Published online Jan 27, 2019. doi: 10.4254/wjh.v11.i1.99
Peer-review started: October 7, 2018
First decision: October 18, 2018
Revised: December 20, 2018
Accepted: December 31, 2018
Article in press: January 1, 2019
Published online: January 27, 2019
Processing time: 113 Days and 16.1 Hours
The impact of platelets on liver transplantation (LT) is well recognized, but not completely understood. Platelets exert dichotomous effects on the graft and on the patient. On the one hand, they are essential for primary hemostasis and tissue repair and regeneration. On the other hand, they support ischemia/reperfusion injury and inflammatory processes. Recent evidence has shown a new role for platelet count (PC) in predicting outcomes after LT.
To evaluate if low PC is a predictor of short- and long-term outcomes after LT.
Four hundred and eighty consecutive LT patients were retrospectively assessed. PC from the preoperative to the seventh postoperative day (POD) were considered. C-statistic analysis defined the ideal cutoff point for PC. Cox regression was performed to check whether low PC was a predictor of death, retransplantation or primary changes in graft function within one year after LT.
The highest median PC was 86 × 109/L [interquartile range (IQR) = 65–100 × 109/L] on seventh POD, and the lowest was 51 × 109/L (IQR = 38–71 × 109/L) on third POD. The C-statistic defined a PC < 70 × 109/L on fifth POD as the ideal cutoff point for predicting death and retransplantation. In the multivariate analysis, platelets < 70 × 109/L on 5POD was an independent risk factor for death at 12 mo after LT [hazard ratio (HR) = 2.01; 95% confidence interval (CI) 1.06-3.79; P = 0.031]. In the Cox regression, patients with PC < 70 × 109/L on 5POD had worse graft survival rates up to one year after LT (HR = 2.76; 95%CI 1.52-4.99; P = 0.001).
PC < 70 × 109/L on 5POD is an independent predictor of death in the first year after LT. These results are in agreement with other studies that indicate that low PC after LT is associated with negative outcomes.
Core tip: Recent evidence shows that low platelet count (PC) can predict outcomes after liver transplantation (LT). We evaluated if a low PC in the immediate postoperative period of LT, defined as a PC < 70 × 109/L on the fifth postoperative day (5POD), is a predictor of death or retransplantation. We retrospectively assessed 480 consecutive LT patients. This study showed that a PC < 70 × 109/L on the 5POD was independently associated with shorter patient and graft survival within one year after LT. These results are in agreement with other studies indicating that thrombocytopenia in the immediate postoperative period of LT is associated with negative outcomes.
- Citation: Beltrame P, Rodriguez S, Brandão ABM. Low platelet count: Predictor of death and graft loss after liver transplantation. World J Hepatol 2019; 11(1): 99-108
- URL: https://www.wjgnet.com/1948-5182/full/v11/i1/99.htm
- DOI: https://dx.doi.org/10.4254/wjh.v11.i1.99
Low platelet count (PC) is common in candidates for liver transplantation (LT). Its etiology is multifactorial, including increased spleen destruction, inability to produce bone marrow and reduced production of thrombopoietin[1-3]. Reduction in PC is also commonly observed in the postoperative period of LT. In this period, there are other possible contributing factors for its occurrence, such as graft and splenic sequestration, hemodilution, use of some medications and immunological reactions[4-8]. After LT, PC reach their lowest level around the third or fifth postoperative day (POD), returning to preoperative values in around two weeks[9]. Low PC after LT is associated with shorter graft and patient survival and higher rates of negative outcomes[10], possibly due to the fact that platelets play an important role in the promotion of angiogenesis[11-14] and hepatic regeneration[15,16].
Lesurtel et al[17] observed that a PC < 60 × 109/L on the fifth POD was an independent risk factor for complications and shorter graft and patient survival within the first 90 d posttransplantation. They then proposed the “60-5 criterion”, where a PC of < 60 × 109/L on 5POD of LT could be used to predict severe complications[17]. Subsequently, Takahashi et al[18] reported that a PC of < 72 × 109/L on 5POD was associated with graft loss and shorter patient survival. The present study aimed to confirm the hypothesis that a low PC in the immediate postoperative period of LT is a predictor of death or retransplantation.
All adult patients consecutively submitted to LT with a deceased donor between June 2006 and June 2016 were eligible at a referral center in southern Brazil. Excluded from the study were patients who underwent double organ transplantation (liver and kidney), late liver retransplantation (more than one year between LT and retransplantation) or LT due to acute liver failure and who had incomplete medical records. The study followed the recommended guidelines for observational studies[19] and was approved by the Institutional Review Board of Santa Casa de Misericórdia de Porto Alegre (No. 1.183.375).
The following variables were analyzed: (1) Demographic characteristics of the recipient and donor (sex, age and body mass index); (2) variables related to the procedure (times of cold and warm ischemia, volume of bleeding and transfusions during transplantation); (3) blood components transfused up to the first week after LT; (4) biochemical tests performed immediately before LT and up to the seventh POD: PC, international normalized ratio (INR), aspartate aminotransferase, alanine aminotransferase (ALT), total bilirubin, factor V, alkaline phosphatase and gamma-GT; and (5) relevant preoperative characteristics of the recipient: Need for ventilation or hemodynamic support, renal replacement therapy, hepatorenal syndrome, cirrhosis etiology, model for end-stage liver disease (MELD) score, and Child-Turcotte-Pugh (CTP) score[20-22].
All patients underwent LT by the same team, where the technique of choice was hepatectomy with preservation of the inferior vena cava (piggy-back technique). The preservation solutions used during the period were: First, the University of Wisconsin solution, then histidine-tryptophan-ketoglutarate and, since 2013, the Institute Georges Lopez-1 solution. The decision to transfuse blood components was based on clinical, laboratory and hemodynamic parameters. Blood loss was restored by transfusion of packed red blood cells, with the goal of maintaining hemoglobin levels between 8.0 and 10.0 g/dL. Platelet concentrate was administered only in patients with platelet dysfunction and persistent bleeding, even after correction of other coagulation factors.
The primary outcome was death, by any cause, within 30, 90 and 365 d after LT. The secondary outcome was the need for liver retransplantation in the same period. We considered any cause of death and retransplantation after LT, which could be primary or secondary. Secondary death/retransplantation was due to technical, immunological, infectious or cardiovascular causes. Primary death/retransplantation was due to delayed graft function or primary graft dysfunction. Delayed graft function was defined as the presence of at least 1 of the following parameters 7 d after LT: A serum bilirubin level ≥ 10 mg/dL and an INR ≥ 1.6 or an ALT level > 2000 IU/L[17]. Primary graft dysfunction was defined as the presence of one or more of the following postoperative laboratory parameters: A serum bilirubin level ≥ 10mg/dL on day 7, INR ≥ 1.6 on day 7, and an ALT level > 2000 IU/L within the first 7 d[23].
Statistical analysis was performed with IBM SPSS software version 22.0 for Windows (IBM, Armonk, NY, United States). Quantitative variables were described by mean and SD, and significance was determined by Student t-test. Categorical variables were described by counts and percentages, and significance was assessed using the chi-square test or Fisher exact, when needed. The C-statistic, equivalent to the area under the receiver operating characteristics (ROC) curve (AUC) assessed on each POD of LT, was adopted to establish the day on which the PC showed the best performance. Recursive analyses of ROC curves, within the day previously detected, allowed us to identify the cutoff point. Univariate and multivariate Cox regression analyses were performed to adjust the PC findings with the potential effect of other factors. The pre-operative variables chosen, with potential confounding effect over PC in the postoperative period, as previously shown in similar studies[17,18,23], were: MELD score before LT > 20, pre-LT PC < 70 × 109/L, age of the recipient > 60 yr, donor age > 40 yr and diagnosis of hepatorenal syndrome at the time of transplantation. In addition, the need for intraoperative transfusion of platelet concentrates, perioperative bleeding > 2500 mL and cold ischemia time > 8 h were considered. Survival curves were obtained using the Kaplan-Meier method. P < 0.05 was considered statistically significant.
Of the 617 eligible patients for study, 137 (22.2%) were excluded from the analysis for the following reasons: Underwent combined liver and kidney transplantation (n = 32; 5.2%); late liver retransplantation (performed one year after the initial LT) (n = 21; 3.4%); transplantation for acute liver failure (n = 21; 3.4%); or incomplete information in medical records up to seventh POD, due to either death, retransplantation or inadequate completion of medical records (n = 63; 10.2%). Therefore, 480 patients were included in the study. Approximately 20% of them were older than 60 yr, with the majority being male, and hepatitis C virus infection was the most frequent etiology of cirrhosis. At the time of LT, the mean MELD score was 16; approximately 49% of patients were in the B classification of the CTP score and 3.5% underwent hemodialysis immediately before transplantation. About 48% of the donors were older than 40, and most times the cold ischemia time was < 8 h (Table 1).
| Variables prior to LT | All patients (n = 480) | Platelets < 70 × 109/L - 5POD (n = 288) | Platelets ≥ 70 × 109/L - 5POD (n = 192) | P-value |
| Related to recipients | ||||
| Age > 60 yr | 97 (20.2) | 65 (22.6) | 32 (16.7) | 0.071 |
| Sex: Male | 311 (64,8) | 189 (65.6) | 122 (63.5) | 0.355 |
| BMI (kg/m2) | 25.7 (± 3.8) | 25.7 (± 3.5) | 25.4 (± 3.4) | 0.410 |
| Hepatitis C | 141 (29.4) | 85 (29.5) | 56 (29.2) | > 0.99 |
| Hepatitis B | 20 (4.2) | 12 (4.2) | 8 (4.2) | > 0.99 |
| MELD score (± SD) | 16.3 (± 5.3) | 16.8 (± 5.4) | 15.7 (± 5.0) | 0.033 |
| CTP score | ||||
| A | 117 (24.5) | 59 (20.6) | 58 (30.4) | 0.014 |
| B | 228 (47.8) | 140 (49) | 88 (46.1) | |
| C | 132 (27.7) | 87 (30.4) | 45 (23.6) | |
| Life support1 | 3 (0.6) | 3 (1) | 0 (0.0) | 0.215 |
| HRS | 45 (9.4) | 27 (9.4) | 18 (9.4) | 0.560 |
| Hemodialysis2 | 17 (3.5) | 10 (3.5) | 7 (3.6) | 0.554 |
| Related to donors | ||||
| Age > 40 yr | 223 (47.2) | 139 (49.3) | 84 (44.2) | 0.161 |
| CIT > 8 h | 192 (40.6) | 116 (41.1) | 76 (39.8) | 0.422 |
| Variables during LT | ||||
| Bleeding > 2500 mL | 276 (57.5) | 172 (59.7) | 104 (54.2) | 0.133 |
| Transfusion of platelets | 180 (37.5) | 109 (37.8) | 71 (36.9) | 0.890 |
| Transfusion of fresh frozen plasma | 288 (60.0) | 171 (59.4) | 117 (60.9) | 0.805 |
The highest median number of PC was observed on seventh POD: 86 × 109/L [interquartile range (IQR) = 65-100 × 109/L], exceeding the preoperative median, i.e., 77 × 109/L (IQR = 57-97 × 109/L). The lowest median was found on the third POD: 51 × 109/L (IQR = 38-71 × 109/L).
C-statistical analysis showed that a PC < 70 × 109/L on 5POD was the ideal cutoff point for predicting death and retransplantation at 365 d after LT [AUC-ROC = 0.632; 95% confidence interval (CI) 0.558-0.705; P = 0.001]. Patients were then stratified into two groups according to PC on 5POD: Patients with ≥ 70 × 109/L platelets and those with < 70 × 109/L platelets (Table 1). In the group with < 70 × 109/L PC, the recipients were transplanted at later stages of their disease according to the CTP score (P = 0.014) and, although without statistical significance, they received organs with a longer time of cold ischemia and from donors over 40 yr old (Table 1).
Univariate analysis (Table 2) showed that patients with a PC of < 70 × 109/L on 5POD had higher all-cause mortality rates at 90 and 365 d after LT, compared to patients with a higher PC. The 90-day mortality rate was 7.5%, higher in patients with a PC < 70 × 109/L on 5POD (9.7% vs 4.2%, P = 0.037). Overall mortality at 365 d post-LT was 13.3%. Likewise, the highest mortality rates occurred in the group with PC < 70 × 109/L on 5POD (17.4% vs 7.3%; P = 0.002). Retransplantation rates at 30, 90 and 365 d were higher in patients with lower PC (< 70 × 109/L) on 5POD. In fact, the need for retransplantation up to 90 d after LT was only observed in the group of patients with a lower PC, and only one patient with a PC of ≥ 70 × 109/L on 5POD required retransplantation within 365 d post-LT (5.2% vs 0.5%; P = 0.004) (Table 2).
| Outcome | Platelet count < 70 × 109/L - 5POD (n = 288) | Platelet count ≥ 70 × 109/L - 5POD (n = 192) | HR (95%CI) | P-value |
| Mortality in 30 d | 17 (5.9) | 7 (3.6) | 1.62 (0.68-3.83) | 0.37 |
| Mortality in 90 d | 28 (9.7) | 8 (4.2) | 2.33 (1.09-5.00) | 0.037 |
| Mortality in 1 yr | 50 (17.4) | 14 (7.3) | 2.38 (1.36-4.18) | 0.002 |
| Retransplantation within 30 d | 10 (3.5) | 0 (0.0) | - | 0.007 |
| Retransplantation within 90 d | 12 (4.2) | 0 (0.0) | - | 0.002 |
| Retransplantation within 1 yr | 15 (5.2) | 1 (0.5) | 10 (1.33-76.92) | 0.004 |
| Delayed graft, function | 28 (9.7) | 15 (7.8) | 1.24 (0.68-2.27) | 0.58 |
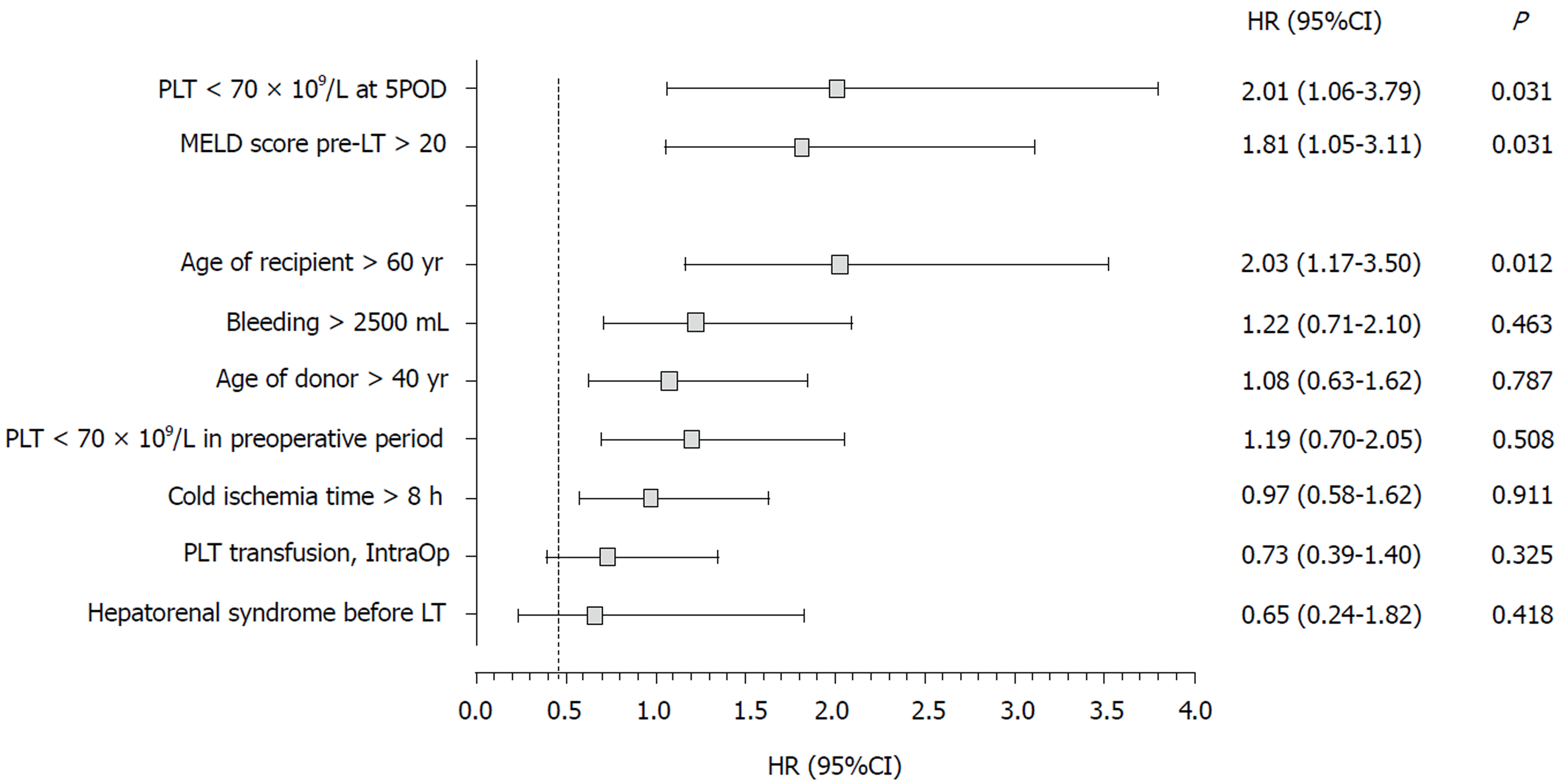
Multivariate analysis (Figure 1) showed that, in the first year after LT, a PC < 70 × 109/L on 5POD was a risk factor for death, independently of age of the recipient > 60 yr, pre-LT MELD score > 20, bleeding volume > 2500 mL intraoperatively and need for transfusion of platelet concentrates during the procedure. Pre-LT MELD > 20 and age of the recipient > 60 yr also appeared to be independent risk factors for mortality up to one year after LT (P = 0.031 and P = 0.012, respectively).
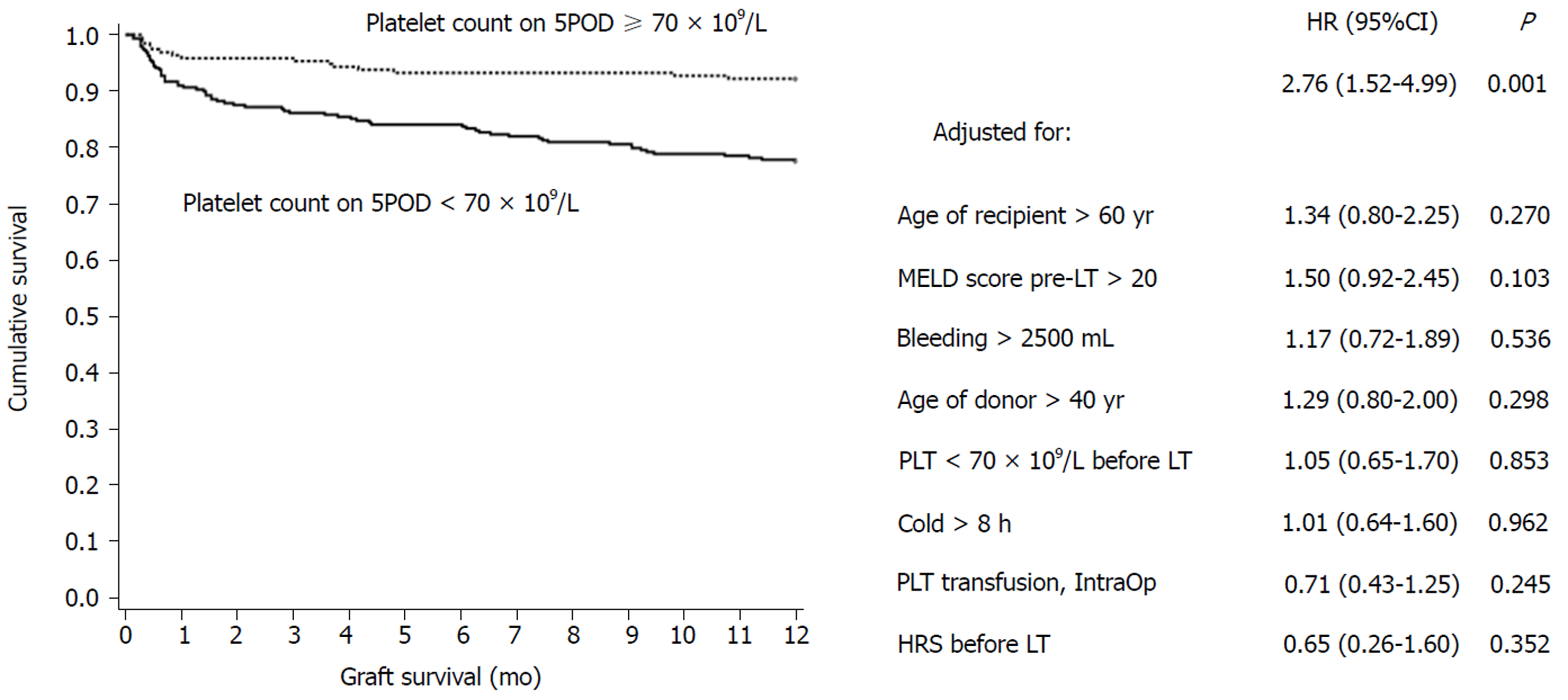
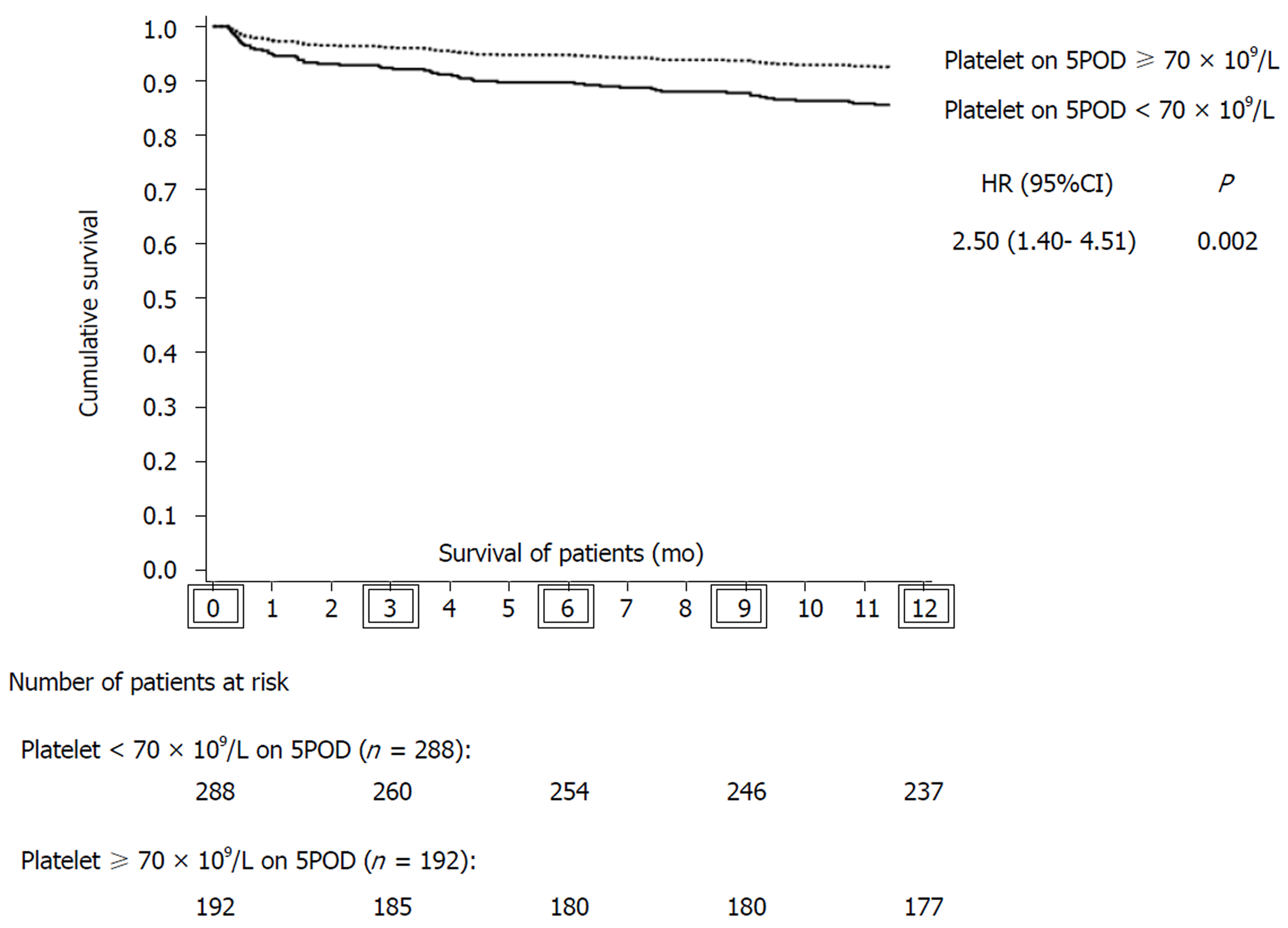
Eighty grafts were lost in up to one year of follow-up: 64 (80.0%) due to death of the recipient and 16 (20.0%) due to the need for liver retransplantation. Of the patients who underwent liver retransplantation, six were due to primary causes: three for primary graft nonfunction (18.75%), two for primary graft dysfunction (12.5%) and one for delayed graft function (6.25%). The most common causes of death were infection (28.1%), cardiovascular events (21.8%) and primary causes related to the graft (7.8%). Graft survival in the first year after LT was significantly lower in the group with PC < 70 × 109/L on 5POD compared to ≥ 70 × 109/L on 5POD group, even when adjusted for the factors used in multivariate analysis (77.5% vs 92.2%, P = 0.001) (Figure 2). Overall survival at 12 mo for patients with a PC ≥ 70 × 109/L on 5POD was higher than for those with a lower PC (92.8% vs 82.7%, P = 0.002) (Figure 3).
This study showed that PC < 70 × 109/L on 5POD of LT were independently associated with shorter patient and graft survival within one year after LT. These results are in agreement with other studies indicating that thrombocytopenia in the immediate postoperative period of LT is associated with negative outcomes[17,18,23].
Platelets contain a marked number of secretory granules, filled with proteins essential for hemostasis and different tissue growth factors, such as platelet-derived growth factor (PDGF), hepatocyte growth factor (HGF), insulin-like growth factor type 1 (IGF-1), vascular endothelial growth factor (VEGF), serotonin, ADP and ATP[14]. Platelets are activated by various types of stimuli and release these substances depending on the situation. In addition to its known role in primary hemostasis and thrombosis, platelets exert other functions, such as promoting liver regeneration and liver protection[10-14]. In liver recipients, it is common to have thrombocytopenia[1,24], a situation that worsens during transplantation, for reasons not completely understood[10]. However, many factors have been identified and associated with thrombocytopenia, such as their consumption in the process of hemostasis and sequestration by reperfusion graft or spleen[4,10,25,26].
The relationship between low PC and events after LT was suggested in the late 1990s, when thrombocytopenia (defined arbitrarily) in the immediate postoperative period of LT was associated with shorter patient and graft survival[10,25,27,28]. However, it was the Lesurtel et al[17] who established a cutoff point in the number of platelets that could be used as a predictor of outcomes after LT. In a retrospective study, Lesurtel et al[17] evaluated a cohort of 257 patients who underwent LT with a deceased donor. They observed that a PC < 60 × 109/L on 5POD was an independent predictor of Clavien-Dindo IIIb/V complications (OR = 1.96; 95%CI 1.07-3.56), graft loss [hazard ratio (HR) = 2.0; 95%CI 1.1-3.6], and lower survival of patients (HR = 2.2; 95%CI 1.4-4.6, P = 0.03) within 90 d after LT. They then proposed the “60-5” criterion, in which the determination of the number of platelets on 5POD could be used to predict outcomes, anticipate complications, and thus be used prophylactically. Later, Takahashi et al[18], also retrospectively analyzing a cohort of transplanted patients with a deceased donor, reported that a PC < 72 × 109/L on 5POD was associated with graft loss and shorter patient survival within one year after LT. The authors postulated that in patients with a greater number of platelets, more thrombocytes accumulate in the graft, which would allow the release of a greater amount of growth factors (PDGF, HGF, IGF-1 and VEGF), improving graft survival and survival in general[18]. In studying 234 adult patients submitted to LT with living donors, a Chinese study[23] concluded that a PC of ≤ 68 × 109/L at any time in the immediate postoperative period was an independent risk factor for early graft dysfunction (OR = 2.88; 95%CI 1.22-6.82, P = 0.016).
The present study validated the “60-5 criterion”, where we found that a PC < 70 × 109/L on 5 POD was independently associated with mortality at 365 d after LT. Overall survival of patients with < 70 × 109/L on 5POD was 2.5-fold lower than for patients with a PC ≥ 70 × 109/L on 5POD. In addition, lower liver graft survival was observed in the first year after surgery: patients with PC < 70 × 109/L on 5POD had a 2.76-fold greater risk of graft loss than patients with a higher PC. Therefore, our results are in line with the “60-5 criterion”, although the cutoff point was different. In fact, another study[29] validating the “60-5 criterion” also identified a cutoff point different from the one originally proposed. At the time of transplantation, patients with a lower PC on 5POD had a more severe disease: The mean MELD was higher (16.8 ± 5.4 vs 15.7 ± 5.0; P = 0.033), and most of them were in the C category of the CTP score (30.4% vs 23.6%, P = 0.014), which could predict the onset of intra- and postoperative complications. However, the intraoperative variables analyzed, such as the requirement for transfusion of blood components (including platelets), were similar in the two groups, and it is unlikely that the severity of the disease at the time of LT showed any clinical significance. Another issue to be pondered is how a decreased PC on 5POD can have an influence on late outcomes. Considering that patients with a reduced number of platelets on 5POD have a higher rate of biliary tract complications[29], and that these are associated with infectious complications, hospital readmissions and endoscopic or surgical interventions, it is possible that such complications may have an impact on the survival of grafts and patients in the long run[18,29].
Theoretically, for patients with post-transplant thrombocytopenia, preventive measures should be taken to avoid or minimize adverse effects: Platelet transfusion, suspension of potentially myelosuppressive drugs, or administration of serotonin or thrombopoietin. However, further studies are needed before these interventions are considered in clinical practice, although transfusion of platelets in transplant recipients seems to be positively associated with graft regeneration[30,31]. In addition, this study pointed out that a pre-LT MELD > 20 and age of the recipient > 60 yr was also independent risk factors for mortality up to one year after LT.
This study had some limitations. One of them is inherent to retrospective studies; this study was carried out in a single center. However, the same team of surgeons and anesthesiologists performed the procedures, and in the immediate postoperative period, patients were treated in an intensive care unit exclusively for patients undergoing organ transplantation. The study design also did not allow us to demonstrate that platelet transfusion could effectively exert a protective effect for the graft and patient. In addition, with this study, it was not possible to clarify, in fact, if low PC in the postoperative period is the cause of negative outcomes after LT or if it is a surrogate marker of another clinical condition, which could be the real problem.
In summary, patients with PC of < 70 × 109/L on 5POD of LT showed higher mortality within 365 d after transplantation and lower graft survival. This study reinforces the need to evaluate the role of interventions to maintain a minimum PC after LT to potentially improve outcomes after LT.
Platelets have several functions and exert dichotomous effects on the graft and on the patient in the context of liver transplantation (LT). Low platelet count (PC) after LT is associated with higher rates of complications. However, it is not clear whether low PC in the postoperative period is the cause or a surrogate marker of negative outcomes.
The accurate prediction of which LT recipients will do well and which ones will have serious complications remains somewhat elusive. Some authors suggest that low PC after LT can predict early posttransplant survival or graft loss. Confirmation of these findings can provide the clinician with the opportunity to intervene early and theoretically change the postoperative course of the patient.
To confirm the hypothesis that a low PC after LT is a predictor of death or graft loss.
We performed a retrospective database analysis. PC from the preoperative to the seventh postoperative day (POD) were considered. C-statistic analysis was adopted to establish the day on which the PC showed the best performance. Recursive analyses of receiver operating characteristics curves allowed us to identify the cutoff point. Cox regression was performed to check whether low PC was a predictor of death, retransplantation or primary changes in graft function within one year after LT.
PC < 70 × 109/L on 5POD was defined as the ideal cutoff point for predicting death and retransplantation. PC < 70 × 109/L on 5POD was an independent risk factor for death at 12 mo after LT. In the Cox regression, patients with PC < 70 × 109/L on 5POD had worse graft survival rates up to one year after LT.
A low PC on 5POD was associated with graft loss and mortality one year after LT. This result is in agreement with previous studies indicating that low PC in the immediate postoperative period of after LT is associated with negative outcomes.
Our results reinforce the need to evaluate the role of interventions to maintain a minimum PC after LT. Preventive measures, such as platelet transfusion, suspension of potentially myelosuppressive drugs, and administration of serotonin or thrombopoietin, could be used in the future in the LT setting. However, further studies are still required before these interventions can be considered in clinical practice.
The authors thank the Liver Transplantation Group at Santa Casa de Misericórdia de Porto Alegre, RS, Brazil.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gonzalez F, Hanna R, Shehata M S- Editor: Yan JP L- Editor: A E- Editor: Tan WW
| 1. | Witters P, Freson K, Verslype C, Peerlinck K, Hoylaerts M, Nevens F, Van Geet C, Cassiman D. Review article: Blood platelet number and function in chronic liver disease and cirrhosis. Aliment Pharmacol Ther. 2008;27:1017-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 123] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 2. | Afdhal N, McHutchison J, Brown R, Jacobson I, Manns M, Poordad F, Weksler B, Esteban R. Thrombocytopenia associated with chronic liver disease. J Hepatol. 2008;48:1000-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 403] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 3. | Kajihara M, Okazaki Y, Kato S, Ishii H, Kawakami Y, Ikeda Y, Kuwana M. Evaluation of platelet kinetics in patients with liver cirrhosis: Similarity to idiopathic thrombocytopenic purpura. J Gastroenterol Hepatol. 2007;22:112-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Richards EM, Alexander GJ, Calne RY, Baglin TP. Thrombocytopenia following liver transplantation is associated with platelet consumption and thrombin generation. Br J Haematol. 1997;98:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Miyata T, Yokoyama I, Todo S, Tzakis A, Selby R, Starzl TE. Endotoxaemia, pulmonary complications, and thrombocytopenia in liver transplantation. Lancet. 1989;2:189-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Munoz SJ, Carabasi AR, Moritz MJ, Jarrell BE, Maddrey WC. Postoperative thrombocytopenia in liver transplant recipients: Prognostic implications and treatment with high dose of gamma-globulin. Transplant Proc. 1989;21:3545-3546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Plevak DJ, Halma GA, Forstrom LA, Dewanjee MK, O’Connor MK, Moore SB, Krom RA, Rettke SR. Thrombocytopenia after liver transplantation. Transplant Proc. 1988;20:630-633. [PubMed] |
| 8. | Bachmann R, Bachmann J, Lange J, Nadalin S, Königsrainer A, Ladurner R. Incidence of heparin-induced thrombocytopenia type II and postoperative recovery of platelet count in liver graft recipients: a retrospective cohort analysis. J Surg Res. 2014;186:429-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | McCaughan GW, Herkes R, Powers B, Rickard K, Gallagher ND, Thompson JF, Sheil AG. Thrombocytopenia post liver transplantation. Correlations with pre-operative platelet count, blood transfusion requirements, allograft function and outcome. J Hepatol. 1992;16:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Takahashi K, Nagai S, Safwan M, Liang C, Ohkohchi N. Thrombocytopenia after liver transplantation: Should we care? World J Gastroenterol. 2018;24:1386-1397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 11. | Lesurtel M, Graf R, Aleil B, Walther DJ, Tian Y, Jochum W, Gachet C, Bader M, Clavien PA. Platelet-derived serotonin mediates liver regeneration. Science. 2006;312:104-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 595] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 12. | Murata S, Ohkohchi N, Matsuo R, Ikeda O, Myronovych A, Hoshi R. Platelets promote liver regeneration in early period after hepatectomy in mice. World J Surg. 2007;31:808-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 138] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 13. | Starlinger P, Assinger A, Haegele S, Wanek D, Zikeli S, Schauer D, Birner P, Fleischmann E, Gruenberger B, Brostjan C, Gruenberger T. Evidence for serotonin as a relevant inducer of liver regeneration after liver resection in humans. Hepatology. 2014;60:257-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 14. | Kurokawa T, Zheng YW, Ohkohchi N. Novel functions of platelets in the liver. J Gastroenterol Hepatol. 2016;31:745-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Nocito A, Georgiev P, Dahm F, Jochum W, Bader M, Graf R, Clavien PA. Platelets and platelet-derived serotonin promote tissue repair after normothermic hepatic ischemia in mice. Hepatology. 2007;45:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 132] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 16. | Myronovych A, Murata S, Chiba M, Matsuo R, Ikeda O, Watanabe M, Hisakura K, Nakano Y, Kohno K, Kawasaki T, Hashimoto I, Shibasaki Y, Yasue H, Ohkohchi N. Role of platelets on liver regeneration after 90% hepatectomy in mice. J Hepatol. 2008;49:363-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Lesurtel M, Raptis DA, Melloul E, Schlegel A, Oberkofler C, El-Badry AM, Weber A, Mueller N, Dutkowski P, Clavien PA. Low platelet counts after liver transplantation predict early posttransplant survival: The 60-5 criterion. Liver Transpl. 2014;20:147-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | Takahashi K, Nagai S, Putchakayala KG, Safwan M, Li AY, Kane WJ, Singh PL, Collins KM, Rizzari MD, Yoshida A, Schnickel GT, Abouljoud MS. Prognostic impact of postoperative low platelet count after liver transplantation. Clin Transplant. 2017;31:e12891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5754] [Cited by in RCA: 9950] [Article Influence: 585.3] [Reference Citation Analysis (0)] |
| 20. | Desai NM, Mange KC, Crawford MD, Abt PL, Frank AM, Markmann JW, Velidedeoglu E, Chapman WC, Markmann JF. Predicting outcome after liver transplantation: Utility of the model for end-stage liver disease and a newly derived discrimination function. Transplantation. 2004;77:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 211] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 21. | Bolondi G, Mocchegiani F, Montalti R, Nicolini D, Vivarelli M, De Pietri L. Predictive factors of short term outcome after liver transplantation: A review. World J Gastroenterol. 2016;22:5936-5949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 64] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 22. | Rana A, Hardy MA, Halazun KJ, Woodland DC, Ratner LE, Samstein B, Guarrera JV, Brown RS, Emond JC. Survival outcomes following liver transplantation (SOFT) score: A novel method to predict patient survival following liver transplantation. Am J Transplant. 2008;8:2537-2546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 350] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 23. | Li L, Wang H, Yang J, Jiang L, Yang J, Wang W, Yan L, Wen T, Li B, Xu M. Immediate Postoperative Low Platelet Counts After Living Donor Liver Transplantation Predict Early Allograft Dysfunction. Medicine (Baltimore). 2015;94:e1373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Pereboom IT, Lisman T, Porte RJ. Platelets in liver transplantation: Friend or foe? Liver Transpl. 2008;14:923-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 25. | Chatzipetrou MA, Tsaroucha AK, Weppler D, Pappas PA, Kenyon NS, Nery JR, Khan MF, Kato T, Pinna AD, O'Brien C, Viciana A, Ricordi C, Tzakis AG. Thrombocytopenia after liver transplantation. Transplantation. 1999;67:702-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Eyraud D, Granger B, Ionescu C, Fratéa S, Darnat S, Vaillant JC, Siksik JM, Hannoun L, Coriat P. Thrombocytopenia, splenomegaly, and portal blood flow in patients who have undergone liver transplantation for cirrhosis. Liver Transpl. 2012;18:340-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Cywes R, Packham MA, Tietze L, Sanabria JR, Harvey PR, Phillips MJ, Strasberg SM. Role of platelets in hepatic allograft preservation injury in the rat. Hepatology. 1993;18:635-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 105] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Chang FY, Singh N, Gayowski T, Wagener MM, Mietzner SM, Stout JE, Marino IR. Thrombocytopenia in liver transplant recipients: Predictors, impact on fungal infections, and role of endogenous thrombopoietin. Transplantation. 2000;69:70-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 86] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Takahashi K, Nagai S, Putchakayala KG, Safwan M, Gosho M, Li AY, Kane WJ, Singh PL, Rizzari MD, Collins KM, Yoshida A, Abouljoud MS, Schnickel GT. Prediction of biliary anastomotic stricture after deceased donor liver transplantation: The impact of platelet counts - a retrospective study. Transpl Int. 2017;30:1032-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Kim J, Yi NJ, Shin WY, Kim T, Lee KU, Suh KS. Platelet transfusion can be related to liver regeneration after living donor liver transplantation. World J Surg. 2010;34:1052-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Han S, Park HW, Song JH, Gwak MS, Lee WJ, Kim G, Lee SK, Ko JS. Association Between Intraoperative Platelet Transfusion and Early Graft Regeneration in Living Donor Liver Transplantation. Ann Surg. 2016;264:1065-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |