Published online Dec 27, 2019. doi: 10.4254/wjh.v11.i12.761
Peer-review started: September 4, 2019
First decision: October 14, 2019
Revised: October 28, 2019
Accepted: November 25, 2019
Article in press: November 25, 2019
Published online: December 27, 2019
Processing time: 112 Days and 11.3 Hours
Hepatic steatosis is a common form of cystic fibrosis associated liver disease (CFLD) seen in an estimated 15%-60% of patients with cystic fibrosis (CF). The pathophysiology and health implications of hepatic steatosis in cystic fibrosis remain largely unknown. In the general population, hepatic steatosis is strongly associated with insulin resistance and type 2 diabetes. Cystic fibrosis related diabetes (CFRD) impacts 40%-50% of CF adults and is characterized by both insulin insufficiency and insulin resistance. We hypothesized that patients with CFRD would have higher levels of hepatic steatosis than cystic fibrosis patients without diabetes.
To determine whether CFRD is associated with hepatic steatosis and to explore the impact of lumacaftor/ivacaftor therapy on hepatic steatosis in CF.
Thirty patients with CF were recruited from a tertiary care medical center for this cross-sectional study. Only pancreatic insufficient patients with CFRD or normal glucose tolerance (NGT) were included. Patients with established CFLD, end stage lung disease, or persistently elevated liver enzymes were excluded. Mean magnetic resonance imaging (MRI) proton density fat fraction (PDFF) was obtained for all participants. Clinical characteristics [age, sex, body mass index, percent predicted forced expiratory volume at 1 s (FEV1), lumacaftor/ivacaftor use] and blood chemistries were assessed for possible association with hepatic steatosis. Hepatic steatosis was defined as a mean MRI PDFF > 5%. Patients were grouped by diabetes status (CFRD, NGT) and cystic fibrosis transmembrane conductance regulator (CFTR) modulator use (lumacaftor/ivacaftor, no lumacaftor/ivacaftor) to determine between group differences. Continuous variables were analyzed with a Wilcoxon rank sum test and discrete variables with a Chi square test or Fisher’s exact test.
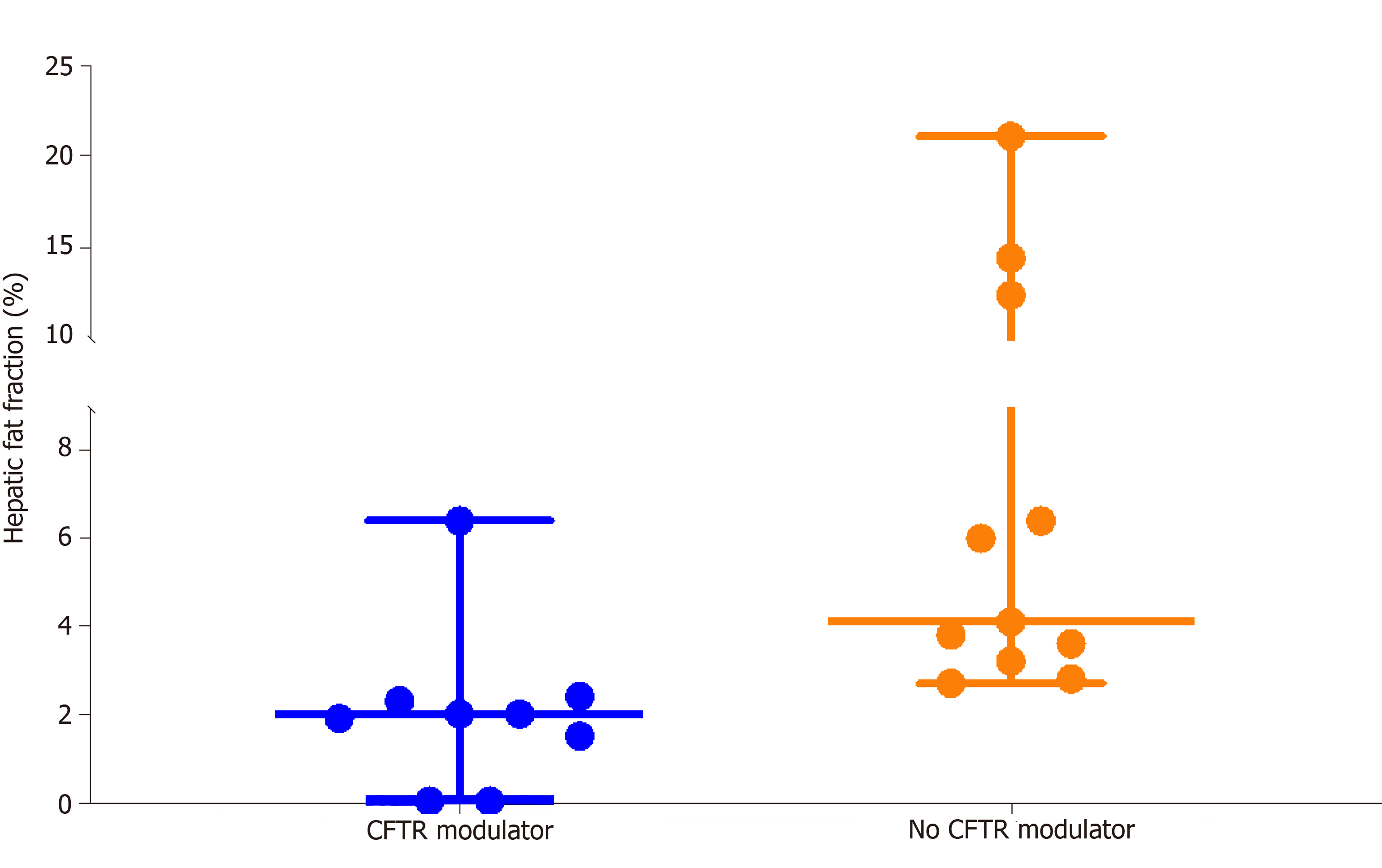
Twenty subjects were included in the final analysis. The median age was 22.3 years (11.3-39.0) and median FEV1 was 77% (33%-105%). Twelve subjects had CFRD and 8 had NGT. Nine subjects were receiving lumacaftor/ivacaftor. The median PDFF was 3.0% (0.0%-21.0%). Six subjects (30%) had hepatic steatosis defined as PDFF > 5%. Hepatic fat fraction was significantly lower in patients receiving lumacaftor/ivacaftor (median, range) (2.0%, 0.0%-6.4%) than in patients not receiving lumacaftor/ivacaftor (4.1%, 2.7-21.0%), P = 0.002. Though patients with CFRD had lower PDFF (2.2%, 0.0%-14.5%) than patients with NGT (4.9%, 2.4-21.0%) this did not reach statistical significance, P = 0.06. No other clinical characteristic was strongly associated with hepatic steatosis.
Use of the CFTR modulator lumacaftor/ivacaftor was associated with significantly lower hepatic steatosis. No association between CFRD and hepatic steatosis was found in this cohort.
Core tip: Hepatic steatosis is a common manifestation of liver disease in cystic fibrosis (CF). It remains unknown whether hepatic steatosis contributes to the development of cirrhosis in patients with CF. Lumacaftor/ivacaftor is a cystic fibrosis transmembrane conductance regulator (CFTR) modulator drug targeting the defective chloride channel that causes CF. In this cross-sectional study, CF patients receiving lumacaftor/ivacaftor had significantly lower magnetic resonance imaging proton density fat fractions than CF patients not receiving the CFTR modulator. CFTR modulator use should be included in future studies of CF liver disease.
- Citation: Kutney K, Donnola SB, Flask CA, Gubitosi-Klug R, O’Riordan M, McBennett K, Sferra TJ, Kaminski B. Lumacaftor/ivacaftor therapy is associated with reduced hepatic steatosis in cystic fibrosis patients. World J Hepatol 2019; 11(12): 761-772
- URL: https://www.wjgnet.com/1948-5182/full/v11/i12/761.htm
- DOI: https://dx.doi.org/10.4254/wjh.v11.i12.761
The life expectancy for cystic fibrosis (CF) patients has improved dramatically over the past several decades, and continued improvement is expected with the widespread use of cystic fibrosis transmembrane conductance regulator (CFTR) modulator therapies[1]. While pulmonary disease remains the leading cause of mortality in CF, extra pulmonary complications such as cystic fibrosis related diabetes (CFRD) and cystic fibrosis associated liver disease (CFLD) have emerged as important sources of morbidity in this population[2-4]. As the life expectancy for CF patients improves, determining the impact of CFTR modulator therapy on extra-pulmonary disease is of critical importance.
Hepatic manifestations of CF are broad, including: Neonatal cholestasis, transaminase elevation, hepatic steatosis, focal biliary cirrhosis, multilobular cirrhosis and portal hypertension[3,5]. Cirrhosis with portal hypertension is the primary cause of morbidity and mortality from CFLD[6]. Debate regarding the optimal diagnostic criteria for CFLD is ongoing[6-9]. While older studies describe CFLD as a childhood-onset disease, recent data demonstrates that adult-onset CFLD is relatively common[8,10,11]. Multilobular cirrhosis with portal hypertension is the end stage manifestation of CFLD and is the third leading cause of death in CF patients[1,6].
Perhaps the most common manifestation of CFLD is hepatic steatosis, with prevalence estimates ranging from 15%-60%[7,12,13]. Historically, hepatic steatosis in CF patients was attributed to malnutrition and considered a benign finding that did not increase risk for hepatic cirrhosis[14]. Outside of CF, hepatic steatosis is strongly associated with obesity and type 2 diabetes[15]. Hepatic steatosis can progress to non-alcoholic steatohepatitis and cirrhosis, which is now a common indication for liver transplantation among the general population in the United States[16]. Little is known about the clinical implications of hepatic steatosis in CF and its relationship to other forms of CFLD[12,17,18].
CFRD is another common extrapulmonary manifestation of CF, with a prevalence of approximately 20% in adolescents and 40%-50% in adults[19]. CFRD is distinct from type 1 diabetes, which is characterized by absolute insulin deficiency, and type 2 diabetes in which peripheral insulin resistance predominates. CFRD is primarily a disease of insulin insufficiency, though insulin resistance occurs during illness and with increasing age[20,21]. Patients with CFRD typically have lower body mass index (BMI), reduced pulmonary function and higher mortality rates. These effects are at least partially mitigated by insulin therapy[22]. The prevalence of CFRD is also higher in CF patients with liver disease[23].
Both CFRD and CFLD are almost exclusively seen in patients carrying two pathogenic CFTR variants that severely limit the chloride channel function[24,25]. CFTR variants are generally categorized into five (or six) groups according to the underlying cause of channel malfunction. Class 1-3 variants result in little or no CFTR function while class 4-6 variants are characterized by residual CFTR function[26]. Individualized CF therapy relies on understanding the functional defect causing CFTR malfunction[27]. CFTR modulator therapies are a revolutionary class of small molecules targeting the underlying defect in CF[26]. Ivacaftor is a CFTR potentiator that increases chloride conductance only if CFTR is present in the cell membrane[28]. Lumacaftor and tezacaftor are correctors which redirect misfolded CFTR protein to the cell surface[29,30]. Lumacaftor/ivacaftor combination therapy was approved in 2015 for patients carrying two copies of the F508del (p.Phe508del, c.1521_1523delCTT) pathogenic variant.
While pulmonary effects of CFTR modulators have been meticulously examined, the extrapulmonary effects are not well characterized[28,30-33]. Two small studies demonstrated improved insulin secretion after modulator therapy; while two other studies failed to show improvement[34-37]. No studies have systematically examined the impact of CFTR modulator therapy on hepatic steatosis or other liver disease in patients with CF. Thus, we sought to determine the impact of CFRD on hepatic steatosis in CF patients and explore other factors associated with elevated hepatic fat, including CFTR modulator use.
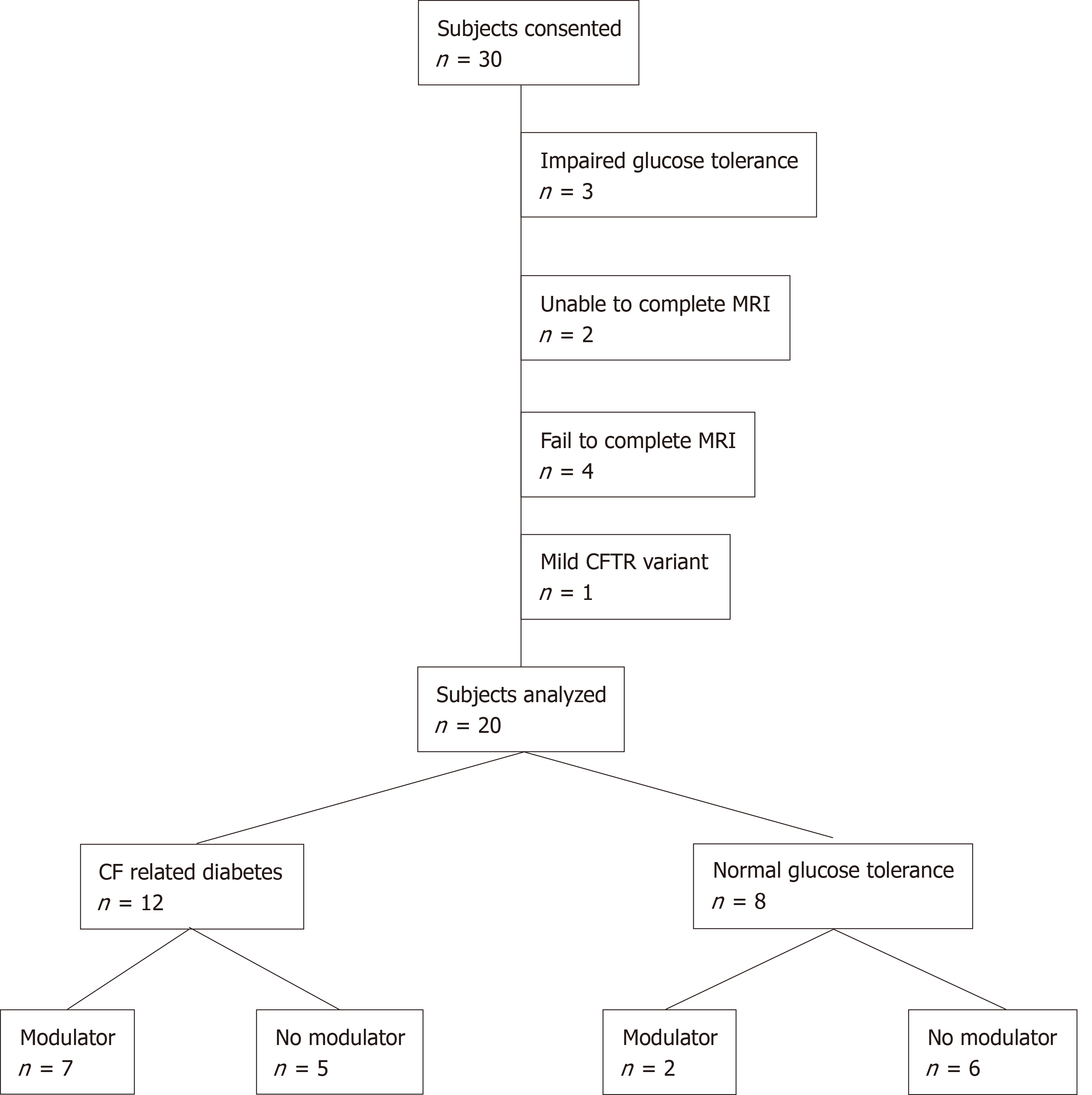
All studies were conducted according to the approved Institutional Review Board protocols at University Hospitals Cleveland Medical Center between January 1 and December 31, 2017. Thirty subjects with CF were recruited from the LeRoy W. Matthews Cystic Fibrosis Center at University Hospitals Cleveland Medical Center/ Rainbow Babies and Children’s Hospital in Cleveland, OH, United States (Table 1 and Supplemental Table 1). Eligible subjects were identified using the local CF database and were approached for study involvement during routine clinic visits or during hospitalization for a CF pulmonary exacerbation. All subjects were diagnosed with cystic fibrosis based on sweat chloride and genetic testing according to established guidelines[38]. Electronic medical records were reviewed to confirm eligibility. Inclusion criteria were age 10-40 years, pancreatic insufficiency and either normal glucose tolerance (NGT) or CFRD[39]. Pancreatic insufficiency was defined by a clinical need for pancreatic enzyme replacement. No fecal elastase testing was performed as part of this study. Subjects with established CFLD, persistent transaminase elevation for greater than one year, low baseline lung function (i.e., FEV1 < 30% predicted) or an ongoing pulmonary exacerbation were excluded. Only subjects with two copies of a class 1-3 pathogenic CFTR variant were included in the final analysis. CFTR variants were classified into classes 1-5 using the CFTR 2 database and existing guidelines for functional classification[40,41]. Subjects with contraindications to MRI scanning (i.e., metal implants, pregnancy) were also excluded. Informed consent was obtained in person prior to commencing study activities.
| All subjects (n = 20) | CFTR modulator (n = 9) | No CFTR modulator (n = 11) | P value | |
| Age at MRI (yr) | 22.3 (11.3-39.0) | 26.4 (16.3-39.0) | 21.9 (11.3-36.1) | 0.29 |
| Genotype | ||||
| F508del/F508del | 10 | 9 | 1 | < 0.01 |
| F508del/other | 9 | 0 | 9 | < 0.01 |
| Other/other | 1 | 0 | 1 | < 0.01 |
| Male sex | 16 (80%) | 7 (78%) | 9 (82%) | 1.00 |
| BMI percentile | 39 (2-96) | 51 (3-77) | 23 (2-96) | 0.21 |
| % predicted FEV1 | 77 (33-105) | 73 (33-89) | 77 (48-105) | 0.24 |
| CFRD | 12 (60%) | 7 (78%) | 5 (45%) | 0.20 |
| Insulin therapy | 10 (50%) | 6 (67%) | 4 (36%) | 0.37 |
Clinical characteristics including BMI, percent predicted FEV1, diabetes status, insulin use and CFTR modulator use were collected from the electronic medical record. Fasting blood chemistries including lipids, hepatic function tests and hemoglobin A1C were assessed during a period of baseline health in ambulatory subjects or after completing treatment for a pulmonary exacerbation in hospitalized subjects. An oral glucose tolerance test (OGTT) was performed in NGT subjects who had not had an OGTT in the past six months. Glucose tolerance testing was performed according to standard guidelines. After an eight hour fast, subjects ingested 1.75 g/kg (maximum 75 g) of glucose dissolved in water. Plasma glucose was evaluated at baseline and 2 h post glucose ingestion[39]. Subjects whose study OGTT demonstrated impaired fasting glucose or impaired glucose tolerance as defined by standard criteria were excluded[42].
All serum chemistries were collected from peripheral venous samples according to standard technique[43]. Glucose, triglycerides, high density lipoprotein (HDL), low density lipoprotein (LDL), aspartate transaminase (AST), alanine transaminase (ALT), gamma-glutamyltransferase (GGT), alkaline phosphatase, and total bilirubin were analyzed using a Beckman AU 5800® analyzer. Hemoglobin A1C was analyzed using a BioRad-D-100® analyzer (University Hospitals Core Lab, Cleveland, OH, United States).
Proton density fat fraction (PDFF) was measured on a Siemens Skyra 3T magnetic resonance imaging (MRI) in the Imaging Research Core at Case Western Reserve University. Briefly, each subject was positioned supine within the MRI scanner. Spine and body array coils were used to obtain uniform images over the entire liver. A single-breathhold VIBE MRI acquisition was used to obtain axial liver PDFF maps for each subject (spatial resolution = 2 mm × 2 mm × 5 mm, 6 echoes). This MRI method also incorporates T2* correction to limit the effects of iron deposition and hepatic fibrosis[44]. All images were exported for offline analysis in Matlab (The Mathworks, Natick, MA, United States). Mean liver PDFF was determined for the central 6-8 imaging slices in each subject using a region of interest (ROI) analysis. The mean liver PDFF in each slice was then averaged over all slices to calculate the overall mean liver PDFF for each subject.
For this study, we considered a PDFF > 5% to be consistent with clinical hepatic steatosis[45]. We grouped subjects by the presence of hepatic steatosis > 5%, diabetes status, and CFTR modulator use to evaluate for significant associations. BMI percentiles were calculated for all subjects to account for age-related variation in BMI. Alkaline phosphatase measurements were standardized by subtracting the age and sex specific mean and dividing by the respective standard deviation. The resulting values were in units of standard deviation. Continuous variables were described with medians and ranges and nominal variables with frequencies and percent. Continuous variables were analyzed with a Wilcoxon rank sum test and nominal variables were analyzed using Chi square test or Fisher’s exact test. Statistical analysis was performed using SAS software version 9.4 (SAS Institute, Cary, NC, United States). The level of significance was set at 0.05. The statistical analyses were performed by MaryAnn O’Riordan PhD, biomedical statistician.
We recruited 30 participants of whom 20 completed the study (Figure 1). Exclusions related to progression to impaired glucose tolerance on OGTT (n = 3), inability to perform breath hold for MRI (n = 2), failure to schedule or complete the MRI (n = 4) and presence of a class 4 CFTR pathogenic variant (n = 1). Participant characteristics are summarized in Table 1. The study population was primarily Caucasian, which is consistent with the demographic of the CF population overall. Eighty percent of the study subjects were male. Median subject age at the time of MRI was 22.3 years with a range from 11.3 to 39.0 years. All participants had two severe class 1-3 CFTR pathogenic variants. Twelve subjects (60%) had CFRD and 8 subjects (40%) had NGT. Ten CFRD subjects (83%) were prescribed insulin therapy. Nine subjects (45%) had received the CFTR modulator lumacaftor/ivacaftor (Orkambi®) for more than 12 mo at the time of MRI. No subject received lumacaftor/ivacaftor for fewer than 12 mo.
The median hepatic fat fraction for all subjects was 3.0% with a range from 0.0%-21.0%. Six subjects (30%) had hepatic steatosis, defined as PDFF > 5%. Subjects with hepatic steatosis showed a trend toward younger age that did not reach statistical significance. Alkaline phosphatase and age-adjusted alkaline phosphatase (z-score) were higher in subjects with hepatic steatosis, P = 0.01 and P = 0.03. LDL and HDL were both higher in patients with hepatic steatosis, P = 0.05 and P = 0.02. Total bilirubin, AST, ALT and GGT did not differ significantly between subjects with and without hepatic steatosis (Table 2). Missing data for alkaline phosphatase, AST, ALT, total bilirubin (1 missing), LDL, HDL, triglyceride (4 missing), and GGT (3 missing) were excluded from all analyses.
| Normal Range | All subjects (n = 20) | Steatosis (n = 6) | No Steatosis (n = 14) | P value | CFTR modulator(n = 9) | No CFTR modulator(n = 11) | P value | CFRD (n = 12) | NGT (n = 8) | P value | |
| Hepatic Fat Fraction | -- | 3.0 | 9.5 | 2.4 | 0.01 | 2.0 | 4.1 | 0.002 | 2.2 | 4.9 | 0.06 |
| 0.0-21.0 | 6.0-21.0 | 0-4.1 | 0.0-6.4 | 2.7-21 | 0.0-14.5 | 2.4-21 | |||||
| Age | -- | 22.3 | 16.7 | 26.0 | 0.08 | 26.4 | 21.9 | 0.29 | 28.2 | 18.0 | 0.04 |
| 11.3-39.0 | 13.4-36.1 | 11.3-39.0 | 16.3-39.0 | 11.3-36.1 | 17.0-39.0 | 11.3-30.6 | |||||
| Sex (male) | -- | 16 | 5 | 11 | 1.00 | 7 | 9 | 1.00 | 8 | 8 | 0.12 |
| 80% | 83% | 78% | 78% | 82% | 67% | 100% | |||||
| BMI | -- | 21.0 | 20.5 | 21.0 | 0.6 | 22.4 18.8-25.7 | 20.0 16.9-32.4 | 0.05 | 21.0 18.8-32.4 | 19.6 16.9-22.4 | 0.05 |
| 16.9-32.4 | 16.9-32.4 | 18.8-25.7 | |||||||||
| BMI percentile | -- | 39 | 50 | 30 | 0.77 | 51 | 23 | 0.21 | 39 | 37.5 | 0.62 |
| 2-96 | 2-96 | 3-77 | 3-77 | 2-96 | 3-96 | 2-73 | |||||
| FEV1 % | -- | 77 | 86 | 74.5 | 0.22 | 73 | 77 | 0.24 | 63.5 | 88 | 0.001 |
| 33-105 | 62-105 | 33-97 | 33-89 | 48-105 | 33-105 | 65-97 | |||||
| CFTR modulator | -- | 9 | 1 | 8 | 0.16 | 9 | 11 | -- | 7 | 2 | 0.20 |
| 45% | 17% | 57% | 100% | 100% | 58% | 25% | |||||
| Hemoglobin A1C (%) | < 5.8 | 5.8 | 5.7 | 6.3 | 0.30 | 6.3 | 5.7 | 0.21 | 6.4 | 5.6 | 0.002 |
| 5.2-8.2 | 5.4-6.4 | 5.2-8.2 | 5.3-8.2 | 5.2-7.4 | 5.4-8.2 | 5.2-5.7 | |||||
| AST (U/L) | 9-39 | 23 | 27 | 23 | 0.93 | 20 | 27 | 0.39 | 20 | 26 | 0.87 |
| 11-45 | 11-45 | 11-42 | 11-42 | 11-45 | 11-42 | 11-45 | |||||
| ALT (U/L) | 10-52 | 22 | 24.5 | 22 | 0.57 | 18 | 29 | 0.19 | 18 | 26 | 0.46 |
| 10-58 | 12-58 | 10-45 | 10-45 | 12-58 | 10-58 | 14-47 | |||||
| GGT (U/L) | 5-64 | 14 | 19 | 13.5 | 0.10 | 12 | 19 | 0.07 | 13.5 | 19 | 0.30 |
| 9-29 | 14-25 | 9-29 | 9-24 | 10-29 | 9-29 | 11-24 | |||||
| Alk Phos (U/L) | 33-120 | 110 | 172 | 90 | 0.01 | 66 | 155 | 0.01 | 90 | 157 | 0.04 |
| 44-310 | 146-310 | 44-234 | 44-178 | 103-310 | 44-178 | 71-310 | |||||
| Alk Phos SD | -2-2 | 1.2 | 3.1 | -0.3 | 0.03 | -0.3 | 1.8 | 0.07 | 0.6 | 1.4 | 0.65 |
| -1.5-4.8 | -0.6-4.8 | -1.5-4.7 | -1.5-4.7 | -0.6-4.8 | -1.5-4.8 | -0.6-3.2 | |||||
| Total Bili (µmol/L) | 0-20.5 | 6.8 | 8.5 | 5.1 | 0.47 | 5.1 | 8.6 | 0.003 | 5.1 | 8.6 | 0.06 |
| 3.4-20.5 | 3.4-15.4 | 3.4-20.5 | 3.4-6.8 | 3.4-20.5 | 3.4-18.8 | 5.1-20.5 | |||||
| Triglyceride (mmol/L) | < 1.7 | 1.02 | 1.25 | 0.68 | 0.07 | 0.9 | 1.25 | 0.79 | 0.68 | 1.28 | 0.15 |
| 0.36-2.55 | 0.69-2.55 | 0.36-1.54 | 0.64-2.55 | 0.36-2.35 | 0.64-2.35 | 0.36-2.55 | |||||
| LDL (mmol/L) | < 3.4 | 1.7 | 1.4 | 1.7 | 0.05 | 1.6 | 1.7 | 0.53 | 1.7 | 1.6 | 0.71 |
| 0.8-2.4 | 0.9-1.6 | 0.8-2.4 | 0.8-2.2 | 0.9-2.4 | 0.9-2.4 | 0.8-2.0 | |||||
| HDL (mmol/L) | > 1.0 | 1.03 | 0.84 | 1.09 | 0.02 | 1.04 | 0.98 | 0.31 | 1.01 | 1.05 | 0.73 |
| 0.53-2.03 | 0.53-1.41 | 0.91-2.03 | 0.86-2.03 | 0.53-1.54 | 0.53-2.03 | 0.84-1.53 |
Hepatic fat fraction was significantly lower in the 9 subjects receiving CFTR modulator therapy (2.0%, 0.0%-6.4%) than in the 11 subjects not receiving CFTR modulators (4.1%, 2.7%-21.0%), P = 0.002 (Figure 2). Two CFRD subjects receiving CFTR modulators had exceptionally low hepatic fat fractions of 0.0%. Subjects receiving CFTR modulator therapy were not significantly different in terms of age, BMI percentile or diabetes status from subjects not receiving modulators. Absolute BMI was higher in the CFTR modulator group, which likely reflects expected age-related change in BMI, P = 0.05 (Table 3).
| Reference range | All subjects, n = 20 | CFTR modulator, n = 9 | No CFTR modulator, n = 11 | P value | |
| PDFF (%) | < 5% | 3.0 (0.0-21.0) | 2.0 (0.0-6.4) | 4.1 (2.7-21.0) | 0.002 |
| HbA1C (%) | < 5.8% | 5.8 (5.2-8.2) | 6.3 (5.3-8.2) | 5.7 (5.2-7.4) | 0.21 |
| Alk Phos (U/L) | 1 | 110 (44-310) | 66 (44-178) | 155 (103-310) | 0.01 |
| Alk Phos (SD) | -2.0-2.0 | 1.2 (-1.5-4.8) | -0.3 (-1.5-4.7) | 1.8 (-0.6-4.8) | 0.07 |
| Total bilirubin (µmol/L)2 | 0-20.5 | 6.8 (3.4-20.5) | 5.1 (3.4-6.8) | 8.6 (3.4-20.5) | 0.003 |
| AST (U/L) | 9-39 | 23 (11-45) | 20 (11-42) | 27 (11-45) | 0.39 |
| ALT (U/L) | 10-52 | 22 (10-58) | 18 (10-45) | 29 (12-58) | 0.19 |
| GGT (U/L) | 5-64 | 14 (9-29) | 12 (9-24) | 19 (10-29) | 0.07 |
| Triglyceride (mmol/L)3 | < 1.7 | 1.02 (0.36-2.55) | 0.9 (0.64-2.55) | 1.25 (0.36-2.35) | 0.79 |
CFTR modulator use was associated with lower total bilirubin than no CFTR modulator use, P = 0.003. Alkaline phosphatase levels were also lower in the CFTR modulator group, but this difference may relate to younger age in the no CFTR modulator group, P = 0.01. Although age-adjusted alkaline phosphatase (z scores) were numerically lower in the CFTR modulator group than the no CFTR modulator group, this did not reach statistical significance, P = 0.07 (Table 3).
The median hepatic fat fraction was not statistically different between subjects with CFRD (median, range) (2.2%, 0.0-14.5%) and NGT (4.9%, 2.4-21.0%), P = 0.06. Subjects with CFRD were older (28.2 years, 17.0-39.0) than NGT subjects (18.0 years, 11.3-30.6), P = 0.04. As expected, the older CFRD cohort demonstrated a higher BMI, P = 0.05, but not BMI percentile, than NGT subjects. Patients with CFRD demonstrated lower percent predicted FEV1, which is known to be associated with CFRD, P = 0.001. Subjects with CFRD also demonstrated higher hemoglobin A1C levels, P = 0.002. Alkaline Phosphatase was lower in the CFRD group compared to the NGT group, P = 0.04; however, age adjusted alkaline phosphatase (z-score) was not different between groups (Table 2).
Importantly, CFTR modulator use was more common among patients with CFRD (7 of 12, 58%) than patients with NGT (2 of 8, 22%). Because we demonstrated that CFTR modulator use is associated with lower hepatic fat, we repeated the fat fraction analysis by diabetes status after excluding subjects receiving CFTR modulator therapy. The median hepatic fat fraction for the 5 CFRD subjects not receiving CFTR modulator was 4.1% (range 2.8%-14.5%) and for the 6 NGT subject not receiving CFTR modulators was 4.9% (range 2.7%-21.0%), which were not significantly different, P = 0.92.
In this cross-sectional study of 20 CF patients aged 11-39 years with either NGT or CFRD, we demonstrate a statistically significant association between use of the CFTR modulator lumacaftor/ivacaftor and reduced hepatic fat. CFTR modulator use was also associated with lower total bilirubin and a trend toward lower age-adjusted alkaline phosphatase levels (z-score). Interestingly, CFRD patients on lumacaftor/ivacaftor demonstrated particularly low hepatic fat fractions (0.0% in two cases), suggesting a particular sensitivity to modulator effects in patients with CFRD (Supplemental Table 1). CFRD was not found to be associated with increased hepatic steatosis as was originally hypothesized. In contrast, patients with CFRD showed a trend toward lower PDFF, which most likely reflects higher rates of CFTR modulator use in the CFRD group. Given the small number of subjects not receiving lumacaftor/ivacaftor, we cannot exclude a relationship between CFRD and hepatic steatosis in CF based on this study.
The multifactorial pathogenesis of hepatic steatosis has not been fully elucidated. While strongly associated with obesity and insulin resistance in the general population, hepatic steatosis has historically been attributed to nutritional deficiencies in CF patients. In 1999, Lindblad reported an association between hepatic steatosis and linoleic acid deficiency in a cohort of 41 CF patients[14]. Others have proposed that carnitine and choline deficiency cause hepatic steatosis in CF[5,46]. In contrast, more recent data suggests that hepatic steatosis in CF is associated with higher BMI and better lung function[13]. Importantly, one case report suggests that CFTR dysfunction may be responsible for hepatic steatosis in CF. Hayes et al[47] reported rapid resolution of severe hepatic steatosis in a 17 year old female (F508del/G511D genotype) after initiation of ivacaftor therapy. Our results further support a role for CFTR dysfunction in the pathogenesis of hepatic steatosis in CF.
We have considered possible explanations for our findings. As CFTR is not expressed in hepatocytes, improvements in hepatic steatosis with CFTR modulators must be mediated by CFTR expression in other tissues[48]. In CF, biliary stasis and impaired enterocyte function contribute to persistent fat malabsorption, even with adequate pancreatic enzyme replacement[49,50]. Chronic fat malabsorption can lead to deficiencies in fat soluble nutrients including linoleic acid and choline-which have previously been associated with hepatic steatosis[46]. Therefore, we theorize that CFTR modulator therapy may lead to resolution of hepatic steatosis by reversing nutritional deficiencies. Further mechanistic studies are needed to test this theory.
It is also possible that lumacaftor/ivacaftor therapy reduces hepatic steatosis through an off target, non-CFTR mediated, mechanism. Additionally, the extremely low hepatic fat seen in subjects receiving lumacaftor/ivacaftor may be secondary to the F508del/F508del genotype itself, rather than the modulator. Although the single F508del/F508del homozygous subject not on lumacaftor/ivacaftor had significant hepatic steatosis, a single observation cannot exclude a genotype effect. Ultimately, longitudinal study is needed to demonstrate that CFTR modulator therapy causes reduced hepatic steatosis and elucidate the mechanism behind this observation.
Prior studies of hepatic steatosis in CF patients have compared varied, qualitative measures of hepatic fat[13,14]. Ours is the first study to utilize a single, precise, quantitative measure of hepatic fat, the MRI PDFF. Other strengths of our study include the collection of detailed biochemical and clinical information. We acknowledge important limitations. As this study utilized a cross sectional design, we can only demonstrate an association between lumacaftor/ivacaftor therapy and reduced hepatic fat. Moreover, we are unable to exclude the possibility that the F508del/F508del genotype, rather than CFTR modulator use, is associated with reduced hepatic steatosis. Prospective, longitudinal study of modulator therapy in patients expressing different pathogenic CFTR variants will help clarify this question. The relatively small sample size limited our power to detect differences in hepatic fat between CFRD and NGT subjects. This study does not eliminate a possible association between CFRD and hepatic steatosis in CF. Further longitudinal study is needed to understand how hepatic steatosis influences insulin sensitivity and risk for progression to CFRD.
In conclusion, we found no evidence that CFRD is associated with increased hepatic steatosis. We provide strong preliminary data suggesting that lumacaftor/ivacaftor is associated with reduced hepatic steatosis in CF patients. This finding raises many questions about the impact of CFTR modulator therapy on nutrient absorption and on the mechanisms of hepatic steatosis in CF patients. Our study raises the possibility that CFTR modulator therapy may impact other forms of CFLD and adds to the small but growing literature on the extrapulmonary impact of CFTR modulator therapy. CFTR modulator status should be included in future studies of hepatic steatosis or CFLD.
Hepatic steatosis is a common form of cystic fibrosis associated liver disease (CFLD). The journal has published previous manuscripts regarding CFLD.
Cystic fibrosis (CF) transmembrane conductance regulator (CFTR) modulators are a revolutionary therapy which target the underlying cause of CF for the first time. Currently, very little is known about the impact of CFTR modulator therapy on hepatic disease in CF, despite liver failure being the third leading cause of death in CF patients.
The objectives of this study were therefore to determine whether CF related diabetes (CFRD) is associated with hepatic steatosis and to identify predictors of hepatic steatosis in CF.
Patients with established CFLD, end stage lung disease, or persistently elevated liver enzymes were excluded. Mean magnetic resonance imaging (MRI) proton density fat fraction (PDFF) was obtained for all participants. Clinical characteristics and blood chemistries were assessed for possible association with hepatic steatosis. Hepatic steatosis was defined as a mean MRI PDFF > 5%. Patients were grouped by diabetes status and CFTR modulator use (lumacaftor/ivacaftor, no lumacaftor/ivacaftor) to determine between group differences. Continuous variables were analyzed with a Wilcoxon rank sum test and discrete variables with a Chi square test or Fisher’s exact test.
Twelve subjects (60%) had CFRD and 8 subjects (40%) had normal glucose tolerance (NGT). The median hepatic fat fraction for all subjects was 3.0% with a range from 0.0%-21.0%. Six subjects (30%) had hepatic steatosis, defined as PDFF > 5%. Hepatic fat fraction was significantly lower in the 9 subjects receiving CFTR modulator therapy (2.0%, 0.0%-6.4%) than in the 11 subjects not receiving CFTR modulators (4.1%, 2.7%-21.0%), P = 0.002. The median hepatic fat fraction was not statistically different between subjects with CFRD (median, range) (2.2%, 0.0-14.5%) and NGT (4.9%, 2.4-21.0%), P = 0.06.
In the enclosed manuscript, we demonstrate that lumacaftor/ivacaftor therapy is associated with reduced hepatic fat in CF patients. While hepatic steatosis has historically been considered a benign finding in CF, the spreading epidemic of liver failure from non-alcoholic steatohepatitis makes this doubtful.
It suggests a previously unrecognized effect of CFTR modulators of CFLD. CFTR modulator status should be included in future studies of hepatic steatosis or CFLD.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Lucarelli M, Rowland M, Tjora E S-Editor: Ma RY L-Editor: A E-Editor: Liu MY
| 1. | Cystic Fibrosis Foundation. 2016 Patient Registry Annual Data Report. Available from: https://www.cff.org/Research/Researcher-Resources/Patient-Registry/2016-Patient-Registry-Annual-Data-Report.pdf. |
| 2. | Brennan AL, Beynon J. Clinical updates in cystic fibrosis-related diabetes. Semin Respir Crit Care Med. 2015;36:236-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Herrmann U, Dockter G, Lammert F. Cystic fibrosis-associated liver disease. Best Pract Res Clin Gastroenterol. 2010;24:585-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Boëlle PY, Debray D, Guillot L, Clement A, Corvol H; French CF Modifier Gene Study Investigators. Cystic Fibrosis Liver Disease: Outcomes and Risk Factors in a Large Cohort of French Patients. Hepatology. 2019;69:1648-1656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 5. | Colombo C. Liver disease in cystic fibrosis. Curr Opin Pulm Med. 2007;13:529-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 111] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 6. | Flass T, Narkewicz MR. Cirrhosis and other liver disease in cystic fibrosis. J Cyst Fibros. 2013;12:116-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 7. | Debray D, Kelly D, Houwen R, Strandvik B, Colombo C. Best practice guidance for the diagnosis and management of cystic fibrosis-associated liver disease. J Cyst Fibros. 2011;10 Suppl 2:S29-S36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 287] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 8. | Koh C, Sakiani S, Surana P, Zhao X, Eccleston J, Kleiner DE, Herion D, Liang TJ, Hoofnagle JH, Chernick M, Heller T. Adult-onset cystic fibrosis liver disease: Diagnosis and characterization of an underappreciated entity. Hepatology. 2017;66:591-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 9. | Debray D, Narkewicz MR, Bodewes FAJA, Colombo C, Housset C, de Jonge HR, Jonker JW, Kelly DA, Ling SC, Poynard T, Sogni P, Trauner M, Witters P, Baumann U, Wilschanski M, Verkade HJ. Cystic Fibrosis-related Liver Disease: Research Challenges and Future Perspectives. J Pediatr Gastroenterol Nutr. 2017;65:443-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 10. | Wilschanski M, Rivlin J, Cohen S, Augarten A, Blau H, Aviram M, Bentur L, Springer C, Vila Y, Branski D, Kerem B, Kerem E. Clinical and genetic risk factors for cystic fibrosis-related liver disease. Pediatrics. 1999;103:52-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 82] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Terlizzi V, Lucarelli M, Salvatore D, Angioni A, Bisogno A, Braggion C, Buzzetti R, Carnovale V, Casciaro R, Castaldo G, Cirilli N, Collura M, Colombo C, Di Lullo AM, Elce A, Lucidi V, Madarena E, Padoan R, Quattrucci S, Raia V, Seia M, Termini L, Zarrilli F. Clinical expression of cystic fibrosis in a large cohort of Italian siblings. BMC Pulm Med. 2018;18:196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Bader RM, Jonas MM, Mitchell PD, Wiggins S, Lee CK. Controlled attenuation parameter: A measure of hepatic steatosis in patients with cystic fibrosis. J Cyst Fibros. 2019;18:280-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Ayoub F, Trillo-Alvarez C, Morelli G, Lascano J. Risk factors for hepatic steatosis in adults with cystic fibrosis: Similarities to non-alcoholic fatty liver disease. World J Hepatol. 2018;10:34-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Lindblad A, Glaumann H, Strandvik B. Natural history of liver disease in cystic fibrosis. Hepatology. 1999;30:1151-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 213] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 15. | Saponaro C, Gaggini M, Gastaldelli A. Nonalcoholic fatty liver disease and type 2 diabetes: common pathophysiologic mechanisms. Curr Diab Rep. 2015;15:607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 16. | Agopian VG, Kaldas FM, Hong JC, Whittaker M, Holt C, Rana A, Zarrinpar A, Petrowsky H, Farmer D, Yersiz H, Xia V, Hiatt JR, Busuttil RW. Liver transplantation for nonalcoholic steatohepatitis: the new epidemic. Ann Surg. 2012;256:624-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 184] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 17. | Cortes-Santiago N, Leung DH, Castro E, Finegold M, Wu H, Patel KR. Hepatic Steatosis Is Prevalent Following Orthotopic Liver Transplantation in Children With Cystic Fibrosis. J Pediatr Gastroenterol Nutr. 2019;68:96-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Haber HP. Cystic fibrosis in children and young adults: findings on routine abdominal sonography. AJR Am J Roentgenol. 2007;189:89-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Moran A, Dunitz J, Nathan B, Saeed A, Holme B, Thomas W. Cystic fibrosis-related diabetes: current trends in prevalence, incidence, and mortality. Diabetes Care. 2009;32:1626-1631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 415] [Cited by in RCA: 439] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 20. | Battezzati A, Bedogni G, Zazzeron L, Mari A, Battezzati PM, Alicandro G, Bertoli S, Colombo C. Age- and Sex-Dependent Distribution of OGTT-Related Variables in a Population of Cystic Fibrosis Patients. J Clin Endocrinol Metab. 2015;100:2963-2971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Colomba J, Boudreau V, Lehoux-Dubois C, Desjardins K, Coriati A, Tremblay F, Rabasa-Lhoret R. The main mechanism associated with progression of glucose intolerance in older patients with cystic fibrosis is insulin resistance and not reduced insulin secretion capacity. J Cyst Fibros. 2019;18:551-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 22. | Moran A, Pekow P, Grover P, Zorn M, Slovis B, Pilewski J, Tullis E, Liou TG, Allen H; Cystic Fibrosis Related Diabetes Therapy Study Group. Insulin therapy to improve BMI in cystic fibrosis-related diabetes without fasting hyperglycemia: results of the cystic fibrosis related diabetes therapy trial. Diabetes Care. 2009;32:1783-1788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 176] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 23. | Minicucci L, Lorini R, Giannattasio A, Colombo C, Iapichino L, Reali MF, Padoan R, Calevo MG, Casciaro R, De Alessandri A, Haupt R. Liver disease as risk factor for cystic fibrosis-related diabetes development. Acta Paediatr. 2007;96:736-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Adler AI, Shine BS, Chamnan P, Haworth CS, Bilton D. Genetic determinants and epidemiology of cystic fibrosis-related diabetes: results from a British cohort of children and adults. Diabetes Care. 2008;31:1789-1794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Ivanov M, Matsvay A, Glazova O, Krasovskiy S, Usacheva M, Amelina E, Chernyak A, Ivanov M, Musienko S, Prodanov T, Kovalenko S, Baranova A, Khafizov K. Targeted sequencing reveals complex, phenotype-correlated genotypes in cystic fibrosis. BMC Med Genomics. 2018;11:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Elborn JS. Cystic fibrosis. Lancet. 2016;388:2519-2531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1068] [Cited by in RCA: 1243] [Article Influence: 138.1] [Reference Citation Analysis (0)] |
| 27. | Cutting GR. Cystic fibrosis genetics: from molecular understanding to clinical application. Nat Rev Genet. 2015;16:45-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 560] [Cited by in RCA: 675] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 28. | McKone EF, Borowitz D, Drevinek P, Griese M, Konstan MW, Wainwright C, Ratjen F, Sermet-Gaudelus I, Plant B, Munck A, Jiang Y, Gilmartin G, Davies JC; VX08-770-105 (PERSIST) Study Group. Long-term safety and efficacy of ivacaftor in patients with cystic fibrosis who have the Gly551Asp-CFTR mutation: a phase 3, open-label extension study (PERSIST). Lancet Respir Med. 2014;2:902-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 171] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 29. | Wainwright CE, Elborn JS, Ramsey BW, Marigowda G, Huang X, Cipolli M, Colombo C, Davies JC, De Boeck K, Flume PA, Konstan MW, McColley SA, McCoy K, McKone EF, Munck A, Ratjen F, Rowe SM, Waltz D, Boyle MP; TRAFFIC Study Group; TRANSPORT Study Group. Lumacaftor-Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del CFTR. N Engl J Med. 2015;373:220-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1058] [Cited by in RCA: 1132] [Article Influence: 113.2] [Reference Citation Analysis (0)] |
| 30. | Taylor-Cousar JL, Munck A, McKone EF, van der Ent CK, Moeller A, Simard C, Wang LT, Ingenito EP, McKee C, Lu Y, Lekstrom-Himes J, Elborn JS. Tezacaftor-Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del. N Engl J Med. 2017;377:2013-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 600] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 31. | Davies JC, Moskowitz SM, Brown C, Horsley A, Mall MA, McKone EF, Plant BJ, Prais D, Ramsey BW, Taylor-Cousar JL, Tullis E, Uluer A, McKee CM, Robertson S, Shilling RA, Simard C, Van Goor F, Waltz D, Xuan F, Young T, Rowe SM; VX16-659-101 Study Group. VX-659-Tezacaftor-Ivacaftor in Patients with Cystic Fibrosis and One or Two Phe508del Alleles. N Engl J Med. 2018;379:1599-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 248] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 32. | Keating D, Marigowda G, Burr L, Daines C, Mall MA, McKone EF, Ramsey BW, Rowe SM, Sass LA, Tullis E, McKee CM, Moskowitz SM, Robertson S, Savage J, Simard C, Van Goor F, Waltz D, Xuan F, Young T, Taylor-Cousar JL; VX16-445-001 Study Group. VX-445-Tezacaftor-Ivacaftor in Patients with Cystic Fibrosis and One or Two Phe508del Alleles. N Engl J Med. 2018;379:1612-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 539] [Article Influence: 77.0] [Reference Citation Analysis (0)] |
| 33. | Konstan MW, McKone EF, Moss RB, Marigowda G, Tian S, Waltz D, Huang X, Lubarsky B, Rubin J, Millar SJ, Pasta DJ, Mayer-Hamblett N, Goss CH, Morgan W, Sawicki GS. Assessment of safety and efficacy of long-term treatment with combination lumacaftor and ivacaftor therapy in patients with cystic fibrosis homozygous for the F508del-CFTR mutation (PROGRESS): a phase 3, extension study. Lancet Respir Med. 2017;5:107-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 217] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 34. | Bellin MD, Laguna T, Leschyshyn J, Regelmann W, Dunitz J, Billings J, Moran A. Insulin secretion improves in cystic fibrosis following ivacaftor correction of CFTR: a small pilot study. Pediatr Diabetes. 2013;14:417-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 35. | Kelly A, De Leon DD, Sheikh S, Camburn D, Kubrak C, Peleckis AJ, Stefanovski D, Hadjiliadis D, Rickels MR, Rubenstein RC. Islet Hormone and Incretin Secretion in Cystic Fibrosis after Four Months of Ivacaftor Therapy. Am J Respir Crit Care Med. 2019;199:342-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 36. | Thomassen JC, Mueller MI, Alejandre Alcazar MA, Rietschel E, van Koningsbruggen-Rietschel S. Effect of Lumacaftor/Ivacaftor on glucose metabolism and insulin secretion in Phe508del homozygous cystic fibrosis patients. J Cyst Fibros. 2018;17:271-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 37. | Li A, Vigers T, Pyle L, Zemanick E, Nadeau K, Sagel SD, Chan CL. Continuous glucose monitoring in youth with cystic fibrosis treated with lumacaftor-ivacaftor. J Cyst Fibros. 2019;18:144-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 38. | Farrell PM, White TB, Ren CL, Hempstead SE, Accurso F, Derichs N, Howenstine M, McColley SA, Rock M, Rosenfeld M, Sermet-Gaudelus I, Southern KW, Marshall BC, Sosnay PR. Diagnosis of Cystic Fibrosis: Consensus Guidelines from the Cystic Fibrosis Foundation. J Pediatr. 2017;181S:S4-S15.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 542] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 39. | Moran A, Brunzell C, Cohen RC, Katz M, Marshall BC, Onady G, Robinson KA, Sabadosa KA, Stecenko A, Slovis B; CFRD Guidelines Committee. Clinical care guidelines for cystic fibrosis-related diabetes: a position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care. 2010;33:2697-2708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 521] [Cited by in RCA: 481] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 40. | Castellani C, Cuppens H, Macek M, Cassiman JJ, Kerem E, Durie P, Tullis E, Assael BM, Bombieri C, Brown A, Casals T, Claustres M, Cutting GR, Dequeker E, Dodge J, Doull I, Farrell P, Ferec C, Girodon E, Johannesson M, Kerem B, Knowles M, Munck A, Pignatti PF, Radojkovic D, Rizzotti P, Schwarz M, Stuhrmann M, Tzetis M, Zielenski J, Elborn JS. Consensus on the use and interpretation of cystic fibrosis mutation analysis in clinical practice. J Cyst Fibros. 2008;7:179-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 455] [Cited by in RCA: 402] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 41. | Castellani C; CFTR2 team. CFTR2: How will it help care? Paediatr Respir Rev. 2013;14 Suppl 1:2-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 42. | Moran A, Pillay K, Becker DJ, Acerini CL; International Society for Pediatric and Adolescent Diabetes. ISPAD Clinical Practice Consensus Guidelines 2014. Management of cystic fibrosis-related diabetes in children and adolescents. Pediatr Diabetes. 2014;15 Suppl 20:65-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 43. | Clinical and Laboratory Standards Institute/NCCLS. Procedures for the Collection of Diagnostic Blood Specimens by Venipuncture, 5th Edition 2003. Available from: https://clsi.org/standards/. |
| 44. | Yokoo T, Shiehmorteza M, Hamilton G, Wolfson T, Schroeder ME, Middleton MS, Bydder M, Gamst AC, Kono Y, Kuo A, Patton HM, Horgan S, Lavine JE, Schwimmer JB, Sirlin CB. Estimation of hepatic proton-density fat fraction by using MR imaging at 3.0 T. Radiology. 2011;258:749-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 237] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 45. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 4951] [Article Influence: 707.3] [Reference Citation Analysis (9)] |
| 46. | Schall JI, Mascarenhas MR, Maqbool A, Dougherty KA, Elci O, Wang DJ, Altes TA, Hommel KA, Shaw W, Moore J, Stallings VA. Choline Supplementation With a Structured Lipid in Children With Cystic Fibrosis: A Randomized Placebo-Controlled Trial. J Pediatr Gastroenterol Nutr. 2016;62:618-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 47. | Hayes D, Warren PS, McCoy KS, Sheikh SI. Improvement of hepatic steatosis in cystic fibrosis with ivacaftor therapy. J Pediatr Gastroenterol Nutr. 2015;60:578-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 48. | Olivier AK, Gibson-Corley KN, Meyerholz DK. Animal models of gastrointestinal and liver diseases. Animal models of cystic fibrosis: gastrointestinal, pancreatic, and hepatobiliary disease and pathophysiology. Am J Physiol Gastrointest Liver Physiol. 2015;308:G459-G471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 49. | Mailhot G, Rabasa-Lhoret R, Moreau A, Berthiaume Y, Levy E. CFTR depletion results in changes in fatty acid composition and promotes lipogenesis in intestinal Caco 2/15 cells. PLoS One. 2010;5:e10446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 50. | Peretti N, Marcil V, Drouin E, Levy E. Mechanisms of lipid malabsorption in Cystic Fibrosis: the impact of essential fatty acids deficiency. Nutr Metab (Lond). 2005;2:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |