Published online Oct 27, 2019. doi: 10.4254/wjh.v11.i10.710
Peer-review started: May 14, 2019
First decision: June 13, 2019
Revised: August 3, 2019
Accepted: October 15, 2019
Article in press: October 15, 2019
Published online: October 27, 2019
Processing time: 166 Days and 2 Hours
Nonalcoholic fatty liver disease (NAFLD) is the hepatic manifestation of the metabolic syndrome (MetS) and is characterized by steatosis in the absence of significant alcohol consumption. However, MetS and significant alcohol intake coexist in certain individuals which may lead to the development of BAFLD.
To assess the clinical characteristics of patients with both alcoholic and NAFLD (BAFLD) in a large cohort in the United States.
Adults from the National Health and Nutrition Examination Survey between 2003-2014 were included. NAFLD was diagnosed based on elevated alanine aminotransferase (ALT) and being overweight or obese in the absence of other liver diseases. BAFLD patients met the criteria for NAFLD but also had either MetS or type 2 diabetes and consumed excessive amounts of alcohol. Univariable and multivariable analysis were performed to assess differences between NAFLD and BAFLD and to compare severity based on a validated fibrosis score (FIB4 index).
The prevalence of NAFLD was at 25.9% (95%CI; 25.1-26.8) and that of BAFLD was 0.84% (0.67, 1.02) which corresponds to an estimated 1.24 million Americans affected by BAFLD. Compared to NAFLD, patients with BAFLD were more likely to be male, smokers, have higher ALT, aspartate aminotransferase, triglycerides, and lower platelets; P < 0.01 for all. More importantly, after adjusting for MetS components, BAFLD patients were significantly more likely to have advanced fibrosis [adjusted OR (95%CI) based on FIB4 index > 2.67 was 3.2 (1.4, 7.0), P = 0.004].
A significant percentage of the American general population is afflicted by BAFLD and these patients tend to have more advanced liver fibrosis.
Core tip: Using the National Health and Nutrition Examination Survey dataset, we studied a new classification of fatty liver disease that we believe is due to risk factors for both non-alcoholic fatty liver disease (NAFLD) and alcoholic liver disease occurring in the same individual. We propose to call this entity Both Alcoholic and NAFLD (BAFLD). As most of the risk factors that lead to BAFLD are potentially modifiable, understanding their reciprocal association and combined effect on the liver may aid in understanding, treating, and preventing BAFLD.
- Citation: Khoudari G, Singh A, Noureddin M, Fritze D, Lopez R, Asaad I, Lawitz E, Poordad F, Kowdley KV, Alkhouri N. Characterization of patients with both alcoholic and nonalcoholic fatty liver disease in a large United States cohort. World J Hepatol 2019; 11(10): 710-718
- URL: https://www.wjgnet.com/1948-5182/full/v11/i10/710.htm
- DOI: https://dx.doi.org/10.4254/wjh.v11.i10.710
Fat accumulation in the liver, known as fatty liver disease, is a major cause of chronic liver disease worldwide[1]. There are two main classifications of fatty liver disease: alcoholic liver disease (ALD) and non-alcoholic fatty liver disease (NAFLD). The well-accepted threshold values of alcohol consumption thought to contribute to ALD are 30 g/d for men and 20 g/d for women[2,3]. NAFLD, on the other hand, is considered as the hepatic manifestation of the metabolic syndrome (MetS)[4] and is characterized by the presence of liver steatosis in the absence of other causes of fatty liver disease, particularly significant alcohol consumption. Recently, in the general population, the prevalence of obesity and MetS has been rising[5]; this increase presumably positively correlates with cases of NAFLD. Globally, NAFLD is estimated to afflict approximately 25.24% (95%CI: 22.10-28.65) of the population[6], and is on track to becoming the leading cause of liver transplantation in the near future. Similarly, the prevalence of ALD is on the rise. Currently, it affects nearly 8% of the general population in the United States. Alcohol appears to differentially induce toxic effects on the liver based on sex: It takes approximately twice the volume of alcohol and a longer duration of alcohol consumption for men to develop ALD compared to women[7]. In men, ethnicity appears to affect rates and outcomes of alcoholic cirrhosis: Incidence is highest in African-Americans followed by Hispanics then Caucasians; however, Hispanic males have the highest mortality rates from alcoholic cirrhosis[8,9].
In a substantial proportion of the population, the risk factors that contribute to each of the two main types of fatty liver disease coexist within a given individual. Alcohol consumption sensitizes the liver to damage induced by MetS and vice versa; this could result in concurrent ALD and NAFLD in the same liver. We propose to call this scenario both alcoholic and NAFLD (BAFLD). We also believe that due to the presence of risk factors for both ALD and NAFLD, patients with BAFLD are at higher risk of advanced fibrosis (AF) and complications related to end-stage liver disease. Studies describing the combined effect of alcohol consumption and MetS on hepatic steatosis are scarce. MetS and excess alcohol consumption are likely responsive to modifiable dietary and lifestyle factors; therefore, understanding their reciprocal interaction and combined effect on the liver might lead to a better understanding of BAFLD pathogenesis as well as strategies for treatment and prevention[10]. The aim of this study was to determine and compare the prevalence and clinical characteristics of BAFLD to NAFLD in a large cohort of subjects in the United States.
All adult (18+ years) subjects who participated in the National Health and Nutrition Examination Survey (NHANES) during 2003-2014 cycles were identified and assessed. The NHANES is a survey program conducted by the National Center for Health Statistics (NCHS), which is part of the Centers for Disease Control and Prevention (CDC). The program is designed to assess the health and nutritional status of adults and children in the United States. It began in the early 1960s and became a continuous program in 1999. It examines a sample of 5000 persons a year from different counties across the United States representing the United States population of all ages. The survey includes interview questionnaires, standardized physical examination, and laboratory tests from blood samples collected at examination centers and analyzed at a central laboratory. The survey was approved by the Institutional Review Board at the Center for Disease Control and Prevention, and informed consent was obtained from all participants.
Diagnosis of NAFLD was made based on elevated alanine aminotransferase (ALT) (> 30 U/L in males and > 19 in females) and being overweight or obese [body mass index (BMI) ≥ 25 kg/m2) in the absence of other causes of chronic liver disease (viral hepatitis, autoimmune liver disease, metabolic liver disease, total parenteral nutrition and medications etc.) Patients with BAFLD met the criteria for NAFLD but also had either MetS or type 2 diabetes and consumed excessive amounts of alcohol defined as ≥ 3 drinks/d for men and ≥ 2 drinks/d for women. The following patients were excluded; reported use of hepatotoxic medications, hepatitis viral infection, missing ALT, missing BMI, no alcohol intake information, missing diabetes status, and missing information on MetS components. MetS was defined as central Obesity plus at least 2 of the following: diabetes, hypertension, hypertriglyceridemia or low high-density lipoprotein cholesterol (HDL).
Demographic variables including age, gender, ethnicity, waist circumference, average drinks a day, smoking status, BMI, central obesity, hypertension, diabetes, triglyceride (TG), HDL, MetS, and other variables were also collected. Laboratory parameters including aspartate aminotransferase (AST), ALT, alkaline phosphatase, platelets, albumin, bilirubin, lipid profile including TG and hemoglobin A1c (HbA1C) were also measured.
To assess for the presence of AF in NAFLD and BAFLD, a non-invasive liver fibrosis score, Fibrosis-4 (FIB-4) index, was calculated using age, AST, ALT and platelet count.
The primary outcome was to assess the prevalence and clinical characteristics of NAFLD and BAFLD. The secondary outcome was to evaluate the prevalence of AF in patients with NAFLD and BAFLD.
Data is presented as the mean ± SE or un-weighted frequency (%). The prevalence of NAFLD and BAFLD was assessed by calculating the percent of participants meeting the definitions of each; the corresponding 95%CIs are reported. A subgroup analysis was performed in subjects with either of the two liver diseases. The unadjusted analysis was implemented to evaluate differences between NAFLD and BAFLD; continuous variables were compared using t-tests, and categorical variables were compared using Rao-Scott chi-square tests. A multivariable regression analysis was performed to compare disease severity between the BAFLD and NAFLD groups, after adjusting for diabetes and other components of the metabolic syndrome. Linear regression was used to model FIB-4 score while logistic regression was used to model the presence of suspected AF based on these scores.
All analyses were performed using SAS survey procedures (version 9.4, The SAS Institute, Cary, NC, United States), which account for the complex sampling design of NHANES and appropriately weight participants in statistical models. The full sample MEC exam weights were used in all analyses; weights for combined cycles were constructed following the guidelines provided in the NHANES analytic guidelines.
A total of 20939 subjects met the inclusion criteria during the 2003-2014 cycles. Overall, the prevalence of NAFLD was 25.9% (95%CI: 25.1-26.8), and that of BAFLD was 0.84% (95%CI: 0.67-1.02), which corresponds to an estimated 1.24 million Americans affected by BAFLD as shown in Table 1.
| Liver disease | Unweighted frequency | Weighted frequency | Prevalence (95%CI) |
| NAFLD | 5351 | 38151562 | 25.9 (25.1, 26.8) |
| BAFLD | 170 | 1243289 | 0.84 (0.67, 1.02) |
| ALD | 81 | 590979 | 0.40 (0.28, 0.52) |
Patient characteristics: The descriptive characteristics of the study participants are shown in Table 2. Compared to patients with NAFLD, patients with BAFLD were more likely to be males, but there were no significant differences in the mean age between the two groups (43.8 vs 44.5 years; P = 0.34). The BAFLD population had approximately 2.5 times more current smokers than NAFLD group and they also had approximately 16 times a greater number of drinks/d. Given the fact that we used MetS and diabetes mellitus type 2 (DM2) as part of the definition of BAFLD, components of the MetS were more common in the BAFLD cohort. For example, central obesity was also more prevalent in BAFLD patients. In addition, we found significantly higher percentage of MetS in BAFLD group compared to NAFLD population, with high prevalence of HTN, DM, hypertriglyceridemia and low HDL, P < 0.001 for all. Patients with BAFLD were more likely to have significantly higher ALT, AST, gamma glutamyl transferase (GGT). Patients with BAFLD also had significantly higher cholesterol and HbA1C than NAFLD patients. Interestingly, compared to NAFLD patients, those with BAFLD had lower platelet counts.
| Factor (unit) | NAFLD | BAFLD | P value |
| Gender, % ± SE | < 0.001 | ||
| Male | 46.9 ± 0.872 | 75.8 ± 3.91 | |
| Current smoker, % ± SE | 17.6 ± 0.662 | 45.3 ± 5.21 | < 0.001 |
| BMI, mean ± SE | 32.4 ± 0.13 | 33.1 ± 0.56 | < 0.001 |
| Overweight (BMI 25+), % ± SE | 100.0 ± 0.00 | 100.0 ± 0.00 | |
| Obese (BMI 30+), % ± SE | 57.6 ± 1.04 | 64.4 ± 4.7 | 0.18 |
| Severely obese (BMI 40+), % ± SE | 11.1 ± 0.63 | 12.3 ± 3.2 | 0.7 |
| Waist circumference (cm), mean ± SE | 106.5 ± 0.322 | 111.9 ± 1.31 | < 0.001 |
| Average number of drinks/d, mean ± SE | 0.26 ± 0.012 | 4.3 ± 0.221 | < 0.001 |
| Central obesity, % ± SE | 79.2 ± 0.862 | 92.3 ± 2.51 | < 0.001 |
| HTN (MS), % ± SE | 43.5 ± 1.032 | 74.7 ± 3.81 | < 0.001 |
| Diabetes (MS), % ± SE | 20.5 ± 0.682 | 38.1 ± 5.01 | < 0.001 |
| Low HDL, % ± SE | 47.2 ± 1.05 | 49.5 ± 4.7 | < 0.001 |
| Hypertriglyceridemia, % ± SE | 48.5 ± 1.022 | 82.3 ± 2.91 | < 0.001 |
| Metabolic Syndrome, % ± SE | 42.6 ± 1.042 | 91.4 ± 2.61 | < 0.001 |
| Platelet count (1000 cells/uL), mean ± SE | 261.4 ± 1.22 | 243.1 ± 5.71 | 0.007 |
| ALT (U/L), mean ± SE | 38.2 ± 0.322 | 53.1 ± 3.61 | < 0.001 |
| AST (U/L), mean ± SE | 31.2 ± 0.342 | 43.3 ± 3.31 | < 0.001 |
| Alkaline phosphatase (U/L), mean ± SE | 71.4 ± 0.51 | 76.7 ± 3.4 | 0.01 |
| Total bilirubin (mg/dL), mean ± SE | 0.72 ± 0.01 | 0.77 ± 0.02 | 0.007 |
| Creatinine (mg/dL), mean ± SE | 0.86 ± 0.00 | 0.86 ± 0.01 | 0.15 |
| Cholesterol (mg/dL), mean ± SE | 206.8 ± 0.792 | 224.7 ± 4.31 | < 0.001 |
| Triglycerides (mg/dL), mean ± SE | 186.6 ± 2.82 | 268.0 ± 17.21 | < 0.001 |
| HDL (mg/dL), mean ± SE | 48.3 ± 0.30 | 48.3 ± 1.7 | < 0.001 |
| LDL (mg/dL), mean ± SE | 121.2 ± 0.71 | 122.8 ± 4.8 | < 0.001 |
| Gamma glutamyl transferase (U/L), mean ± SE | 36.6 ± 0.622 | 94.6 ± 16.41 | 0.002 |
| Glycohemoglobin (%), mean ± SE | 5.6 ± 0.02 | 5.7 ± 0.09 | < 0.001 |
| FIB-4, mean ± SE | 0.94 ± 0.012 | 1.2 ± 0.091 | 0.001 |
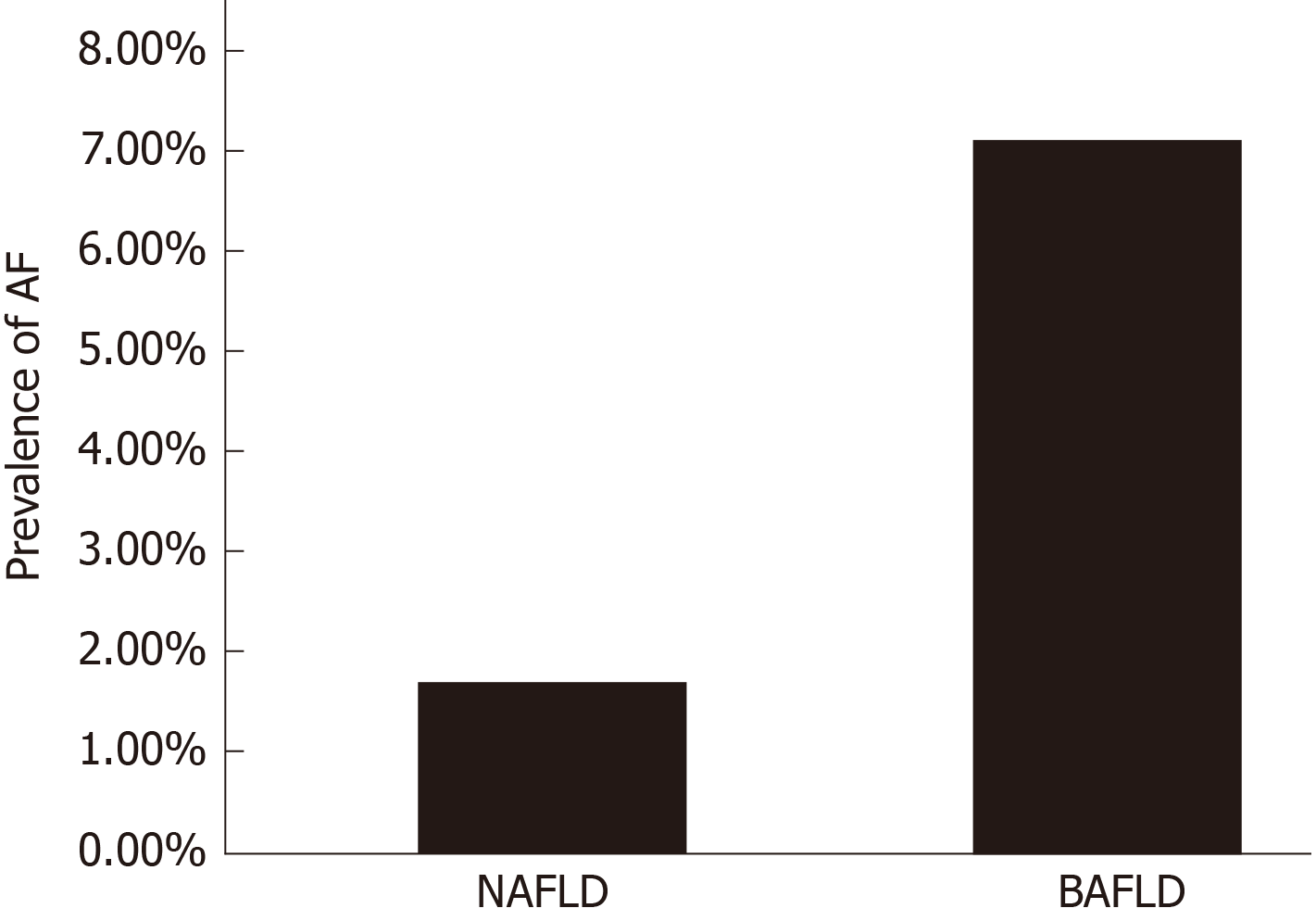
| FIB-4 > 2.67, % ± SE | 1.7 ± 0.22 | 7.1 ± 2.5 | 0.045 |
Based on FIB-4 index of 2.67 or higher, patients with BAFLD were found to have significantly higher prevalence of AF compared to the NAFLD group, (7.1% ± 2.5 in the BAFLD group vs 1.7% ± 0.22 in the NAFLD group, P = 0.045) as seen in Figure 1. Even after adjusting for all the components of MetS, subjects with BAFLD had 3.2 times higher odds of having AF than those with NAFLD [OR (95%CI) for FIB-4 > 2.67 = 3.2 (1.4, 7.0, P = 0.004)].
To the best of our knowledge, the current study provides novel data on the prevalence and clinical characteristics of BAFLD in the United States adult population. The main findings of our study are the following: (1) BAFLD is common in the United States with an estimated 1.24 million Americans being affected; (2) Compared to NAFLD, patients with BAFLD were more likely to be male and active smokers; (3) They also have higher ALT (53.1 vs 38.2 U/L), AST (43.3 vs 31.2 U/L), and lower platelet counts (243 vs 261 K/uL) (P < 0.05 for all); and (4) The prevalence of AF was also significantly higher in patients with BAFLD than subjects with NAFLD with an adjusted OR of having AF being 3.2 (1.4, 7.0) based on the FIB-4 index.
Several studies have highlighted the strong bidirectional association between MetS components and NAFLD[4,11,12]. Obesity is a principle risk factor for both NAFLD and MetS. NAFLD was reported to occur in > 95% of patients with severe obesity undergoing bariatric surgery[13,14]. Between 1980 and 2014, the World Health Organization (WHO) reported a doubling of obesity, with 39% of adults being considered overweight in 2014. In light of this, it is unsurprising that the prevalence of NAFLD is estimated to be close to 25% of the adult population[4,15], which is consistent with our results.
Nearly 70% of the adult population worldwide is estimated to consume alcohol; the highest consumption levels are found in the developed world, particularly Europe and North America[16]. This suggests that the prevalence of ALD in the United States would likely be high as well. Diseases of excess alcohol largely affect men, which could be explained by the fact that men drink more frequently, in larger quantities, and have fewer abstainers than women[16]. However, females are more prone to alcohol-related liver injury upon consuming lesser quantities of alcohol for shorter durations. Determining the relative clinical contributions of alcohol consumption and MetS to fatty liver disease is difficult, especially when both risk factors are present in the same patient. Indeed, it is well known that patients with NAFLD can consume higher amounts of alcohol than previously thought[17]. Obesity/MetS and excessive alcohol consumption may coexist in a significant proportion, affecting the liver in ways that may lead to the development of BAFLD.
Although, there are data addressing the effect of alcohol or obesity on liver[18-21], the interaction between alcohol consumption and obesity/MetS and their effect on liver biochemical variables are not well characterized. Age, sex, and ethnicity affect NAFLD prevalence; the likelihood of men having NAFLD is two times higher than women[6]. In the present study, patients with BAFLD were more likely to be males (75.8% ± 3.9% vs 46.9% ± 0.87%) and smokers (45.3% vs 17.6%) than patients with NAFLD alone. This is likely due to the larger number of males with alcoholic liver disease and high percentage of male smokers.
Furthermore, our data demonstrate that patients with BAFLD, when compared to the NAFLD group, had higher liver enzymes and lower platelets counts, suggesting more advanced liver disease in these patients. Our results are in concordance with previous studies that implied that the effect of alcohol consumption on hepatic steatosis, as measured by the examination of serum liver enzymes, increased with increasing BMI[22-25]. Hepatic steatosis was only found in 16% of lean controls in the Dionysos study in Northern Italy; however, this prevalence was increased to 46% in subjects with an alcohol intake > 60 g/d and to 76% in the obese participants[26].
Notably, in our study, BAFLD patients had three-times the risk of AF than the NAFLD group. This likely suggests that the combined effect of ALD and NAFLD have a synergistic unfavorable impact on hepatic fibrosis. The mechanism underlying this interaction is not known. Few small studies have assessed the effect of alcohol consumption on the underlying hepatic histopathology in subjects diagnosed with NAFLD. In a cohort of 112 patients with liver biopsies, Petersen et al[27] found that the only positive association between weight and fatty liver wass in patients who were overweight and had moderate alcohol consumption. Ekstedt et al[28] found faster fibrosis progression in NAFLD patients who consumed alcohol in moderate amounts. Binge drinking and insulin resistance have been reported to be independent risk factors associated with the progression of fibrosis over a mean of 13.8-year interval. In 1997, Naveau et al[29] and Raynard et al[30] showed that BMI is positively associated with AF in ALD. Obesity may sensitize an individual to alcohol-induced liver injury at a much lower dose. We estimated 1.24 million Americans might be affected by BAFLD with the concomitant three-fold increase in AF; therefore, this is a significant public health issue with important policy implications.
Our results showed significantly higher blood TG (268 mg/dL vs 186 mg/dL) in patients with BAFLD compare to patients with NAFLD. We propose that there may be a combined additive or synergistic effect of alcohol and NAFLD on triglycerides. NAFLD is characterized by increases in TG, very-low-density lipoprotein (LDL), apolipoprotein B to apolipoprotein A1 ratio, and small dense LDL in conjunction with low HDL[31]. Similarly, alcohol consumption is a well-known cause of secondary hypertriglyceridemia and it may exaggerate hypertriglyceridemia in primary lipid disorders[32,33]. Therefore, BAFLD patients may have increased cardio vascular disease (CVD) risk and mortality compared to patients with NAFLD. However, these findings should be interpreted with caution given the fact that MetS and DM2 were included in the definition of BAFLD.
Our study has several limitations. It was a cross-sectional study using a large national database; thus, chronological relationships could not be established. The use of elevated ALT and BMI to define patients with NAFLD is imperfect and may have included patients who do not actually have NAFLD and excluded lean subjects that have non-obese NAFLD. However, given the large population-based study sample, we considered this definition to be the most suitable for this study. We used the non-invasive fibrosis score to predict AF which has been previously used in NHANES studies, as it was impossible to perform liver biopsies or even fibro-scans in such a large cohort[34]; however, it is not the gold-standard. We did not use the AST/ ALT ratio or aspartate aminotransferase to platelet ratio index (APRI) to diagnose AF given their heavy reliance on AST values, which are known to be affected by alcohol consumption. Similarly, we did not use the NAFLD fibrosis score because diabetes is one of its components and this may have biased our estimates of AF. Using a large, national, population-based sample allows for the ability to generalize our results to the United States population and offsets these limitations.
In conclusion, a substantial percentage of the general American population may have BAFLD. Patients with BAFLD tend to have more advanced disease and may have a higher risk of progression to cirrhosis or end-stage liver disease. Therefore, future research should aim to identify the burden of liver disease in this population and intervene in a timely fashion. The risk of the combined effects of MetS and alcohol consumption should be taken seriously in all patients with suspected NAFLD. Importantly, screening for risky, often under-reported, alcohol consumption should be considered. Data on safe alcohol consumption in NAFLD is conflicting and needs further assessment in future prospective studies.
Fatty liver disease caused by excess alcohol consumption is called alcoholic liver disease (ALD) whereas fatty liver disease caused by metabolic disease is called non-alcoholic fatty liver disease (NAFLD). Often, risk factors for both types of fatty liver diseases occur in the same individual, especially as the prevalence of both of these diseases is on the rise. The presence of both types of fatty liver disease in one individual may lead to the development of a new condition we call both alcoholic and NAFLD (BAFLD). We believe that patients with BAFLD are at a higher risk of advanced fibrosis and complications related to end-stage liver disease. Studying and understanding BAFLD has important public health and policy implications.
A new fatty liver entity, we call BAFLD, occurs when both ALD and NAFLD risk factors are present in the same individual. We reported on the clinical characteristics and degree of liver fibrosis in BAFLD patients compared to NAFLD patients. As most of the risk factors that lead to BAFLD are modifiable dietary and lifestyle choices, understanding their reciprocal interaction and combined effect on the liver might lead to a better understanding of BAFLD pathogenesis, treatment, and prevention. This has important public health and policy implications.
This study aimed to identify the prevalence of NAFLD and BAFLD and to assess the clinical characteristics of patients with BAFLD in comparison to those with NAFLD in a large cohort of subjects in the United States.
This is a cross-sectional study that was done using National Health and Nutrition Examination Survey between 2003-2014. NAFLD and BAFLD patients were identified. Univariable and multivariable analysis were performed to assess differences between NAFLD and BAFLD and to compare severity based on a validated fibrosis score (FIB4 index).
The prevalence of NAFLD was at 25.9% and that of BAFLD was 0.84% which corresponds to an estimated 1.24 million Americans affected by BAFLD. Compared to NAFLD, patients with BAFLD were more likely to be male, smokers, have higher ALT, AST, triglycerides, and lower platelets; P < 0.01 for all. More importantly, after adjusting for MetS components, BAFLD patients were significantly about three times more likely to have advanced fibrosis based on FIB4 index > 2.67, P = 0.004].
In conclusion, a substantial percentage of the general American population may have BAFLD. Patients with BAFLD tend to have more advanced disease and may have a higher risk of progression to cirrhosis and end-stage liver disease. Therefore, special attention should be paid to this population to identify the burden of liver disease and intervene in a timely fashion.
The possibility of the combined effects of MetS and alcohol consumption should be considered in all patients with suspected NAFLD. Vitally, consideration should be given to the role of screening for identification of risky, often under-reported, alcohol consumption. Data on safe alcohol consumption in NAFLD is conflicting and needs further assessment in future prospective studies.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Grgurevic I, Jamali R, Xin YN S-Editor: Ma YJ L-Editor: A E-Editor: Ma YJ
| 1. | Souza MR, Diniz Mde F, Medeiros-Filho JE, Araújo MS. Metabolic syndrome and risk factors for non-alcoholic fatty liver disease. Arq Gastroenterol. 2012;49:89-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 147] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 2. | European Association for the Study of the Liver (EASL). European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3174] [Article Influence: 352.7] [Reference Citation Analysis (4)] |
| 3. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ; American Gastroenterological Association; American Association for the Study of Liver Diseases; American College of Gastroenterologyh. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2413] [Cited by in RCA: 2611] [Article Influence: 200.8] [Reference Citation Analysis (1)] |
| 4. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7522] [Article Influence: 835.8] [Reference Citation Analysis (0)] |
| 5. | World Health Organization. Global status report on noncommunicable diseases 2014. 2014;176 Available from: https://www.who.int/nmh/publications/ncd-status-report-2014/en/. |
| 6. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 4936] [Article Influence: 705.1] [Reference Citation Analysis (9)] |
| 7. | Sato N, Lindros KO, Baraona E, Ikejima K, Mezey E, Järveläinen HA, Ramchandani VA. Sex difference in alcohol-related organ injury. Alcohol Clin Exp Res. 2001;25:40S-45S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Basra S, Anand BS. Definition, epidemiology and magnitude of alcoholic hepatitis. World J Hepatol. 2011;3:108-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (3)] |
| 9. | Stinson FS, Grant BF, Dufour MC. The critical dimension of ethnicity in liver cirrhosis mortality statistics. Alcohol Clin Exp Res. 2001;25:1181-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 97] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Sanyal AJ; American Gastroenterological Association. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1705-1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 759] [Cited by in RCA: 781] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 11. | Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62:S47-S64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1516] [Cited by in RCA: 2137] [Article Influence: 213.7] [Reference Citation Analysis (0)] |
| 12. | Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, Srishord M. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9:524-530.e1; quiz e60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 790] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 13. | Sasaki A, Nitta H, Otsuka K, Umemura A, Baba S, Obuchi T, Wakabayashi G. Bariatric surgery and non-alcoholic Fatty liver disease: current and potential future treatments. Front Endocrinol (Lausanne). 2014;5:164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 14. | Subichin M, Clanton J, Makuszewski M, Bohon A, Zografakis JG, Dan A. Liver disease in the morbidly obese: a review of 1000 consecutive patients undergoing weight loss surgery. Surg Obes Relat Dis. 2015;11:137-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 15. | Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4054] [Cited by in RCA: 3783] [Article Influence: 540.4] [Reference Citation Analysis (2)] |
| 16. | World Health Organisation. Global status report on alcohol and health 2014: Glob status Rep alcohol. 2014;1-392 Available from: URL: http://apps.who.int/iris/bitstream/10665/112736/1/9789240692763_eng.pdf?ua=1. |
| 17. | Marks P, Williams R. Calorie and alcohol consumption in nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2012;24:527-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Daeppen JB, Smith TL, Schuckit MA. Influence of age and body mass index on gamma-glutamyltransferase activity: a 15-year follow-up evaluation in a community sample. Alcohol Clin Exp Res. 1998;22:941-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Lawlor DA, Sattar N, Smith GD, Ebrahim S. The associations of physical activity and adiposity with alanine aminotransferase and gamma-glutamyltransferase. Am J Epidemiol. 2005;161:1081-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 135] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Rohrer JE, Rohland BM, Denison A, Way A. Frequency of alcohol use and obesity in community medicine patients. BMC Fam Pract. 2005;6:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Stranges S, Dorn JM, Muti P, Freudenheim JL, Farinaro E, Russell M, Nochajski TH, Trevisan M. Body fat distribution, relative weight, and liver enzyme levels: a population-based study. Hepatology. 2004;39:754-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 172] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 22. | Alatalo PI, Koivisto HM, Hietala JP, Puukka KS, Bloigu R, Niemelä OJ. Effect of moderate alcohol consumption on liver enzymes increases with increasing body mass index. Am J Clin Nutr. 2008;88:1097-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Ruhl CE, Everhart JE. Joint effects of body weight and alcohol on elevated serum alanine aminotransferase in the United States population. Clin Gastroenterol Hepatol. 2005;3:1260-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 184] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 24. | Shen Z, Li Y, Yu C, Shen Y, Xu L, Xu C, Xu G. A cohort study of the effect of alcohol consumption and obesity on serum liver enzyme levels. Eur J Gastroenterol Hepatol. 2010;22:820-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Loomba R, Bettencourt R, Barrett-Connor E. Synergistic association between alcohol intake and body mass index with serum alanine and aspartate aminotransferase levels in older adults: the Rancho Bernardo Study. Aliment Pharmacol Ther. 2009;30:1137-1149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 26. | Bellentani S, Saccoccio G, Masutti F, Crocè LS, Brandi G, Sasso F, Cristanini G, Tiribelli C. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 842] [Cited by in RCA: 863] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 27. | Petersen P. Fatty liver in patients with moderate alcohol consumption, diabetes mellitus and overweight. Scand J Gastroenterol. 1977;12:781-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Ekstedt M, Franzén LE, Holmqvist M, Bendtsen P, Mathiesen UL, Bodemar G, Kechagias S. Alcohol consumption is associated with progression of hepatic fibrosis in non-alcoholic fatty liver disease. Scand J Gastroenterol. 2009;44:366-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 29. | Naveau S, Giraud V, Borotto E, Aubert A, Capron F, Chaput JC. Excess weight risk factor for alcoholic liver disease. Hepatology. 1997;25:108-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 436] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 30. | Raynard B, Balian A, Fallik D, Capron F, Bedossa P, Chaput JC, Naveau S. Risk factors of fibrosis in alcohol-induced liver disease. Hepatology. 2002;35:635-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 254] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 31. | Corey KE, Misdraji J, Gelrud L, Zheng H, Chung RT, Krauss RM. Nonalcoholic steatohepatitis is associated with an atherogenic lipoprotein subfraction profile. Lipids Health Dis. 2014;13:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Berglund L, Brunzell JD, Goldberg AC, Goldberg IJ, Sacks F, Murad MH, Stalenhoef AF; Endocrine society. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:2969-2989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 588] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 33. | Klop B, Wouter Jukema J, Rabelink TJ, Castro Cabezas M. A physician's guide for the management of hypertriglyceridemia: the etiology of hypertriglyceridemia determines treatment strategy. Panminerva Med. 2012;54:91-103. [PubMed] |
| 34. | Wang A, Lazo M, Carter HB, Groopman JD, Nelson WG, Platz EA. Association between Liver Fibrosis and Serum PSA among U.S. Men: National Health and Nutrition Examination Survey (NHANES), 2001-2010. Cancer Epidemiol Biomarkers Prev. 2019;28:1331-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |