Published online Nov 27, 2019. doi: 10.4254/wjh.v11.i11.743
Peer-review started: July 21, 2019
First decision: August 7, 2019
Revised: August 19, 2019
Accepted: October 2, 2019
Article in press: October 2, 2019
Published online: November 27, 2019
Processing time: 112 Days and 23.8 Hours
Pancreaticobiliary maljunction (PBM) can be classified into two categories, PBM with congenital biliary dilatation (CBD) or PBM without biliary dilatation, and the management of PBM is often controversial. The treatment for PBM with CBD is prophylactic flow diversion surgery, and some authors have reported that the incidence of cancer after extrahepatic bile duct excision is less than 1%. A very rare case of intrahepatic cholangiocarcinoma 6 years after flow diversion surgery for PBM with CBD is reported.
A 30-year-old man was diagnosed as having PBM with CBD, Todani classification type IVA, because of abnormal liver enzyme profiles. He underwent flow diversion surgery and cholecystectomy, and the specimen showed adenocarcinoma foci, pT1, pStage IA. Five and a half years passed without any recurrence of bile duct cancer. However, 6 years after his operation, computed tomography showed a gradually growing nodule in the bile duct. Fluorodeoxyglucose positron emission tomography showed high uptake, and magnetic resonance imaging showed restricted diffusion signals. On double balloon enteroscopy, the nodule at the posterior bile duct-jejunum anastomosis was directly visualized, and its biopsy specimen showed adenocarcinoma. The patient underwent right lobectomy and biliary reconstruction. The pathological diagnosis was intraductal papillary neoplasm with high-grade intraepithelial neoplasia, pTis, pN0, pStage 0. The patient’s postoperative course was uneventful, and he has had no recurrence up to the present time.
This case suggests the necessity of careful observation after flow diversion surgery, especially when PBM with CBD is detected in adulthood.
Core tip: Pancreaticobiliary maljunction (PBM) is one of the risk factors for biliary tract cancer. A rare case of intrahepatic cholangiocarcinoma 6 years after flow diversion surgery for PBM with congenital biliary dilatation (CBD) is presented. Careful follow-up after flow diversion surgery is important to detect cholangiocarcinoma in its early stage, especially when PBM with CBD is detected in adulthood, and when cancer has already developed in the bile duct.
- Citation: Ataka R, Ito T, Masui T, Seo S, Ishii T, Ogiso S, Yagi S, Taura K, Uemoto S. Cholangiocarcinoma after flow diversion surgery for congenital biliary dilatation: A case report and review of literature. World J Hepatol 2019; 11(11): 743-751
- URL: https://www.wjgnet.com/1948-5182/full/v11/i11/743.htm
- DOI: https://dx.doi.org/10.4254/wjh.v11.i11.743
Pancreaticobiliary maljunction (PBM) is an uncommon congenital anomaly defined as junction of the pancreatic and bile ducts outside the duodenal wall, allowing reciprocal reflux of pancreatic juice and bile, which is carcinogenic. Thus, PBM is a high-risk factor for biliary tract cancer, and preventive surgery is necessary. Congenital biliary dilatation (CBD), one type of PBM, is usually treated with prophylactic flow diversion surgery, and the extent of intrahepatic and extrahepatic bile duct resection is often controversial[1,2]. A rare case of a young man who developed cholangiocarcinoma 6 years after flow diversion surgery for PBM with CBD is described.
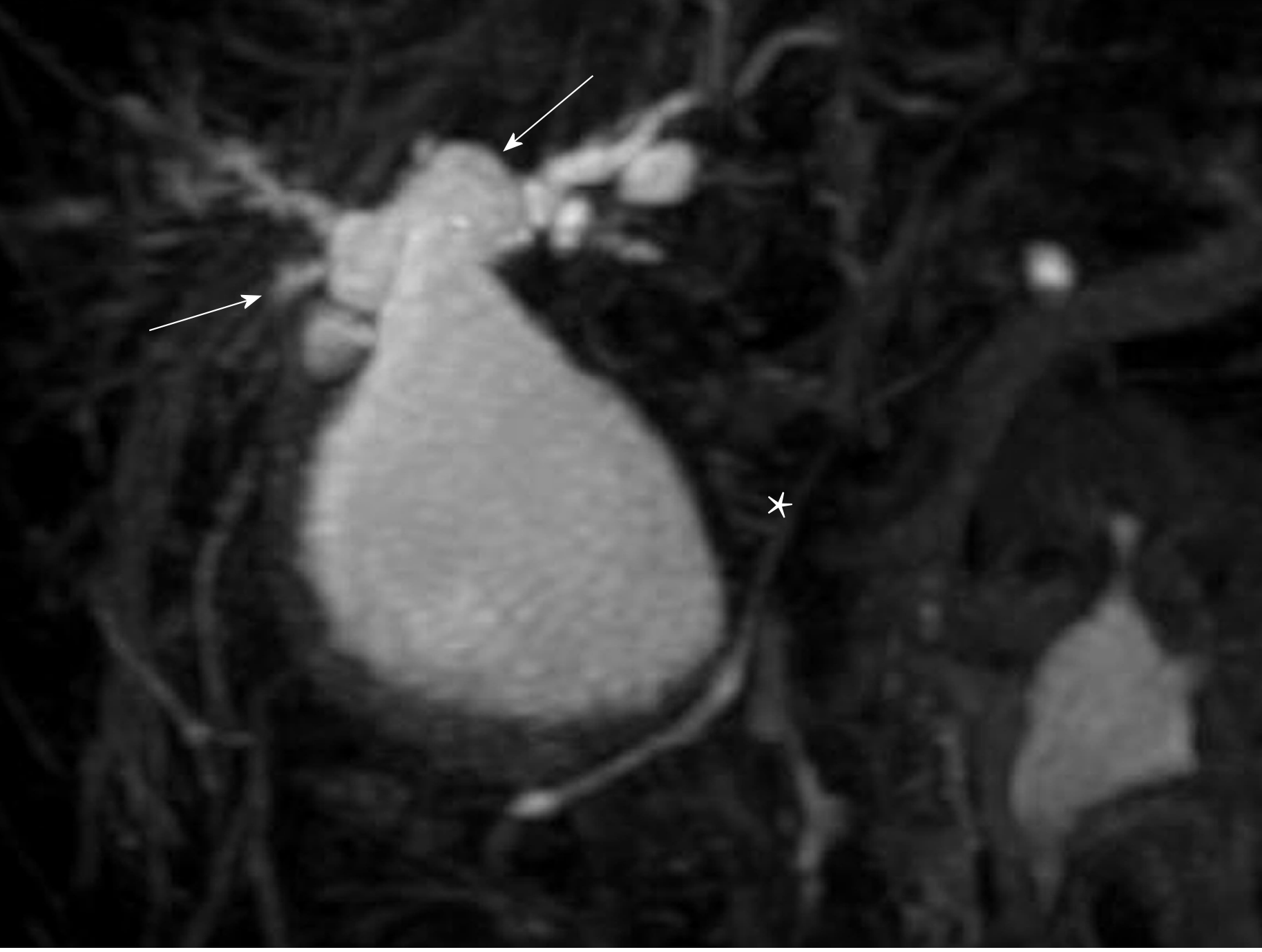
A 30-year-old Japanese man was found to have liver dysfunction at a medical examination. He had no remarkable medical history, was not on any medications, and had no allergies to any food or drug. He visited a nearby hospital and was diagnosed as having PBM with CBD. His common bile duct showed cystic dilatation accompanied by intrahepatic bile duct dilatation, which was classified as Todani type IVA (Figure 1). In the same year, he underwent cholecystectomy and a flow diversion operation that consisted of excision of the extrahepatic bile duct, and biliary and Roux-en-Y reconstruction. According to the Union for International Cancer Control (UICC) 7th edition, the histopathological specimen showed adenocarcinoma foci, pT1, pNx, pStage IA. He did not have any adjuvant chemotherapy and five and a half years passed without any recurrence of biliary tract cancer.
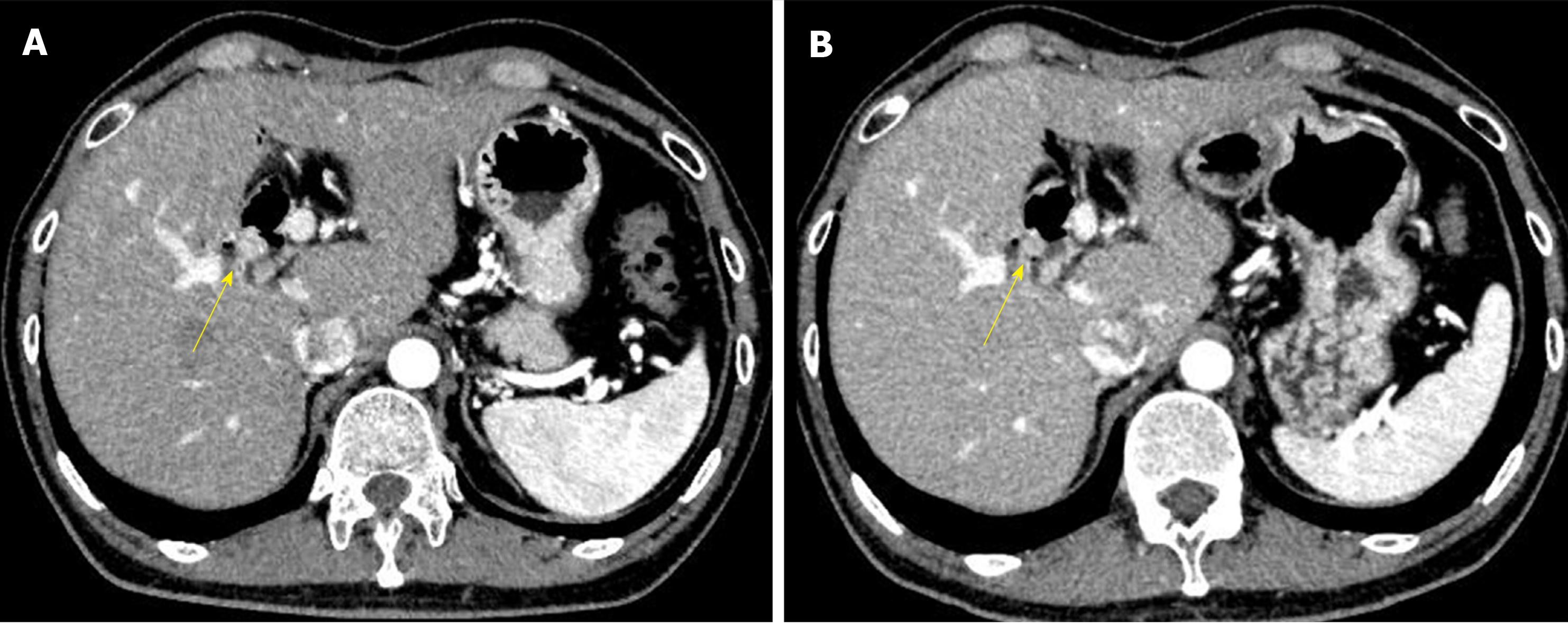
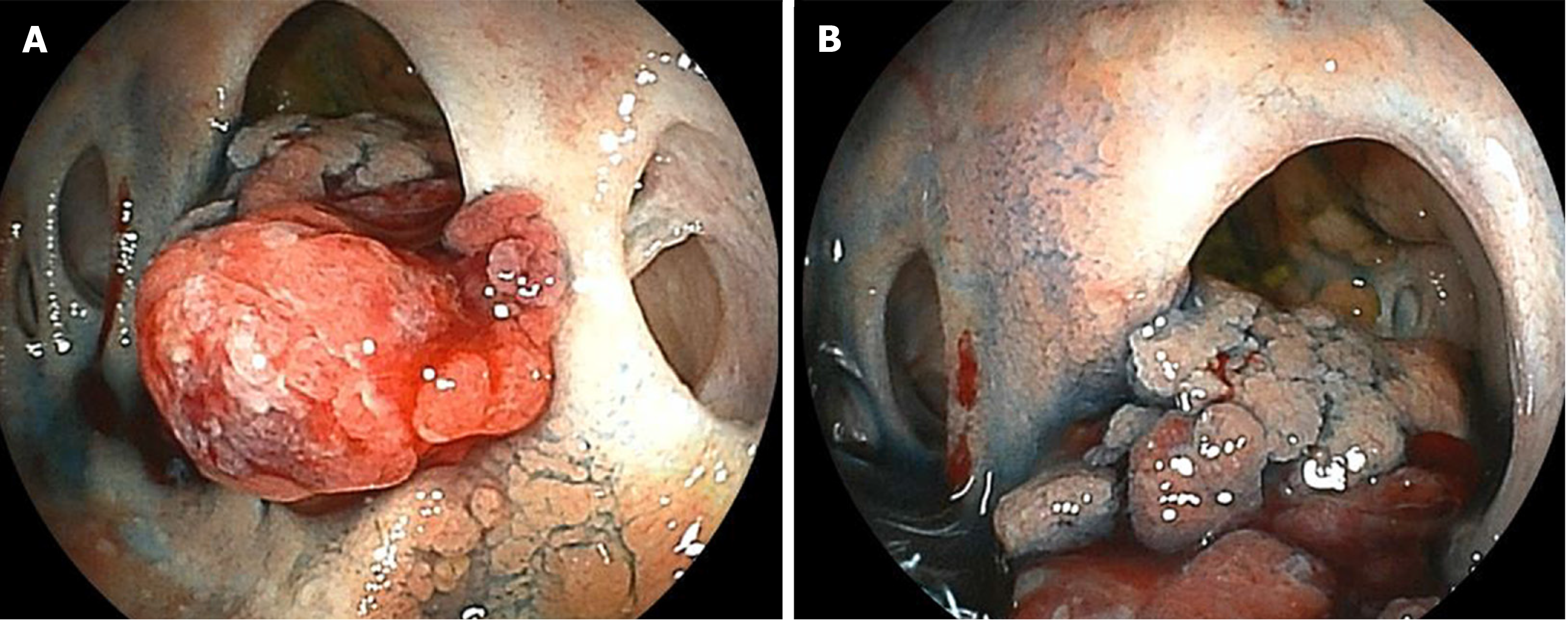
However, six years after his operation, follow-up computed tomography (CT) showed a gradually growing nodule, about 10 mm in diameter, at the bile duct-jejunum anastomosis (Figure 2). Physical examination showed no abnormalities except for his previous operative scar. Laboratory tests were unremarkable, including liver and biliary enzyme profiles. Tumor markers were normal (carcinoembryonic antigen 0.8 ng/mL and carbohydrate 19-9 6.4 U/mL). Fluorodeoxyglucose positron emission tomography (FDG-PET) showed high FDG uptake, and magnetic resonance imaging showed restricted diffusion signals at the same nodular lesion. Double balloon enteroscopy showed the lesion directly. There were four holes anastomoses at the cholangiojejunostomy. A reddish and hemorrhagic tumor protruded from the posterior hole, and the other three holes were intact; the biopsy specimen showed adenocarcinoma (Figure 3).
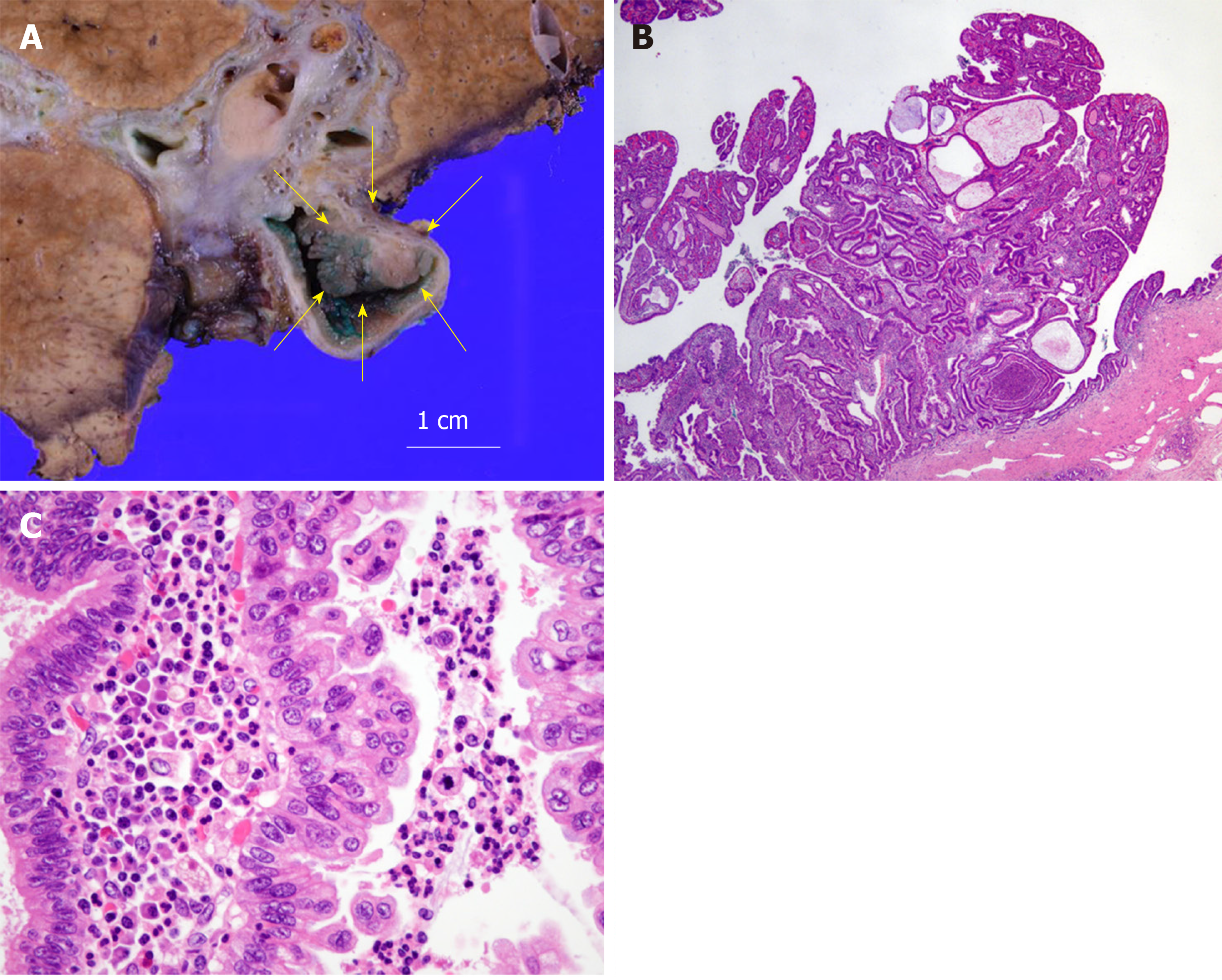
One month after percutaneous transhepatic portal embolization to prevent postoperative liver failure due to small remnant liver volume less than 30%, he underwent right lobectomy, biliary reconstruction, and regional lymph node dissection. At laparotomy, there were severe adhesions due to his previous operation. Right lobectomy was performed with dissection of the right hepatic artery, right portal vein, right hepatic vein, hilar bile ducts, and Roux-en-Y jejunum with cholangiojejunostomy. The remaining bile ducts in the hilar plate were three holes of B1, B2 plus B3, and B4. The biliary reconstruction was performed by new cholangiojejunostomy with a one-hole anastomosis. The Roux-en-Y reconstruction was also performed by a new jejunojejunostomy. The histopathological diagnosis was intraductal papillary neoplasm of the bile duct with high-grade intraepithelial neoplasia. There were no other abnormal findings in other intrahepatic bile ducts, including the anterior and posterior branches, with a negative bile duct margin. The dissected lymph nodes showed no evidence of malignancy. According to the UICC 8th edition, the pTNM classification was pTis, pN0, pStage 0 (Figure 4).
The patient’s postoperative course was uneventful, and he was discharged on the 24th postoperative day. There has been no evidence of re-recurrence up to the present time.
PBM is a congenital anomaly defined as junction of the pancreatic and bile ducts outside the duodenal wall. Because of the abnormally located junction, the sphincter of Oddi does not work effectively. Reciprocal reflux of pancreatic juice and bile occurs, which introduces carcinogenic chemicals such as activated phospholipase A2 and secondary bile acids. These chemicals usually flow into the biliary tract because the hydropressure in the pancreatic duct is greater than that in the bile duct. The anomaly can also cause chronic cholangitis and chronic bacterbilia. As a result, patients with PBM have a higher risk of biliary tract cancer[3].
PBM can be divided into two categories: PBM with congenital biliary dilatation (CBD), which was previously called congenital choledochal cyst; or PBM without biliary dilatation (BD)[4]. Todani et al[5,6] classified this disease into five categories, and the Todani classification is now commonly used worldwide.
The essential management of PBM is prophylactic surgery to avoid carcinogenesis, and it should be performed immediately after the diagnosis of PBM[1]. Since bile stasis in the dilated biliary tract is the most important factor for the development of malignant changes in biliary epithelial cells[7], the treatment for PBM with CBD is prophylactic flow diversion surgery, and that for PBM without BD is prophylactic cholecystectomy. However, there is no consensus of opinion on whether intrahepatic dilated bile ducts should be resected for PBM with CBD, or whether extrahepatic non-dilated bile ducts should be resected for PBM without BD[1,8].
The present case showed a rare clinical course of PBM with CBD. Watanabe et al[9] reported that the incidence of cancer after extrahepatic bile duct excision is less than 1%. There seem to be two reasons for the carcinogenesis in this case. The first reason is that the flow diversion surgery left the dilated right hepatic duct in the hilar plate. In the first operation, the dilated left hepatic duct was removed, but the dilated right hepatic duct was used for cholangiojejunostomy. Since 30 years had passed before his diagnosis of CBD, there must have been some histological changes in the biliary epithelial cells, such as hyperplasia, metaplasia, and dysplasia. Damage and repair of the dilated biliary mucosa were likely to occur even after the first operation, resulting in carcinogenesis at the site of the cholangiojejunostomy. The second reason is that the reflux of intestinal juice at the cholangiojejunostomy probably played an important role in carcinogenesis. The stasis of bile and intestinal juice in the dilated biliary tract induces bacterial overgrowth and generation of unconjugated secondary bile acids[10]. The carcinogenic toxicity of secondary bile acids still remains unclear, but some authors report the following mechanism in mice and its relationship to humans. Secondary bile acids suppress the expression of chemokine ligand 16 (CXCL16), which recruits natural killer T cells, by liver sinusoidal endothelial cells. Then, the suppressed expression of CXCL16 weakens immunological defenses and leads to the development of malignancy and progression[11,12].
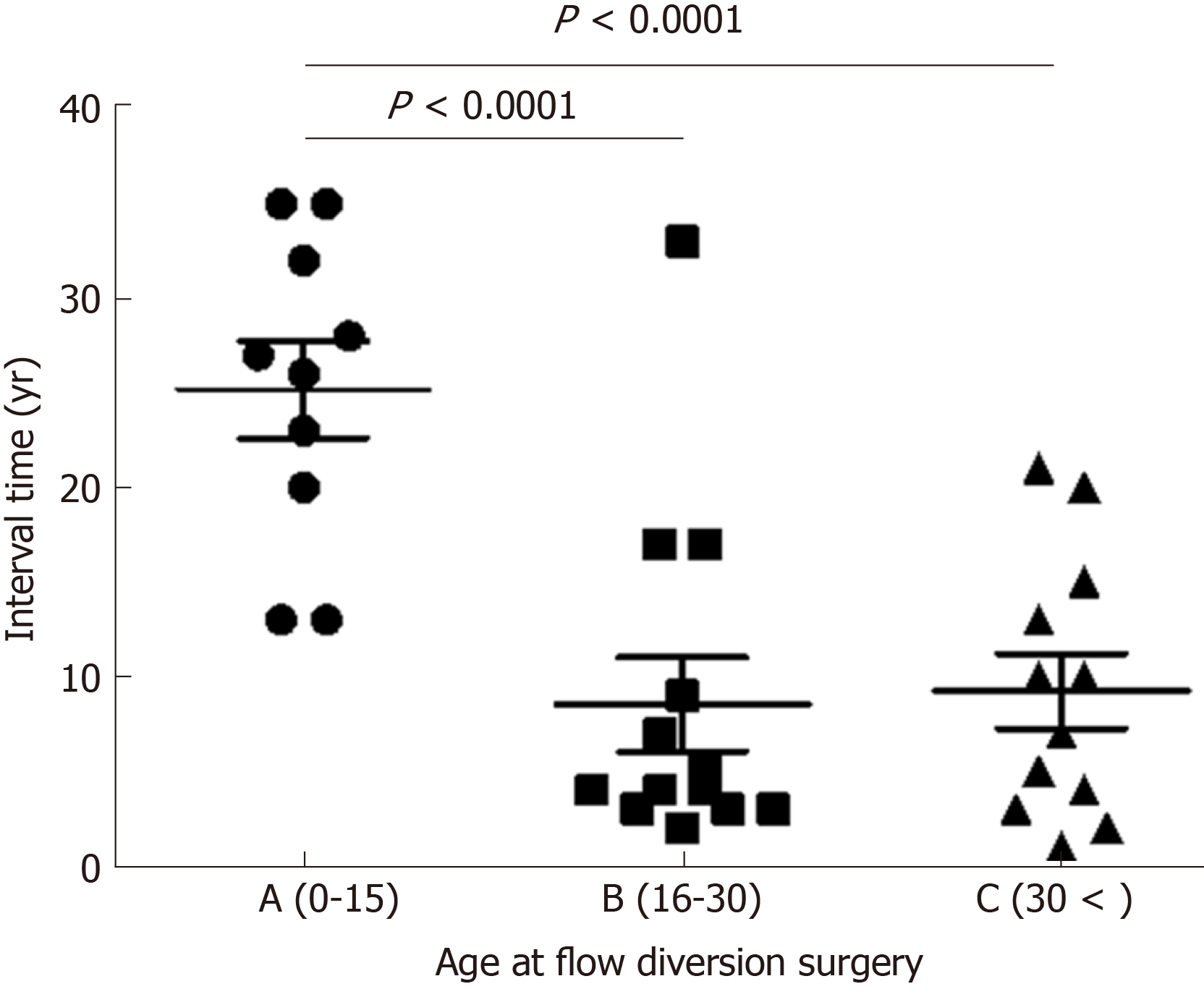
There have been 41 reported cases of biliary tract cancer after flow diversion surgery for PBM with CBD in the English literature from 1967 to 2016[7,13-41]. The characteristics of these patients are shown below (Table 1). Of the 41 cases reported, 35 reported details of the interval between age at flow diversion surgery and age at detection of biliary tract cancer. These 35 cases were divided into three groups by age (A, child group, 0-15; B, adolescent/young adult group, 16-30; and C, adult group, > 30 years old), and the interval times are shown in the figure (Figure 5). There were significant differences between Group A and Group B (median 26.5 vs 4.0 years, P < 0.0001), and between Group A and Group C (median 26.5 vs 8.5 years, P < 0.0001), with no difference between Group B and Group C. These data mean that patients who undergo flow diversion surgery in adulthood can develop biliary tract cancer earlier than patients who undergo it in childhood. In adulthood, furthermore, interval time does not always depend on age at flow diversion surgery, and it is short, most often less than 10 years. This fact suggests that the damage to the remaining biliary epithelial cells due to carcinogenic chemicals is greater in adulthood than in childhood. In adulthood, the interval time may depend on the size of the dilated bile duct, on the extent of reflux, on the flow diversion procedures, or on lifestyle. The details remain unknown, and further similar cases need to be studied.
In the future, the current patient has a high probability of re-recurrence of cholangiocarcinoma. Although Tsuchida et al[42] reported that chemoprevention can be effective for PBM to prevent postoperative carcinogenesis, this is still controversial.
Follow-up throughout the life of a patient after flow diversion surgery is recommended[4], but this is difficult in some cases. This case suggests the necessity of careful observation after flow diversion surgery, especially when PBM with CBD is detected in adulthood, and when cancer has already developed in the bile duct.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dourakis SP, Hoyos SS, Kozarek RA, Lleo A S-Editor: Ma YJ L-Editor:A E-Editor: Ma YJ
| 1. | Kamisawa T, Ando H, Suyama M, Shimada M, Morine Y, Shimada H; Working Committee of Clinical Practice Guidelines for Pancreaticobiliary Maljunction; Japanese Study Group on Pancreaticobiliary Maljunction. Japanese clinical practice guidelines for pancreaticobiliary maljunction. J Gastroenterol. 2012;47:731-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 133] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 2. | Kamisawa T, Ando H, Hamada Y, Fujii H, Koshinaga T, Urushihara N, Itoi T, Shimada H; Japanese Study Group on Pancreaticobiliary Maljunction. Diagnostic criteria for pancreaticobiliary maljunction 2013. J Hepatobiliary Pancreat Sci. 2014;21:159-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 3. | Kamisawa T, Takuma K, Anjiki H, Egawa N, Kurata M, Honda G, Tsuruta K, Sasaki T. Pancreaticobiliary maljunction. Clin Gastroenterol Hepatol. 2009;7:S84-S88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | Hamada Y, Ando H, Kamisawa T, Itoi T, Urushihara N, Koshinaga T, Saito T, Fujii H, Morotomi Y. Diagnostic criteria for congenital biliary dilatation 2015. J Hepatobiliary Pancreat Sci. 2016;23:342-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 5. | Todani T, Watanabe Y, Narusue M, Tabuchi K, Okajima K. Congenital bile duct cysts: Classification, operative procedures, and review of thirty-seven cases including cancer arising from choledochal cyst. Am J Surg. 1977;134:263-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 934] [Cited by in RCA: 835] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 6. | Todani T. Congenital choledochal dilatation: classification, clinical features, and long-term results. J Hepatobiliary Pancreat Surg. 1997;4:276-282. [RCA] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Fujii H, Yang Y, Tang R, Kunitomo K, Itakura J, Mogaki M, Matsuda M, Suda K, Nobukawa B, Matsumoto Y. Epithelial cell proliferation activity of the biliary ductal system with congenital biliary malformations. J Hepatobiliary Pancreat Surg. 1999;6:294-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Kawarada Y, Das BC, Tabata M, Isaji S. Surgical treatment of type IV choledochal cysts. J Hepatobiliary Pancreat Surg. 2009;16:684-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Watanabe Y, Toki A, Todani T. Bile duct cancer developed after cyst excision for choledochal cyst. J Hepatobiliary Pancreat Surg. 1999;6:207-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 145] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Reveille RM, Van Stiegmann G, Everson GT. Increased secondary bile acids in a choledochal cyst. Possible role in biliary metaplasia and carcinoma. Gastroenterology. 1990;99:525-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, Agdashian D, Terabe M, Berzofsky JA, Fako V, Ritz T, Longerich T, Theriot CM, McCulloch JA, Roy S, Yuan W, Thovarai V, Sen SK, Ruchirawat M, Korangy F, Wang XW, Trinchieri G, Greten TF. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. 2018;360:29798856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 1034] [Article Influence: 147.7] [Reference Citation Analysis (0)] |
| 12. | Schramm C. Bile Acids, the Microbiome, Immunity, and Liver Tumors. N Engl J Med. 2018;379:888-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Thistlethwaite JR, Horwitz A. Choledochal cyst followed by carcinoma of the hepatic duct. South Med J. 1967;60:872-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Gallagher PJ, Millis RR, Mitchinson MJ. Congenital dilatation of the intrahepatic bile ducts with cholangiocarcinoma. J Clin Pathol. 1972;25:804-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 68] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Chaudhuri PK, Chaudhuri B, Schuler JJ, Nyhus LM. Carcinoma associated with congenital cystic dilation of bile ducts. Arch Surg. 1982;117:1349-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 45] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Nagorney DM, McIlrath DC, Adson MA. Choledochal cysts in adults: clinical management. Surgery. 1984;96:656-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Yoshikawa K, Yoshida K, Shirai Y, Sato N, Kashima Y, Coutinho DS, Koyama S, Muto T, Yamagiwa I, Iwafuchi M. A case of carcinoma arising in the intrapancreatic terminal choledochus 12 years after primary excision of a giant choledochal cyst. Am J Gastroenterol. 1986;81:378-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Rossi RL, Silverman ML, Braasch JW, Munson JL, ReMine SG. Carcinomas arising in cystic conditions of the bile ducts. A clinical and pathologic study. Ann Surg. 1987;205:377-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 69] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Joseph VT. Surgical techniques and long-term results in the treatment of choledochal cyst. J Pediatr Surg. 1990;25:782-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 56] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Young WT, Thomas GV, Blethyn AJ, Lawrie BW. Choledochal cyst and congenital anomalies of the pancreatico-biliary junction: the clinical findings, radiology and outcome in nine cases. Br J Radiol. 1992;65:33-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Scudamore CH, Hemming AW, Teare JP, Fache JS, Erb SR, Watkinson AF. Surgical management of choledochal cysts. Am J Surg. 1994;167:497-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Yamamoto J, Shimamura Y, Ohtani I, Ohtani H, Yano M, Fukuda K, Nagata T, Ishii M, Ohmura M, Todani T. Bile duct carcinoma arising from the anastomotic site of hepaticojejunostomy after the excision of congenital biliary dilatation: a case report. Surgery. 1996;119:476-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Yamataka A, Ohshiro K, Okada Y, Hosoda Y, Fujiwara T, Kohno S, Sunagawa M, Futagawa S, Sakakibara N, Miyano T. Complications after cyst excision with hepaticoenterostomy for choledochal cysts and their surgical management in children versus adults. J Pediatr Surg. 1997;32:1097-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 86] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Kobayashi S, Asano T, Yamasaki M, Kenmochi T, Nakagohri T, Ochiai T. Risk of bile duct carcinogenesis after excision of extrahepatic bile ducts in pancreaticobiliary maljunction. Surgery. 1999;126:939-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 109] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Fujisaki S, Akiyama T, Miyake H, Amano S, Tomita R, Fukuzawa M, Yamagami H, Tsubaki K, Arakawa Y, Aleemuzzaman S, Nemoto N. A case of carcinoma associated with the remained intrapancreatic biliary tract 17 years after the primary excision of a choledochal cyst. Hepatogastroenterology. 1999;46:1655-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 26. | Jan YY, Chen HM, Chen MF. Malignancy in choledochal cysts. Hepatogastroenterology. 2000;47:337-340. [PubMed] |
| 27. | Goto N, Yasuda I, Uematsu T, Kanemura N, Takao S, Ando K, Kato T, Osada S, Takao H, Saji S, Shimokawa K, Moriwaki H. Intrahepatic cholangiocarcinoma arising 10 years after the excision of congenital extrahepatic biliary dilation. J Gastroenterol. 2001;36:856-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Koike M, Yasui K, Shimizu Y, Kodera Y, Hirai T, Morimoto T, Yamamura Y, Kato T. Carcinoma of the hepatic hilus developing 21 years after biliary diversion for choledochal cyst: a case report. Hepatogastroenterology. 2002;49:1216-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 29. | Mabrut JY, Partensky C, Gouillat C, Baulieux J, Ducerf C, Kestens PJ, Boillot O, de la Roche E, Gigot JF. Cystic involvement of the roof of the main biliary convergence in adult patients with congenital bile duct cysts: a difficult surgical challenge. Surgery. 2007;141:187-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Ono S, Sakai K, Kimura O, Iwai N. Development of bile duct cancer in a 26-year-old man after resection of infantile choledochal cyst. J Pediatr Surg. 2008;43:E17-E19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Palanivelu C, Rangarajan M, Parthasarathi R, Amar V, Senthilnathan P. Laparoscopic management of choledochal cysts: technique and outcomes--a retrospective study of 35 patients from a tertiary center. J Am Coll Surg. 2008;207:839-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Shimamura K, Kurosaki I, Sato D, Takano K, Yokoyama N, Sato Y, Hatakeyama K, Nakadaira K, Yagi M. Intrahepatic cholangiocarcinoma arising 34 years after excision of a type IV-A congenital choledochal cyst: report of a case. Surg Today. 2009;39:247-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Shah OJ, Shera AH, Zargar SA, Shah P, Robbani I, Dhar S, Khan AB. Choledochal cysts in children and adults with contrasting profiles: 11-year experience at a tertiary care center in Kashmir. World J Surg. 2009;33:2403-2411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Nishiyama R, Shinoda M, Tanabe M, Masugi Y, Ueno A, Hibi T, Takano K, Fujisaki H, Kitago M, Itano O, Kawachi S, Aiura K, Tanimoto A, Sakamaoto M, Kitagawa Y. Intrahepatic cholangiocarcinoma arising 33 years after excision of a choledochal cyst: report of a case. Int Surg. 2011;96:320-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Lee SE, Jang JY, Lee YJ, Choi DW, Lee WJ, Cho BH, Kim SW; Korean Pancreas Surgery Club. Choledochal cyst and associated malignant tumors in adults: a multicenter survey in South Korea. Arch Surg. 2011;146:1178-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 36. | Takeshita N, Ota T, Yamamoto M. Forty-year experience with flow-diversion surgery for patients with congenital choledochal cysts with pancreaticobiliary maljunction at a single institution. Ann Surg. 2011;254:1050-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Ong J, Campbell W, Taylor MA. Metastatic cholangiocarcinoma following choledochal cyst excision: an unusual cause of abdominal pain in a 35-year-old female. Ulster Med J. 2013;82:21-22. [PubMed] |
| 38. | Ohashi T, Wakai T, Kubota M, Matsuda Y, Arai Y, Ohyama T, Nakaya K, Okuyama N, Sakata J, Shirai Y, Ajioka Y. Risk of subsequent biliary malignancy in patients undergoing cyst excision for congenital choledochal cysts. J Gastroenterol Hepatol. 2013;28:243-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 39. | Kumamoto T, Tanaka K, Takeda K, Nojiri K, Mori R, Taniguchi K, Matsuyama R, Ueda M, Sugita M, Ichikawa Y, Nagashima Y, Endo I. Intrahepatic cholangiocarcinoma arising 28 years after excision of a type IV-A congenital choledochal cyst: report of a case. Surg Today. 2014;44:354-358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Baik H, Park YH, Seo SH, An MS, Kim KH, Bae KB, Choi CS, Oh SH, Choi YK. Cholangiocarcinoma in choledochal cyst after cystoenterostomy: how a mistreated choledochal cyst can progress to malignancy. Ann Hepatobiliary Pancreat Surg. 2016;20:201-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Yamada M, Ebata T, Sugawara G, Igami T, Mizuno T, Shingu Y, Nagino M. Hepatopancreatoduodenectomy for local recurrence of cholangiocarcinoma after excision of a type IV-A congenital choledochal cyst: a case report. Surg Case Rep. 2016;2:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 42. | Tsuchida A, Itoi T. Carcinogenesis and chemoprevention of biliary tract cancer in pancreaticobiliary maljunction. World J Gastrointest Oncol. 2010;2:130-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |