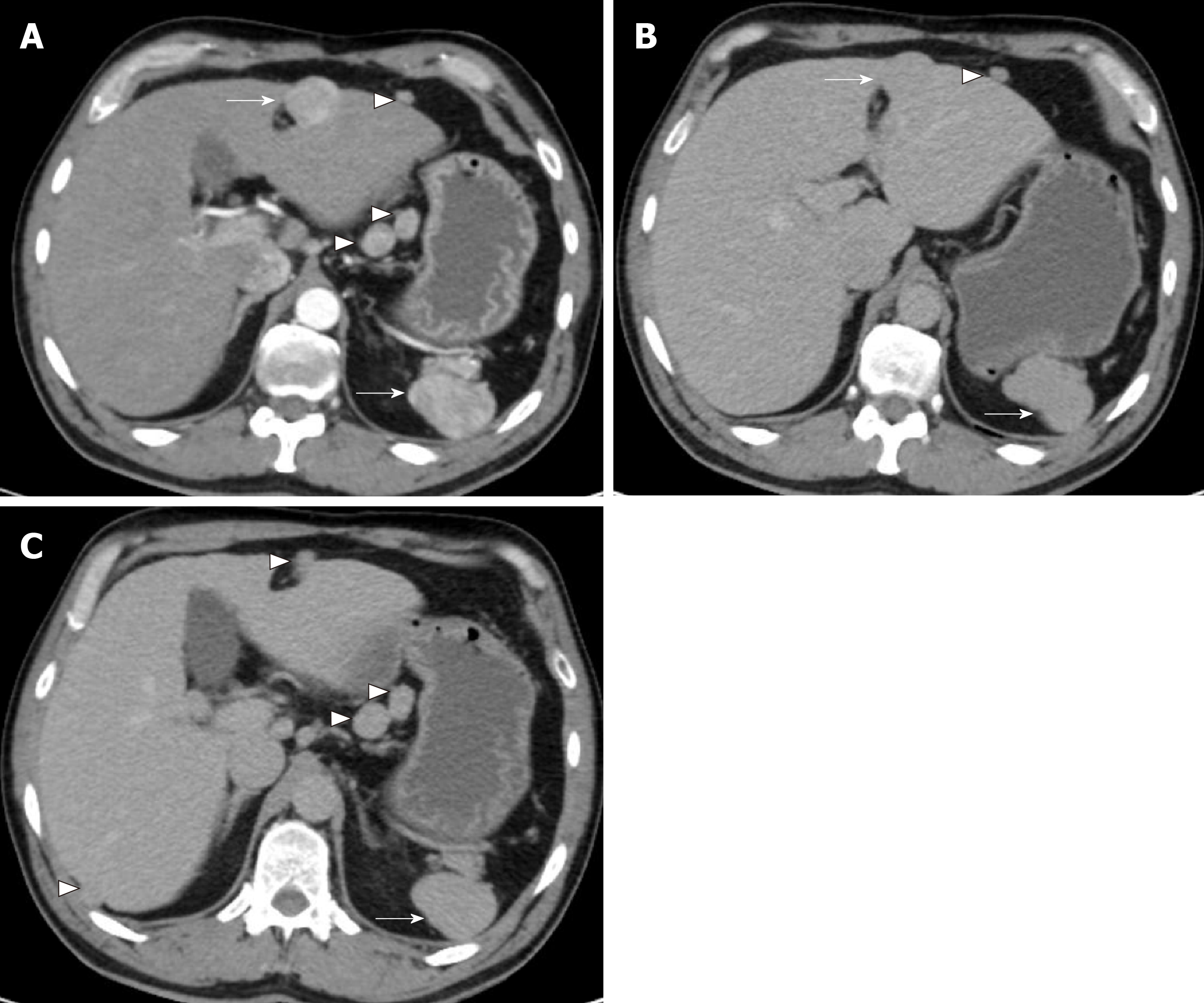
A CT angiogram was performed to rule out aortic dissection due to his acute presentation. No evidence of the latter was seen but there was a 3-cm large arterially enhancing lesion in segment IV of the liver (Figure 1A). The lesion was arterialised with faint hypoenhancement in the portal venous phase (Figure 1B and C). Multiple arterially enhancing peritoneal, lesser sac and retroperitoneal nodules were additionally seen following the same enhancement pattern. The patient’s spleen was observed to be lobulated and the right inferior ribs and right iliac crest had an abnormal appearance suggestive of previous trauma.
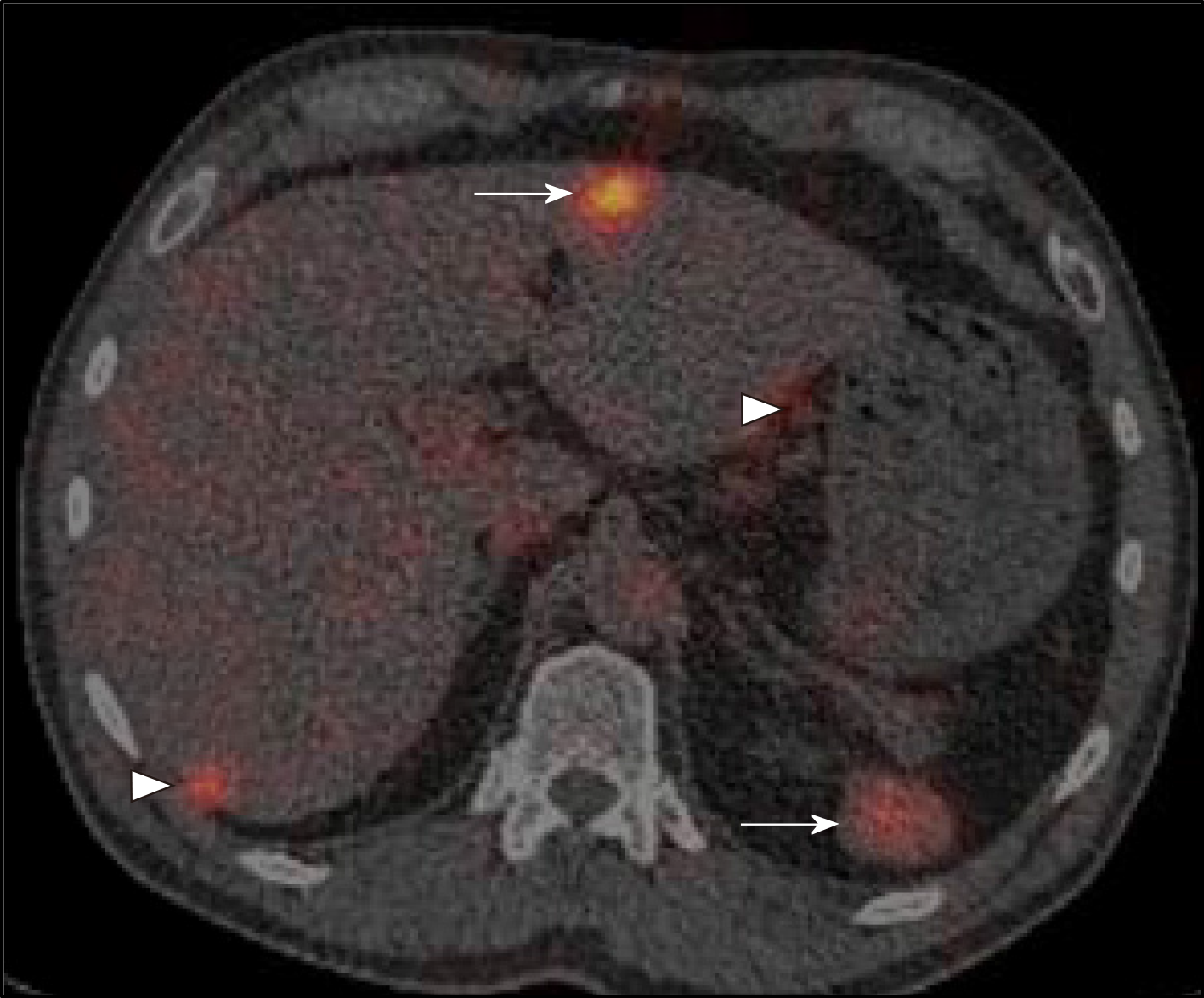
Following discussion of these imaging findings and the patient’s history in a specialist multidisciplinary team meeting, the possibility of intrahepatic splenosis with additional intra-abdominal splenosis was considered. This diagnosis was confirmed using scintigraphy with technetium-99m heat-denatured red blood cells (Tc-99 DRBC), which demonstrated uptake of the radiolabelled red blood cells by the multiple peritoneal nodules, as well as the lesion within the liver and the anterior abdominal wall (Figure 2).
Due to the extensively reported benign nature of this condition, treatment was not required. The patient was informed of the incidental imaging finding and reassured. He was also informed that his chest pain was likely to be musculoskeletal in nature.
We describe a case of intrahepatic splenosis diagnosed radiologically, in a patient with a history of trauma. To the authors’ knowledge, this is the first case of intrahepatic splenosis diagnosed without the need for histological analysis, thereby avoiding the potential risk of complications secondary to invasive investigations such as a liver biopsy or laparoscopic surgery.
To date, 21 case reports of intrahepatic splenosis have been described in the literature. We specifically review 13 cases which include CT and MRI (Table 1). Nine cases describe solitary lesions[9-17], whilst four cases involved multiple lesions[18-21]. The nodules ranged in size from 1.5-5.0 cm and were primarily found in the left lobe of the liver. Additional abdominal splenic nodules were reported in four cases to be located in close proximity to the upper pole of the left kidney[19], pancreatic tail[19], mesentery of the colon[20], paravesical space[21], caecum[21] and abdominal wall[15].
| Ref. | Age/sex | Blood results | Liver disease | Existing malignancy | Initial diagnosis | CT | MRI-T1 | MRI-T2 | Enhance-ment | Technique for diagnosis |
| [9] | 60/M | Abnormal LFTs, ↑AFP | Chronic HepC | None | HCC | NA | NA | NA | A: Hyper V: Hyper | Laparoscopic surgery |
| [10] | 54/M | Normal | None | None | NA | NA | Hypo | Slightly hyper | A: Hyper V: Hypo | Laparotomy |
| [11] | 54/M | Normal | None | Gastric cancer | Liver metastasis | ND | Hypo | Slightly hyper | NA | Laparotomy |
| [12] | 52/M | Normal | None | None | Neurendocrine tumour | NA | NA | NA | Hypervascular | Surgery |
| [13] | 53/M | ↑γGT | NASH | None | HCC/ hepatic adenoma | NA | NA | NA | A: Hyper V: Hyper | Laparoscopic surgery |
| [14] | 58/M | ↑AST, ↑ALT ↑AFP, ↓PT, +HepC | Chronic HepC | None | HCC | Hypo | NA | Hyper | A: Hyper V: Hypo | Surgery |
| [15] | 67/F | Slightly abnormal LFTs, ↑AFP | Chronic HepC | None | HCC | Hypo | Slightly hyper | Slightly hyper | A: Hyper V: Hypo | Surgery |
| [16] | 42/M | +HepB +HepC | NASH | None | HCC | Hypo | Hypo | Hyper | A: Hyper V: Hyper | Laparotomy |
| [17] | 54/M | Normal | None | None | HCC | Slightly hypo | Slightly hypo | Slightly hypo | A: Hyper V: Hypo | Surgery |
| [18] | 32/M | ↑AFP, ↑AST, +HepB | Chronic HepB | None | HCC | NA | Hypo | Hyper | A: Hyper V: Slightly hyper | Laparotomy |
| [19] | 39/M | Normal | NA | None | Renal malignancy | Hypo | Hypo | Slightly hyper | A: Hyper V: Iso | Surgery |
| [20] | 49/F | Normal | None | None | Hepatic malignancy | NA | NA | NA | A: Hyper V: Hypo | Laparotomy |
| [21] | 69/M | Normal | None | None | Intrahepatic, abdominal splenosis | NA | Hypo | Slightly hyper | Hypovasc-ular | Percutaneousbiopsy |
All patients except one[14] had undergone a splenectomy in the past. Notably, a wrong diagnosis was made in all but one case[21], primarily of hepatocellular carcinoma (HCC)[9,13-18,20], leading to unnecessary surgery with the correct diagnosis only being made following post-operative histological analysis. This is partly due to the fact that splenosis is rare and hence is often not considered amongst the differential diagnosis. Additionally, six of the patients included in the literature review had chronic liver disease[9,13-16,18] including hepatitis B; a major risk factor for the development of HCC and four patients had raised tumour markers[9,14,15,18]. In such cases, HCC presents a more likely diagnosis rather than hepatic splenosis. In the isolated case where splenosis was correctly suspected, percutaneous biopsy was still carried out for confirmation[21].
Imaging is a useful diagnostic tool to distinguish splenosis from other lesions such as HCC, hepatic metastasis, haemangioma and focal nodular hyperplasia (FNH). CT and MRI provide panoramic imaging of the abdomen and can identify the size, location and enhancement characteristics of all lesions. Critically, all the splenic deposits exhibit an enhancement pattern identical to the native spleen on all imaging, with a heterogenous classical striped arterial hyperenhancement. However, this may be difficult to characterise if the native spleen is small or has been removed.
Classically, on unenhanced CT splenic tissue appears hypointense relative to the liver, whilst on MRI it appears hypodense on T1 and hyperintense on T2-weighted images. Five and six cases in the literature review exhibited these CT[14-17,19] and MRI[10,11,16,18,19,21] findings respectively (Table 1). On administration of contrast, splenic nodules are hyperintense in the arterial phase[9,10,13-20] often with a striated appearance as seen in our patient. They vary in appearance in the portal venous phase and may be hypointense[10,14,15,17,20], isointense[19] or hyperintense[9,13,16,18].
HCC has a variable appearance on both CT and MRI depending on biological characteristics including their degree of differentiation[22]. Since their blood supply is derived from the neoangiogenesis of non-triadal arteries, HCC, like splenosis typically appear hyperenhancing in the arterial phase with portal venous washout[22] (Table 2).
| Enhancement pattern | |
| Splenosis | Arterially hyperenchancing; variable venous enhancement |
| HCC | Arterially hyperenhancing; venous hypoenhancement |
| Hepatic metastasis | Arterially variable enhancement; venous hypoenhancement |
| Haemangioma | Arterially peripheral nodular enhancement; venous infilling |
| FNH | Arterially hyperenhancing; venous iso/hyperenhancing with late enhancement of scar on MRI |
| Hepatic adenoma | Arterially hyperenhancing; variable venous enhancement |
Hepatic metastasis also varies widely in appearance depending on the location of the primary tumour. They may appear hypo or hypervascular but typically show portal venous washout[23]. Haemangioma, FNH and adenoma are benign lesions which typically show arterial hyperenhancement[24-26] and hence may be mimicked by splenosis.
Of note, MRI using superparamagnetic iron oxide contrast instead of gadopentetate dimeglumine has been used to distinguish hepatic splenosis from malignancy[27]. Following intravenous administration, these particles are removed from the circulation specifically by the reticuloendothelial cells of the liver and spleen, leading to a reduction in signal intensity of the hepatic and splenic parenchyma on T2-weighted MRI. Such a reduction in signal intensity is however not seen in malignant lesions except some well differentiated HCCs[[18,28]. Splenic nodules still have a higher intensity than the hypointense liver as they take up more contrast. Nonetheless, uptake of contrast still occurs in FNH and so the specificity of this technique is limited in isolation[29].
Scintigraphy using Tc-99 DRBC is the current diagnostic tool of choice. This is due to its high specificity in identifying splenic tissue. It involves intravenous injection of heat denatured erythrocytes labelled with Tc-99. The majority, as many as 90% of the erythrocytes are sequestered in splenic tissue, whilst normal liver tissue or malignant lesions have relatively modest uptake of the radioactive isotope[30]. This technique is therefore a reliable means of distinguishing splenic tissue from other hepatic lesions and avoids subjecting a patient to invasive procedures such as biopsy or surgery which are associated with their own risks.
Patients with splenosis are typically asymptomatic. Hence, surgery is only indicated if rare complications such as infarction[31], bleeding[32] or adhesions resulting in bowel obstruction occur[33]. It is suggested that splenosis may even be beneficial, providing some degree of immunological protection[13]. As it is a benign condition, it is often only diagnosed incidentally decades after the initial trauma following imaging for an unrelated condition. It is therefore difficult to ascertain the time taken for splenosis to occur. However, the process of splenic cells seeding in and growing on the serosal surface of the liver after recruiting nearby hepatic vasculature is likely to take several years. In the literature cases of intra-hepatic splenosis were diagnosed from a range of 5 to 46 years after trauma or splenectomy[17].
In conclusion, splenosis should be considered as a possible differential diagnosis for a hepatic nodule in any patients with a history of abdominal trauma or previous splenectomy, especially when the nodules are located near the capsule of the liver and are associated with multiple intra-abdominal deposits. Scintigraphy using Tc-99 DRBC is a reliable technique to diagnose splenosis and should be carried out in all patients suspected of the condition before more invasive diagnostic procedures are considered. Greater awareness of this condition could reduce the high incidence of unnecessary invasive interventions in these patients.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aoki H, Goral V, Surucu E, Tsoulfas G S-Editor: Ma RY L-Editor: A E-Editor: Liu MY
| 1. | Arena R, Gasperoni S, Lisotti A, Petrini CAA, Brancaccio ML, Triossi O, Mussetto A. An unusual cause of gastrointestinal bleeding and intestinal obstruction. Turk J Gastroenterol. 2018;29:365-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Guedes TP, Fernandes B, Pedroto I. Hepatobiliary and Pancreatic: Symptomatic hepatic splenosis. J Gastroenterol Hepatol. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Guan B, Li XH, Wang L, Zhou M, Dong ZW, Luo GJ, Meng LP, Hu J, Jin WY. Gastric fundus splenosis with hemangioma masquerading as a gastrointestinal stromal tumor in a patient with schistosomiasis and cirrhosis who underwent splenectomy: A case report and literature review. Medicine (Baltimore). 2018;97:e11461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Bastidas AB, Holloman D, Lankarani A, Nieto JM. Endoscopic Ultrasound-Guided Needle-Based Probe Confocal Laser Endomicroscopy (nCLE) of Intrapancreatic Ectopic Spleen. ACG Case Rep J. 2016;3:196-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Ha YJ, Hong TH, Choi YS. Thoracic Splenosis after Splenic and Diaphragmatic Injury. Korean J Thorac Cardiovasc Surg. 2019;52:47-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Page JB, Lenz DL, Wong C. Right-sided intrarenal splenosis mimicking a renal carcinoma. ScientificWorldJournal. 2006;6:2442-2444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Disanto MG, Mercalli F, Palicelli A, Arnulfo A, Boldorini R. A unique case of bilateral ovarian splenosis and review of the literature. APMIS. 2017;125:844-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Kim JM, Lee SM, Choi J, Lyu J, Lee MS. Two Rare Cases of Intrathoracic Splenosis and Subcutaneous Splenosis: Spleen Scintigraphy Avoided the Need for Invasive Procedures. Nucl Med Mol Imaging. 2016;50:76-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Abu Hilal M, Harb A, Zeidan B, Steadman B, Primrose JN, Pearce NW. Hepatic splenosis mimicking HCC in a patient with hepatitis C liver cirrhosis and mildly raised alpha feto protein; the important role of explorative laparoscopy. World J Surg Oncol. 2009;7:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Yu H, Xia L, Li T, Ju M, Liu L, Wu Z, Tang Z. Intrahepatic splenosis mimicking hepatoma. BMJ Case Rep. 2009;2009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Kang KC, Cho GS, Chung GA, Kang GH, Kim YJ, Lee MS, Kim HK, Park SJ. Intrahepatic splenosis mimicking liver metastasis in a patient with gastric cancer. J Gastric Cancer. 2011;11:64-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Leong CW, Menon T, Rao S. Post-traumatic intrahepatic splenosis mimicking a neuroendocrine tumour. BMJ Case Rep. 2013;2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Inchingolo R, Peddu P, Karani J. Hepatic splenosis presenting as arterialised liver lesion in a patient with NASH. Eur Rev Med Pharmacol Sci. 2013;17:2853-2856. [PubMed] |
| 14. | Sato N, Abe T, Suzuki N, Waragai M, Teranishi Y, Takano Y, Sato A, Azami A, Gotoh M. Intrahepatic splenosis in a chronic hepatitis C patient with no history of splenic trauma mimicking hepatocellular carcinoma. Am J Case Rep. 2014;15:416-420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Li T, Yang XY, Tang ZY. Intrahepatic and intraperitoneal splenosis mimicking hepatocellular carcinoma with abdominal wall metastasis in a patient with hepatitis C cirrhotic liver. Surgery. 2015;157:954-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Wang MY, Li B, Chen D, Liu AL, Qamar S, Sun MY. Spleen implanting in the fatty liver mimicking hepatocarcinoma in a patient with hepatitis B&C: A case report and literature review. Medicine (Baltimore). 2017;96:e7217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Xuan Z, Chen J, Song P, Du Y, Wang L, Wan D, Zheng S. Management of intrahepatic splenosis:a case report and review of the literature. World J Surg Oncol. 2018;16:119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Choi GH, Ju MK, Kim JY, Kang CM, Kim KS, Choi JS, Han KH, Park MS, Park YN, Lee WJ, Kim BR. Hepatic splenosis preoperatively diagnosed as hepatocellular carcinoma in a patient with chronic hepatitis B: a case report. J Korean Med Sci. 2008;23:336-341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Imbriaco M, Camera L, Manciuria A, Salvatore M. A case of multiple intra-abdominal splenosis with computed tomography and magnetic resonance imaging correlative findings. World J Gastroenterol. 2008;14:1453-1455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Liu Y, Ji B, Wang G, Wang Y. Abdominal multiple splenosis mimicking liver and colon tumors: a case report and review of the literature. Int J Med Sci. 2012;9:174-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Vergara D, Ginolfi F, Moscati S, Giordano B, Ferrara N, Panico C, Imbriaco M. Multiple intra-hepatic and abdominal splenosis: an easy call if you know about it. Acta Radiol Open. 2018;7:2058460118772324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Choi JY, Lee JM, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part I. Development, growth, and spread: key pathologic and imaging aspects. Radiology. 2014;272:635-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 349] [Article Influence: 31.7] [Reference Citation Analysis (1)] |
| 23. | Sica GT, Ji H, Ros PR. CT and MR imaging of hepatic metastases. AJR Am J Roentgenol. 2000;174:691-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 118] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Klotz T, Montoriol PF, Da Ines D, Petitcolin V, Joubert-Zakeyh J, Garcier JM. Hepatic haemangioma: common and uncommon imaging features. Diagn Interv Imaging. 2013;94:849-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 25. | Mortelé KJ, Praet M, Van Vlierberghe H, Kunnen M, Ros PR. CT and MR imaging findings in focal nodular hyperplasia of the liver: radiologic-pathologic correlation. AJR Am J Roentgenol. 2000;175:687-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 117] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 26. | Faria SC, Iyer RB, Rashid A, Whitman GJ. Hepatic adenoma. AJR Am J Roentgenol. 2004;182:1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Kondo M, Okazaki H, Takai K, Nishikawa J, Ohta H, Uekusa T, Yoshida H, Tanaka K. Intrahepatic splenosis in a patient with chronic hepatitis C. J Gastroenterol. 2004;39:1013-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | De Vuysere S, Van Steenbergen W, Aerts R, Van Hauwaert H, Van Beckevoort D, Van Hoe L. Intrahepatic splenosis: imaging features. Abdom Imaging. 2000;25:187-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Zheng WW, Zhou KR, Chen ZW, Shen JZ, Chen CZ, Zhang SJ. Characterization of focal hepatic lesions with SPIO-enhanced MRI. World J Gastroenterol. 2002;8:82-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Massey MD, Stevens JS. Residual spleen found on denatured red blood cell scan following negative colloid scans. J Nucl Med. 1991;32:2286-2287. [PubMed] |
| 31. | Ksiądzyna D. A case report of abdominal splenosis - a practical mini-review for a gastroenterologist. J Gastrointestin Liver Dis. 2011;20:321-324. [PubMed] |
| 32. | Basile RM, Morales JM, Zupanec R. Splenosis. A cause of massive gastrointestinal hemorrhage. Arch Surg. 1989;124:1087-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | El-Kheir A, Abdelnour M, Boutros JG. Simultaneous small bowel and colon obstruction due to splenosis. A case report and review of literature. Int J Surg Case Rep. 2019;58:63-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |