Published online Jan 27, 2019. doi: 10.4254/wjh.v11.i1.37
Peer-review started: September 27, 2018
First decision: October 16, 2018
Revised: November 14, 2018
Accepted: December 31, 2018
Article in press: January 1, 2019
Published online: January 27, 2019
Processing time: 120 Days and 4.9 Hours
Slaughterhouse workers (SHW) are at increased risk of hepatitis which can occur due to different organisms and should be investigated for viral, bacterial, and parasitic organisms. Slaughter house personnel including butchers are at a higher risk of infections from cuts and blood-letting, with the possible risk of the transmission of blood-borne pathogens to their colleagues. The objective of this review is to evaluate the common etiologies of hepatitis in SHW which will assist in the assessment of these patients presenting with transaminitis. Types of Microorganisms causing hepatitis with their reservoirs, routes of transmission, laboratory diagnosis, clinical features, treatment options and preventive strategies are included in this review. Proper investigation and awareness is of utmost importance as it causes significant financial constraints derived from workers health cost and from livestock production losses when the disease is confirmed. The work up is essential because infected workers might be a source of infections to other colleagues, family and the consumers.
Core tip: Butchers and other personnel of slaughterhouse belong to a high-risk occupation and are at increased risk of transmissible diseases. This group of patients presenting to the healthcare providers with hepatitis require extensive work up to find the causative agent. In this review article, we have searched a list of organisms associated with hepatitis in slaughterhouse workers. We have also proposed an algorithm for the evaluation and management of hepatitis in these workers. It is critical to work up hepatitis in these infective patients because they might be a source of transmissible diseases to their colleagues, family members and consumers.
- Citation: Tariq H, Kamal MU, Makker J, Azam S, Pirzada UA, Mehak V, Kumar K, Patel H. Hepatitis in slaughterhouse workers. World J Hepatol 2019; 11(1): 37-49
- URL: https://www.wjgnet.com/1948-5182/full/v11/i1/37.htm
- DOI: https://dx.doi.org/10.4254/wjh.v11.i1.37
Slaughterhouse workers (SHW) are at a higher risk of infectious hepatitis that can be multifactorial and should be evaluated for viral, bacterial, and parasitic organisms. Viral infections are commonly sustained by certain reservoirs e.g., the hepatitis B virus (HBV) has been found in gorillas, monkeys and cattle[1,2]. Slaughter house personnel including butchers are at a higher risk of infections from cuts and blood-letting, with the possible risk of the transmission of blood-borne pathogens to their colleagues[2,3].
The objective of this review is to evaluate the common etiologies of hepatitis in SHW that will be helpful for the assessment of the patients coming with hepatitis. It causes significant financial constraints derived from workers health cost and from livestock production losses if the disease is confirmed. This is also certainly important, as, the infected workers might be a source of infections to other colleagues, family, and the consumers.
HBV: Several cases of HBV infection have been reported in the SHW. This not only affects their colleagues but also their families[4]. HBV can be transmitted parenterally, perinatally, sexually, and horizontally. Horizontal transmission occurs via open wounds and by saliva, which is an important concern for the SHW as studies have reported infection in the SHW via this transmission[5].
SHW is also a high-risk group for HBV infections like the surgeons and blood donors etc. Many studies have compared rates of HBV infection among the high-risk groups, including the SHW, in the hospital and community based locations and found that HBV infection was quite higher in the community than reported in the hospital cases[6]. Butchers are exposed to the public during encounters of sale; also, infection of the cattle from the SHW is a concern, which can affect the community. SHW should be considered high-risk population for HBV infection, like health care workers and be recommended HBV vaccination[3]. A recent update by American Association for the Study of Liver Diseases in regard to the high-risk who should be screened for HBV infection , identifies the slaughter house workers at risk , in view of the blood or the body fluid exposure and requires post-exposure prophylaxis[7].
Hepatitis E virus (HEV): HEV infection in humans is usually rare in developed countries but is more frequent in many developing countries[8,9]. Majority of HEV infections are unremarkable and self-limiting but can lead to acute liver failure in immunosuppressed patients[10,11].
HEV is reported in the wild boar, camels, cows and goats. In addition to the fecal-oral route, consumption of contaminated water, raw or undercooked animal tissues or organs such as liver can be a source of HEV infection[12-14].
Rift Valley fever virus (RVF): RVF virus is an RNA virus responsible for causing significant illness both in humans and animals[15]. Mostly the patients are asymptomatic or have mild flu like disease, but small percentages of patients develop a life-threatening illness with ocular disease, hepatitis, encephalitis or hemorrhagic fever[16,17].
Humans are mostly affected by having contact with the blood and the fluids of the animals infected with RVF virus during slaughtering, taking care of sick animals or during the animal birth. Therefore, SHW and cattlemen are at an increased for the infection due to direct exposure to the infected animals[18,19].
Q fever: Q fever is a zoonotic disease caused by the bacterium, Coxiella burnetii. The disease was first seen in Australia among the meat packers and mentioned as “abattoir fever”[20]. The organism has extensive and worldwide reservoir, mostly ungulates, can be transmitted in the urine, feces, milk and parturition products of infected animals[21]. Humans dealing with animals are mostly infected after the inhalation of contaminated aerosols in the air[22].
The disease may present acutely with a wide range of symptoms like fever, pneumonia, hepatitis and different neurologic manifestations ranging from simple headache to meningitis, encephalitis or both. Chronic disease can manifest later, after an initial infection, as endocarditis, or chronic fatigue syndrome[21,23]. Many outbreaks have been reported in the different parts of the world. Wilson et al[24] investigated the largest outbreak of Q fever in Scotland reporting about 110 cases, which occurred in the setting of the co-located slaughterhouse. In Korea, Chu et al[25] also reported the seroconversion of about 10.2% among SHW for Q fever. In their research, the critical risk factor was the contact with cattle blood especially around the mouth. Esmaeili et al[26] found a higher seroprevalence among butchers and SHW in Iran in a large cross-sectional study. The total seroprevalence of Q fever among subjects in the study was 22.5%.
Salmonellosis: Typhoid fever, a common infectious disease, typically manifests as acute systemic disease involving multiple body organs, and difficult to distinguish clinically from other infections. The liver can also be affected resulting in hepatomegaly and transaminitis. Although, acute hepatitis due to salmonellosis is a rare entity, delay in the treatment leads to increase mortality[27]. This is a very common foodborne illness and associated with contaminated poultry meat and pork[28]. Multiple studies through the world have evaluated the risks factors causing the transmission of salmonella to the humans[29,30]. SHW dealing with the poultry directly are at a higher risk for getting infected.
Campylobacter jejuni:Campylobacter jejuni, a gram-negative bacterium, is responsible for causing a major food borne gastroenteritis in humans[31]. It also causes a variety of extra-intestinal manifestations including meningitis, hepatitis, gram negative bacteremia and cardiac complications[32]. The common mode of transmission to humans include eating and handling of contaminated poultry[33]. Broiler flocks are infected in the poultry houses. Many studies have discussed factors responsible for the infection of the broiler flocks[34-36].
Leptospirosis: Leptospirosis is a zoonotic disease caused by spirochetes belonging to the genus Leptospira. Different domestic animals host this bacterium and include cattle, pigs, and sheep[37]. Humans are infected through the broken skin or exposure to contaminated water and soil from infected urine of animals[38]. SHW are increasingly exposed to Leptospira species and have noted to have the higher seroprevalence values twice those of other non-risk occupations[39,40]. The identified factors leading to increasing prevalence in SHW are smoking, drinking at work, and poor hygiene at work[38,41]. Most of the infected cases are mild but more severe clinical spectrum of leptospirosis include hepatitis, and Weil’s disease with renal failure and jaundice[42]. Esmaeilli et al did a serological survey of leptospirosis among different population groups in Iran. The major risk factors associated with higher prevalence included eating hare meat and exposure to dead animals[43].
Bovine tuberculosis: Although, an uncommon cause of hepatitis in slaughter workers, Mycobacterium bovis (M. Bovis) is transmitted from animals to humans, either through the ingestion of animal products or through the airborne inhalation of spores[44]. Both domestic and domesticated animals are infested by M. Bovis and include cattle, sheep, pigs, goats, cats, dogs and horses[45]. The disease spectrum is similar to that of Mycobacterium tuberculosis and includes fever, night sweats, and weight loss. The other symptoms result, depending on the tissue of the body infected by the organism[46].
Brucellosis: Brucellosis, a zoonotic bacterial illness affects both animals and humans worldwide. It spreads systemically and mainly affects the lymph nodes, liver, spleen and bone marrow. The intracellular location of the bacterium is responsible for chronic infections. It commonly infects the gastrointestinal tract, but brain, nerves, GU, skin and hepatobiliary systems are also involved[47-49]. Brucella hepatitis usually occurs in the chronic granulomatous form with mild transaminitis, but acute cases have also been reported[49-51]. Brucellosis is transmitted to humans via the intake of contaminated milk products or during physical contact with infected tissues of the animals or inhalation of contaminated aerosols[52,53]. SHW, shepherds, veterinary doctors, meat packing staff and lab staff are at an increased risk for the infection, due to increased exposure to the contaminated tissues[53].
Clostridium perfringens (C. perfringens): C. perfringens cause food poisoning from food contaminated with the organism such as eating undercooked meats. Toxin mediated illness is usually self-limited and cause abdominal pain, nausea and diarrhea and last for about 6-24 h[54]. The worst form of illness is gas gangrene. Gas gangrene with C. perfringens typically presents with necrosis of the soft tissue, gas production, and septicemia. It rarely involves internal solid viscera like liver, kidneys, heart, etc. Hepatic infection possibly results from the extension of infection into the biliary tree and then into the liver[55,56]. Immunocompromised patients, such as liver transplant patients, are at an increased risk of infection with C. perfringens[55,57]. Very few cases of C. perfringens causing allograft failure are reported in the literature[58-60]. The disease has also been reported in the animals especially broiler and may be a source of transmission to the immunocompromised SHW[61].
Chlamydia psittaci: Ornithosis is a bacterial disease caused by Chlamydia psittaci and transmitted from infected parrots, pigeons, sparrows and many other bird species. The patients usually inhale the infected organism in the form of aerosolized respiratory secretions or dried feces or contact from the infected tissues of the birds[62,63]. The common symptoms of Psittacosis include influenza-like illness but can worsen to severe pneumonia and other non-respiratory health problems. Transaminitis with hepatomegaly and jaundice has been reported in the literature[62,64].
Echinococcosis: Echinococcosis is a zoonotic parasitic infection caused by larval form of different species belonging to the Echinococcus tapeworms[65]. Cystic echinococcosis represents a persistent zoonosis and one of the etiologies of parasitic hepatitis. Humans are mostly infected via ingesting parasitic eggs excreted within the feces of the definitive hosts, resulting in the development of cysts, primarily in the lungs and liver. This causes damage as they enlarge resulting in hepatitis and pneumonitis[65]. Some cases are reported in France and Moldova but the disease is likely present worldwide[66,67].
The prevalence in South America ranges from 20%-95% in some areas[68]. Although intermediate hosts are variable, the common ones include sheep, goats, pigs, camels, horses, and cattle 65]. Human Liver is mainly affected by the sheep strain (G1) resulting in echinococcal cysts[69].
Toxoplasma gondii: Toxoplasma gondii is a common parasitic infection with varied clinical presentations. The disease ranges from symptomless stage to a wide spectrum of clinical presentation ranging from fever and lymphadenopathy to multi-organ involvement including hepatitis, encephalomyelitis or myocarditis[70,71]. Toxoplasma gondii is commonly transmitted via drinking water or eating undercooked/raw meat contaminated with tissue cysts[72]. Many studies have reported an increased prevalence of infection in the SHW[70,73].
Trichinosis: Trichinosis is a parasitic infection transmitted commonly by ingestion of partially cooked/uncooked or raw pork contaminated with the cysts or larvae[74]. The incubation period is variable from a few days to weeks depending on the stage of the transmission (enteral phase or parenteral phase). The disease has acute and chronic phases. The earlier manifestations of trichinellosis include gastrointestinal upset with diarrhea along with, fever, muscle aches and persists while the larvae migrate throughout the body[74]. Larval tissue penetration and migration in the body is responsible for immune-mediated inflammatory reaction resulting in eosinophilia. Severe illness causes cardiac, neurological, hepatic manifestations and thromboembolic disease. Hepatic involvement is rare but has been reported in the literature[75,76].
Candidiasis and other fungal infections can be transmitted to the SHW but rarely cause hepatitis in an immunocompetent patient. The possibility of hepatitis is usually in the patient with systemic candidiasis or severe sepsis due to candidiasis.
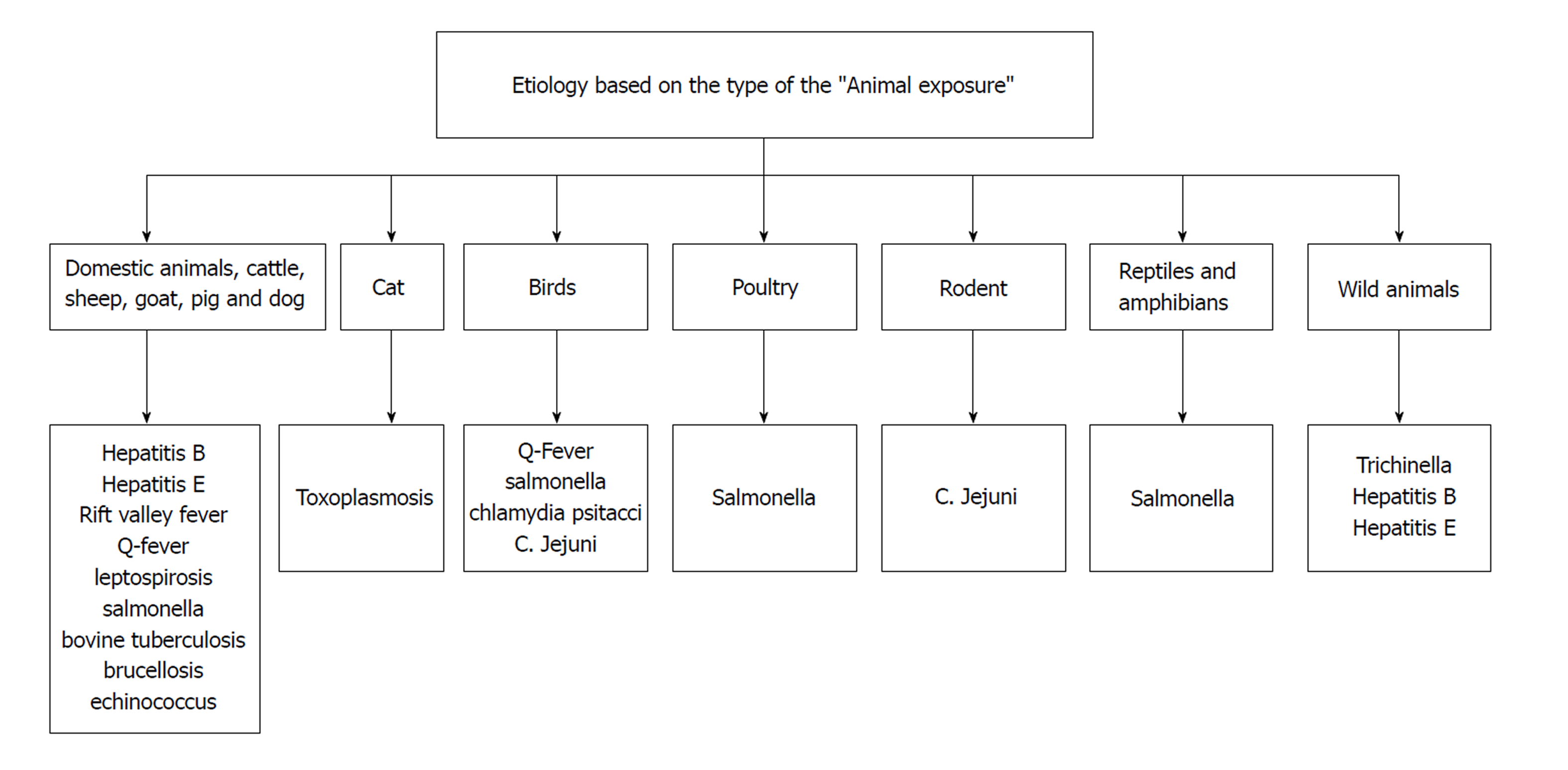
There is a wide array of the etiologies causing hepatitis in the patients working in the slaughter house (Table 1). The initial evaluation of hepatitis should guide whether it is an isolated presentation, or a manifestation of the systemic illness. The predominant hepatitis presentation is usually viral in etiology which includes HBV or HEV. Management is usually observant in acute disease, requires clinical monitoring to evaluate for possible liver failure. As per current guideline , the anti-viral medication are served for the fulminant presentation. The inquiry regarding the prevalent livestock diseases in the community can help in diagnosis. The type of the animal exposure will assist in identifying the causative organism. This is discussed in Figure 1.
| Microorganisms | Reservoirs of infection | Routes of transmission | Laboratory diagnosis | Common clinical features | Treatment options | Preventive strategies |
| HBV | (1) Gorillas; (2) Chimpanzees; and (3) Cows[77] | (1) Parenterally; (2) Perinatally; (3) Sexually; and (4) Horizontally[5] | Serology: (1) HBsAg; (2) HBeAg; (3) Anti-HBc IgM; (4) Anti-HBc IgG; (5) Anti-HBe; and (6) HBV DNA[77] | (1) Constitutional symptoms; (2) Anorexia; (3) Nausea; (4) Vomiting; (5) Low-grade fever; (6) Myalgia; (7) Disordered gustatory acuity and smell; (8) RUQ pain; (9) Hepatic encephalopathy; (10) Ascites; (11) Gastrointestinal bleeding; and (12) Coagulopathy[77] | (1) NtRTIs: (a) Tenofovir; and (b) Adefovir; (2) NRTIs: (a) Entecavir; (b) Elbivudine; (c) Lamivudine; and (3) PEG- interferon -a 2a, interferon alfa-2b[78] | (1) Pre-exposure vaccination; and (2) Post exposure prophylaxis with vaccination and immunoglobulin’s depending on clinical status[3] |
| HEV | (1) Wild boar; (2) Camels; (3) Cows; (4) Goats; and (5) Pigs[13] | Fecal-oral route[14] | Anti-HEV IgM[14] | (1) Prodromal-phase; (2) Myalgia; (3) Arthralgia; (4) Fever; (5) Anorexia; (6) Nausea/vomiting; (7) Weight loss; (8) Right upper quadrant pain; (9) Icteric-phase; (10) Jaundice; (11) Dark urine; (12) Light-colored stools; (13) Pruritus; and (14) Right upper quadrant tenderness and hepatomegaly[12,14] | (1) Mostly are self-limited; and (2) Current treatment options: (a) Ribavirin; (b) Pegylated interferon for chronic infection in immune-compromised[79] | (1) Hygiene; and (2) Recombinant vaccines have demonstrated efficacy against HEV. (Available in China)[79] |
| RVF | Livestock[15] | (1) Contact with the blood and the fluids of the animals; and (2) Infected mosquitoes[15] | (1) Both IgM and IgG antibodies are specific to RVF virus; and (2) PCR of the antigens[17] | Mostly the patients are asymptomatic or have mild flu like disease, but a small percentage of patients may develop life threatening illness with ocular disease, hepatitis, encephalitis or hemorrhagic fever[16,17] | (1) Most human cases of RVF are mild and self-limiting; and (2) A specific treatment for RVF has not established[80] | (1) Avoid contact with blood, body fluids, or tissues of infected animals and protecting themselves against mosquitoes and other bloodsucking insects; and (2) Use of mosquito repellents and bed nets are two effective methods[80] |
| Q fever | (1) Domestic mammals, especially ungulates (cattle, sheep, and goats); and (2) Also has been found in wild mammals, birds, and arthropods[21] | (1) Transmitted via the urine, feces, milk and parturition products of infected animals; and (2) Aerosolized breathing in dust that has been contaminated by infected animal feces, urine, milk, and birth products[22] | (1) Serologic testing with PCR in the early stages of acute illness[81]; and (2) A fourfold rise in IgG antibody titer between acute and convalescent samples | (1) Acute: (a) Fever; (b) Pneumonia; (c) Hepatitis; and (d) Neurologic manifestations ranging from a simple headache to meningitis, encephalitis or both; and (2) Chronic infection: (a) Endocarditis; and (b) Chronic fatigue syndrome[21,23] | (1) Acute illness: Self-limited but 2 wk of doxycycline recommender; and (2) Chronic Q fever: Requires several months of antibiotics with a combination of antibiotics including doxycycline and hydroxychloroquine[81] | (1) Avoiding contact with animals, especially while animals are giving birth; and (2) Do not consume raw milk or raw milk products[81] |
| Salmonella | Intestinal tracts of humans and other animals, including poultry, other birds, amphibians, and reptiles[82] | Foodborne illness associated with contaminated poultry meat and pork[28-30] | (1) Serotyping and DNA fingerprinting; (2) Blood cultures; (3) PCR using H1-d primers; and (4) The Widal test or Typhidot for serology is rarely used now[82] | (1) Systemic disease involving multiple body organs; and (2) Liver can also be affected resulting in hepatomegaly and transaminitis[27,28] | Antibiotics based on sensitivities[82] | (1) Do not eat or drink foods containing raw eggs, or raw (unpasteurized) milk; and (2) Wash hands, kitchen work surfaces, and utensils with soap and water immediately after they have been in contact with raw meat or poultry[82] |
| Campylobacter jejuni | Wildlife reservoirs: (a) Wild birds species include migratory birds—ranes, ducks, geese and seagulls; and (b) Rodents and insects[83] | (1) Eating and handling of contaminated poultry, water and milk; and (2) Contact through the feces of a dog or cat[33] | (1) Serological diagnosis with ELISA; (2) PCR to detect Campylobacter jejuni in stool; and (3) Detection of antigens in stool specimens[84] | (1) Food borne gastroenteritis; (2) Extra intestinal manifestations; (3) Meningitis; (4) Hepatitis; (5) Bacteremia; and (6) Cardiac complications[31,32] | (1) Azithromycin and Fluoroquinolones; and (2) Antimicrobial susceptibility testing is essential before treatment[84] | Good hygiene[84] |
| Leptospirosis | (1) Domestic animals; (2) Cattle; (3) Pigs; and (4) Sheep[37] | Humans are infected through the broken skin or exposure to contaminated water and soil from infected urine of animals[38] | (1) DNA PCR; (2) Urine is the most reliable body fluid to study because the urine contains leptospires paired; (3) Antileptospira antibodies; and (4) MAT of acute and convalescent serum specimens[85] | (1) Most of the infected cases are mild; (2) Severe disease; (2) Hepatitis; and (3) Weil’s disease with renal failure and jaundice[42] | (1) Mild leptospirosis; (2) Doxycycline; (3) Ampicillin; (4) Amoxicillin; (5) Severe leptospirosis; (6) Intravenous penicillin G; (7) Third generation cephalosporin i.e. cefotaxime and ceftriaxone; and (8) Alternative regimens include ampicillin, amoxicillin, or erythromycin[86] | Good hygiene[42] |
| Bovine tuberculosis | (1) Both domestic and domesticated animals; (2) Cattle; (3) Sheep; (4) Pigs; (5) Goats; (7) Cats; (8) Dogs; and (9) Horses[45] | (1) Animals to humans; (2) Ingestion of animal products; and (3) Airborne inhalation of spores[44] | (1) AFB staining; (2) Mycobacterial cultures; (3) Molecular testing for mycobacterial DNA; (4) TST; and (5) IGRAs[87] | (1) Fever; (2) Night sweats; (3) Weight loss; and (4) The other symptoms depend on the tissue of the body infected by the organism[46] | Two months of isoniazid, rifampin, and ethambutol , followed by seven months of isoniazid and rifampin[46] | (1) Immunization with BCG vaccine; and (2) Treatment of latent infection[88] |
| Brucellosis | (1) Domestic animals; (2) B. abortus in cattle; (3) B. melitensis in sheep, goats, and camels; and (4) B. suis in swine[47,48] | (1) Intake of contaminated milk products; (2) Physical contact with infected tissues of the animals; and (3) Inhalation of contaminated aerosols[48] | (1) Cultures; (2) Serology; and (3) PCR[48] | (1) It commonly infects the GI tract but brain, nerves, GU, skin and hepatobiliary systems can also be involved; and (2) Brucella hepatitis usually occurs in the chronic granulomatous form with mild transaminitis but acute cases have been reported also[48-50] | (1) Combination of antibiotics is used; (2) Doxycycline and streptomycin; and (3) Based on the severity and location of infection, multiple combinations and longer durations are needed[89] | (1) Vaccination of domestic livestock, serologic testing, quarantine of herds, and slaughter of infected animals; (2) Protection of slaughterhouse workers; (3) Separated areas for killing from other processing areas; (4) Use of protective clothing and disinfectants; (5) Control of air circulation; and (6) Pasteurization of milk[89] |
| C. perfringens | (1) Soil; (2) Water; (3) Air; (4) Feces of healthy and infected individuals; (5) Gastrointestinal tract of humans and animals; and (7) Variety of dehydrated and processed foods[90] | (1) Food Poisoning; (2) Ingestion of large number of C. perfringens vegetative cells present in the contaminated food; (3) Gas gangrene; and (4) Contamination of wounds with dirt or any foreign material contaminated with C. perfringens[90] | (1) Cultures; (2) Imaging of the infected sites; and (3) Tissue biopsy with culture and Gram stain[90] | (1) Gastroenteritis[54,55]; (2) Gas gangrene; (3) Necrosis of the soft tissue; (4) Septicemia; (5) It rarely involves internal solid viscera like liver, kidneys, heart, etc.; and (6) Hepatic infection possibly results from the extension of infection into the biliary tree and then into the liver[56] | (1) Treatment of gas gangrene; (2) Surgical debridement; (3) Antibiotic therapy; and (4) Supportive measures[90] | (1) Protective materials like Lab coat, gloves and eye protection when dealing with infected materials[90]; and (2) Vaccination of non-immunized trauma individuals |
| Chlamydia psittaci | (1) Infected birds; (2) Parrots; (3) Pigeons; and (4) Sparrows[91] | (1) Aerosolized respiratory secretions; (2) Dried feces; and (3) Contact from the infected tissues of the birds[62,63] | (1) Complement fixation; (2) Micro immunofluorescence; and (3) PCR[91] | (1) Mild disease; (2) Influenza-like illness; (3) Severe disease; (4) Pneumonia; (5) Transaminitis with hepatomegaly and jaundice; and (6) Cardiac involvement[62,64] | (1) Antibiotics; (2) Tetracycline; and (3) Macrolides[91] | Follow precautions when handling and cleaning birds and cage[91] |
| Echinococcosis | (1) Sheep; (2) Goats; (3) Pigs; (4) Camels; (5) Horses; (6) Cattle; and (7) Human Liver is mainly affected by the sheep strain (G1) resulting in echinococcal cysts[65,69] | Ingestion of Echinococcal eggs excreted within the feces of the definitive host[92] | (1) Indirect hemagglutination test; (2) ELISA; and (3) Imaging tests for the location and size of the cysts[92] | (1) Development of cysts, primarily in the lungs and liver; and (2) This causes damage as they enlarge resulting in hepatitis and pneumonitis[65] | (1) Surgical removal of intact cysts; (2) Chemotherapy; (3) Albendazole and/or Praziquantel; (4) Chemotherapy is recommended 4 wk before, and for 1 mo after the surgery; and (5) Percutaneous aspiration, injection, re-aspiration is used in patients with inoperable intra-parenchymatous cysts[92] | Protective materials like Lab coat, gloves and eye protection when dealing with infected materials[92] |
| Toxoplasma gondii | (1) The definitive hosts are cats; and (2) The intermediate hosts are warm-blooded animals, including most mammals and birds[93] | (1) Consumption of poorly-cooked infected meat; (2) Ingestion of water, food, or milk contaminated with oocysts; (3) Inhalation of aerosols containing oocysts; (4) Contact with sand or soil contaminated by cat feces; (5) Transmission is also possible through blood transfusions and organ transplants; and (6) Transplacental if mother is infected[72] | (1) Positive serology for IgM and IgG antibodies; and (2) PCR[93] | (1) Fever; (2) Lymphadenopathy; (3) Multiorgan involvement; (4) Hepatitis; (5) Encephalomyelitis; and (6) Myocarditis[70,71] | (1) Spiramycin; (2) Can be taken by women in their first trimester to prevent transplacental transmission; (3) Sulfadiazine and folinic acid; and (4) For pregnant women in their third trimester[93] | (1) Protective materials like Lab coat, gloves and eye protection when dealing with infected materials[92]; and (2) Testing for toxoplasmosis in females before pregnancy[93] |
| Trichinosis | Different species are found world-wide in carnivorous and omnivorous animals like bears, foxes, walruses, hyenas, Pigs and cougars[94] | Ingestion of partially cooked/ uncooked or raw pork contaminated with the cysts or larvae[69] | (1) ELISAs; (2) Indirect immunofluorescence; (3) Latex agglutination; (4) Western blot; (5) Muscle biopsy; and (6) PCR[94] | (1) Diarrhea; (2) Fever; (3) Muscle aches; (4) Cardiac, neurological, and thromboembolic disease; and (5) Hepatic involvement is rare but has been reported in the literature[69] | (1) Albendazole; and (2) Mebendazole[94] | Consumption of properly cooked meat[94] |
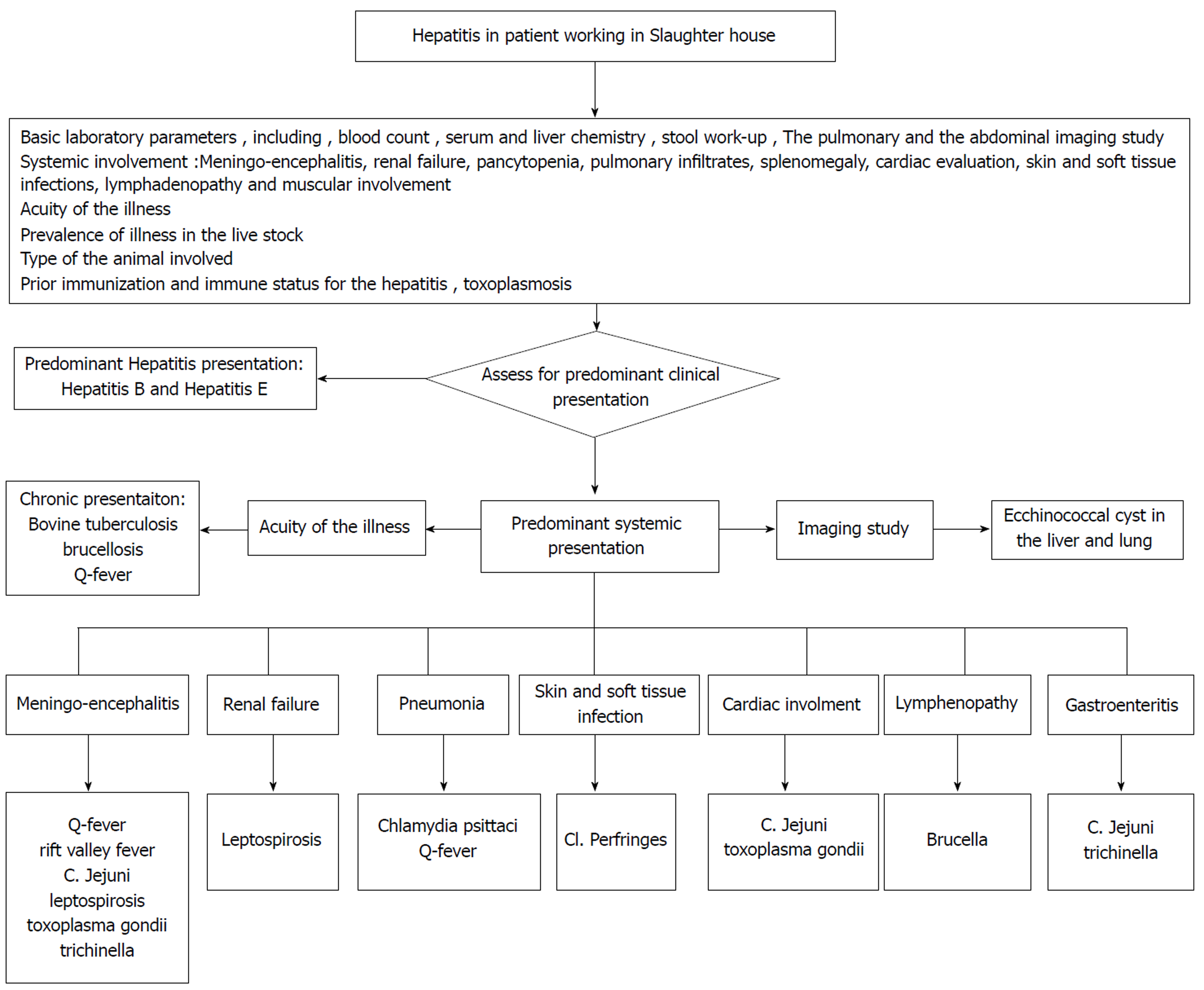
The systemic manifestation should be evaluated for meningoencephalitis, renal failure, pancytopenia, pulmonary infiltrates, splenomegaly, cardiac diseases, skin and soft tissue infections, lymphadenopathy and muscular diseases. Management (anti-microbial) largely varies if the infection is bacterial or parasitic, hence the initial serology and imaging studies should guide to differentiate between the etiologies. Some bacterial diseases present as acute systemic illness and are usually from the atypical organism. Thus, empiric treatment with doxycycline may be considered while awaiting the bacterial serology. The bacterial infections, like bovine tuberculosis, will have chronic onset and will need thorough evaluation before consideration of anti-tuberculosis treatment. The parasitic etiology can be suspected based on characteristic imaging finding like the echinococcal cysts in liver or lungs. The other parasitic causes can be ruled out based on the serum serology and stool examination for the ova and parasites. Figure 2 elaborates a proposed algorithm for the assessment and management of slaughter house workers presenting with hepatitis.
This review concludes that SHW are high-risk occupational group for hepatic infections and there should be regular screening tests against the transmissible infections. All SHW should be instructed to see medical attention as soon as they there an event that might lead to transmission of disease. This is especially important for the workers directly involved in animal slaughtering. Individuals involved in transportation and handling of animal residues, or inspection the carcasses may be at a lower risk.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Qi XS Sirin G S- Editor: Wang JL L- Editor: A E- Editor: Tan WW
| 1. | Makuwa M, Souquière S, Telfer P, Bourry O, Rouquet P, Kazanji M, Roques P, Simon F. Hepatitis viruses in non-human primates. J Med Primatol. 2006;35:384-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Starkman SE, MacDonald DM, Lewis JC, Holmes EC, Simmonds P. Geographic and species association of hepatitis B virus genotypes in non-human primates. Virology. 2003;314:381-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Mevorach D, Brezis M, Ben Yishai F, Sadeh T, Shouval D, Eliakim R. Increased risk of exposure to hepatitis B infection among butchers sharing knives. Am J Med. 1999;106:479-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Mijch AM, Barnes R, Crowe SM, Dimitrakakis M, Lucas CR. An outbreak of hepatitis B and D in butchers. Scand J Infect Dis. 1987;19:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Mevorach D, Eliakim R, Brezis M. Hepatitis B--an occupational risk for butchers? Ann Intern Med. 1992;116:428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Ola SO, Otegbayo JA, Yakubu A, Odaibo GN, Olaleye DO. Risk of hepatitis B virus in the slaughter house. Trop Doct. 2008;38:249-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 2845] [Article Influence: 406.4] [Reference Citation Analysis (0)] |
| 8. | Clemente-Casares P, Ramos-Romero C, Ramirez-Gonzalez E, Mas A. Hepatitis E Virus in Industrialized Countries: The Silent Threat. Biomed Res Int. 2016;2016:9838041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Ofori-Asenso R, Agyeman AA. Hepatitis E infection among Ghanaians: a systematic review. Infect Dis Poverty. 2017;6:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Li S, Liu M, Cong J, Zhou Y, Miao Z. Detection and Characterization of Hepatitis E Virus in Goats at Slaughterhouse in Tai'an Region, China. Biomed Res Int. 2017;2017:3723650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Davern TJ, Chalasani N, Fontana RJ, Hayashi PH, Protiva P, Kleiner DE, Engle RE, Nguyen H, Emerson SU, Purcell RH, Tillmann HL, Gu J, Serrano J, Hoofnagle JH; Drug-Induced Liver Injury Network (DILIN). Acute hepatitis E infection accounts for some cases of suspected drug-induced liver injury. Gastroenterology. 2011;141:1665-72.e1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 253] [Article Influence: 18.1] [Reference Citation Analysis (2)] |
| 12. | Purcell RH, Emerson SU. Hepatitis E: an emerging awareness of an old disease. J Hepatol. 2008;48:494-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 494] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 13. | Okano H, Takahashi M, Isono Y, Tanaka H, Nakano T, Oya Y, Sugimoto K, Ito K, Ohmori S, Maegawa T, Kobayashi M, Nagashima S, Nishizawa T, Okamoto H. Characterization of sporadic acute hepatitis E and comparison of hepatitis E virus genomes in acute hepatitis patients and pig liver sold as food in Mie, Japan. Hepatol Res. 2014;44:E63-E76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Panda SK, Thakral D, Rehman S. Hepatitis E virus. Rev Med Virol. 2007;17:151-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 167] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 15. | Bird BH, Ksiazek TG, Nichol ST, Maclachlan NJ. Rift Valley fever virus. J Am Vet Med Assoc. 2009;234:883-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 315] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 16. | Madani TA, Al-Mazrou YY, Al-Jeffri MH, Mishkhas AA, Al-Rabeah AM, Turkistani AM, Al-Sayed MO, Abodahish AA, Khan AS, Ksiazek TG, Shobokshi O. Rift Valley fever epidemic in Saudi Arabia: epidemiological, clinical, and laboratory characteristics. Clin Infect Dis. 2003;37:1084-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 341] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 17. | de St Maurice A, Nyakarahuka L, Purpura L, Ervin E, Tumusiime A, Balinandi S, Kyondo J, Mulei S, Tusiime P, Manning C, Rollin PE, Knust B, Shoemaker T. Rift Valley Fever: A survey of knowledge, attitudes, and practice of slaughterhouse workers and community members in Kabale District, Uganda. PLoS Negl Trop Dis. 2018;12:e0006175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Anyangu AS, Gould LH, Sharif SK, Nguku PM, Omolo JO, Mutonga D, Rao CY, Lederman ER, Schnabel D, Paweska JT, Katz M, Hightower A, Njenga MK, Feikin DR, Breiman RF. Risk factors for severe Rift Valley fever infection in Kenya, 2007. Am J Trop Med Hyg. 2010;83:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 129] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 19. | Lancelot R, Béral M, Rakotoharinome VM, Andriamandimby SF, Héraud JM, Coste C, Apolloni A, Squarzoni-Diaw C, de La Rocque S, Formenty PB, Bouyer J, Wint GR, Cardinale E. Drivers of Rift Valley fever epidemics in Madagascar. Proc Natl Acad Sci U S A. 2017;114:938-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Derrick EH. "Q" fever, a new fever entity: clinical features, diagnosis and laboratory investigation. Rev Infect Dis. 1983;5:790-800. [PubMed] |
| 21. | Maurin M, Raoult D. Q fever. Clin Microbiol Rev. 1999;12:518-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1471] [Cited by in RCA: 1438] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 22. | van Woerden HC, Mason BW, Nehaul LK, Smith R, Salmon RL, Healy B, Valappil M, Westmoreland D, de Martin S, Evans MR, Lloyd G, Hamilton-Kirkwood M, Williams NS. Q fever outbreak in industrial setting. Emerg Infect Dis. 2004;10:1282-1289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Angelakis E, Raoult D. Emergence of q Fever. Iran J Public Health. 2011;40:1-18. [PubMed] |
| 24. | Wilson LE, Couper S, Prempeh H, Young D, Pollock KG, Stewart WC, Browning LM, Donaghy M. Investigation of a Q fever outbreak in a Scottish co-located slaughterhouse and cutting plant. Zoonoses Public Health. 2010;57:493-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Chu H, Yoo SJ, Hwang KJ, Lim HS, Lee K, Park MY. Seroreactivity to Q Fever Among Slaughterhouse Workers in South Korea. J Prev Med Public Health. 2017;50:195-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Esmaeili S, Naddaf SR, Pourhossein B, Hashemi Shahraki A, Bagheri Amiri F, Gouya MM, Mostafavi E. Seroprevalence of Brucellosis, Leptospirosis, and Q Fever among Butchers and Slaughterhouse Workers in South-Eastern Iran. PLoS One. 2016;11:e0144953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 27. | Pramoolsinsap C, Viranuvatti V. Salmonella hepatitis. J Gastroenterol Hepatol. 1998;13:745-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Botteldoorn N, Heyndrickx M, Rijpens N, Grijspeerdt K, Herman L. Salmonella on pig carcasses: positive pigs and cross contamination in the slaughterhouse. J Appl Microbiol. 2003;95:891-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 141] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 29. | Heyndrickx M, Vandekerchove D, Herman L, Rollier I, Grijspeerdt K, De Zutter L. Routes for salmonella contamination of poultry meat: epidemiological study from hatchery to slaughterhouse. Epidemiol Infect. 2002;129:253-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 134] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | Giombelli A, Gloria MB. Prevalence of Salmonella and Campylobacter on broiler chickens from farm to slaughter and efficiency of methods to remove visible fecal contamination. J Food Prot. 2014;77:1851-1859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Kita E, Oku D, Hamuro A, Nishikawa F, Emoto M, Yagyu Y, Katsui N, Kashiba S. Hepatotoxic activity of Campylobacter jejuni. J Med Microbiol. 1990;33:171-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Korman TM, Varley CC, Spelman DW. Acute hepatitis associated with Campylobacter jejuni bacteraemia. Eur J Clin Microbiol Infect Dis. 1997;16:678-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Pearson AD, Greenwood MH, Donaldson J, Healing TD, Jones DM, Shahamat M, Feltham RK, Colwell RR. Continuous source outbreak of campylobacteriosis traced to chicken. J Food Prot. 2000;63:309-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Herman L, Heyndrickx M, Grijspeerdt K, Vandekerchove D, Rollier I, De Zutter L. Routes for Campylobacter contamination of poultry meat: epidemiological study from hatchery to slaughterhouse. Epidemiol Infect. 2003;131:1169-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 114] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 35. | Hue O, Allain V, Laisney MJ, Le Bouquin S, Lalande F, Petetin I, Rouxel S, Quesne S, Gloaguen PY, Picherot M, Santolini J, Bougeard S, Salvat G, Chemaly M. Campylobacter contamination of broiler caeca and carcasses at the slaughterhouse and correlation with Salmonella contamination. Food Microbiol. 2011;28:862-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Franz E, van der Fels-Klerx HJ, Thissen J, van Asselt ED. Farm and slaughterhouse characteristics affecting the occurrence of Salmonella and Campylobacter in the broiler supply chain. Poult Sci. 2012;91:2376-2381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14:296-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1940] [Cited by in RCA: 1837] [Article Influence: 76.5] [Reference Citation Analysis (0)] |
| 38. | Campagnolo ER, Warwick MC, Marx HL, Cowart RP, Donnell HD, Bajani MD, Bragg SL, Esteban JE, Alt DP, Tappero JW, Bolin CA, Ashford DA. Analysis of the 1998 outbreak of leptospirosis in Missouri in humans exposed to infected swine. J Am Vet Med Assoc. 2000;216:676-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Schoonman L, Swai ES. Risk factors associated with the seroprevalence of leptospirosis, amongst at-risk groups in and around Tanga city, Tanzania. Ann Trop Med Parasitol. 2009;103:711-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Sharma S, Vijayachari P, Sugunan AP, Natarajaseenivasan K, Sehgal SC. Seroprevalence of leptospirosis among high-risk population of Andaman Islands, India. Am J Trop Med Hyg. 2006;74:278-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 41. | Dreyfus A, Benschop J, Collins-Emerson J, Wilson P, Baker MG, Heuer C. Sero-prevalence and risk factors for leptospirosis in abattoir workers in New Zealand. Int J Environ Res Public Health. 2014;11:1756-1775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 42. | Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, Levett PN, Gilman RH, Willig MR, Gotuzzo E, Vinetz JM; Peru-United States Leptospirosis Consortium. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 2003;3:757-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1363] [Cited by in RCA: 1443] [Article Influence: 68.7] [Reference Citation Analysis (1)] |
| 43. | Esmaeili S, Hashemi Shahraki A, Bagheri Amiri F, Karimi M, Mostafavi E. Serological survey of leptospirosis among different groups in western Iran. Trop Doct. 2017;47:124-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 44. | Phillips CJ, Foster CR, Morris PA, Teverson R. The transmission of Mycobacterium bovis infection to cattle. Res Vet Sci. 2003;74:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 166] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 45. | Cousins DV. Mycobacterium bovis infection and control in domestic livestock. Rev Sci Tech. 2001;20:71-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 153] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 46. | Scott C, Cavanaugh JS, Pratt R, Silk BJ, LoBue P, Moonan PK. Human Tuberculosis Caused by Mycobacterium bovis in the United States, 2006-2013. Clin Infect Dis. 2016;63:594-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 47. | Seleem MN, Boyle SM, Sriranganathan N. Brucellosis: a re-emerging zoonosis. Vet Microbiol. 2010;140:392-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 442] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 48. | Franco MP, Mulder M, Gilman RH, Smits HL. Human brucellosis. Lancet Infect Dis. 2007;7:775-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 716] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 49. | Ozaras R, Celik AD, Demirel A. Acute hepatitis due to brucellosis in a laboratory technician. Eur J Intern Med. 2004;15:264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 50. | Cervantes F, Bruguera M, Carbonell J, Force L, Webb S. Liver disease in brucellosis. A clinical and pathological study of 40 cases. Postgrad Med J. 1982;58:346-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 49] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 51. | Denk A, Ozden M. A case of brucellosis presenting with acute hepatitis and bicytopenia. Infez Med. 2015;23:178-181. [PubMed] |
| 52. | Almuneef MA, Memish ZA, Balkhy HH, Alotaibi B, Algoda S, Abbas M, Alsubaie S. Importance of screening household members of acute brucellosis cases in endemic areas. Epidemiol Infect. 2004;132:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 53. | Khalili M, Sami M, Aflatoonian MR and Shahabi-Nejad N. Seroprevalence of brucellosis in slaughterhouse workers in Kerman city, Iran. Asian Pac J Trop Dis. 2012;2:448-450. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 54. | Grass JE, Gould LH, Mahon BE. Epidemiology of foodborne disease outbreaks caused by Clostridium perfringens, United States, 1998-2010. Foodborne Pathog Dis. 2013;10:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 160] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 55. | Bergert H, Illert T, Friedrich K, Ockert D. Fulminant liver failure following infection by Clostridium perfringens. Surg Infect (Larchmt). 2004;5:205-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 56. | Doblecki-Lewis S, Palaios E, Bejarano PA, Tzakis AG, Selvaggi G, Morris MI. Hepatic gas gangrene following orthotopic liver transplantation: three cases treated with re-transplantation and a review of the literature. Transpl Infect Dis. 2008;10:280-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 57. | Lu J, Wu XT, Kong XF, Tang WH, Cheng JM, Wang HL. Gas gangrene without wound: both lower extremities affected simultaneously. Am J Emerg Med. 2008;26:970.e3-970.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 58. | Hadem J, Westerkamp V, Trautwein C, Winkler M, Manns MP, Hafer C. Hepatic gas gangrene following liver transplantation. Liver Transpl. 2007;13:468-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 59. | Buimer MG, Spillenaar Bilgen EJ. Gas gangrene of the liver after a choledocho-jejunostomy. Dig Surg. 2008;25:260-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 60. | Eigneberger B, Königsrainer I, Kendziorra H, Riessen R. Fulminant liver failure due to Clostridium perfringens sepsis 9 years after liver transplantation. Transpl Int. 2006;19:172-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 61. | Sasaki J, Goryo M, Makara M, Nakamura K, Okada K. Necrotic hepatitis due to Clostridium perfringens infection in newly hatched broiler chicks. J Vet Med Sci. 2003;65:1249-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 62. | Carella G, Marra L, Vallot T. [Hepatic psittacosis: a case of liver abnormality diagnosed by ultrasonography]. Presse Med. 1996;25:197-198. [PubMed] |
| 63. | Compendium of measures to control Chlamydia psittaci infection among humans (psittacosis) and pet birds (avian chlamydiosis), 1998. Center for Disease Control and Prevention. MMWR Recomm Rep. 1998;47:1-14. [PubMed] |
| 64. | Ragnaud JM, Dupon M, Echinard E, Lacut JY, Aubertin J. [Hepatic manifestations of psittacosis]. Gastroenterol Clin Biol. 1986;10:234-237. [PubMed] |
| 65. | Otero-Abad B, Torgerson PR. A systematic review of the epidemiology of echinococcosis in domestic and wild animals. PLoS Negl Trop Dis. 2013;7:e2249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 188] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 66. | Grenouillet F, Umhang G, Arbez-Gindre F, Mantion G, Delabrousse E, Millon L, Boué F. Echinococcus ortleppi infections in humans and cattle, France. Emerg Infect Dis. 2014;20:2100-2102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 67. | Chihai O, Umhang G, Erhan D, Boué F, Tălămbuţă N, Rusu Ş, Zamornea M. Slaughterhouse survey of cystic echinococcosis in cattle and sheep from the Republic of Moldova. J Helminthol. 2016;90:279-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 68. | Brunetti E, Kern P, Vuitton DA, Writing Panel for the WHO-IWGE. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010;114:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1335] [Article Influence: 89.0] [Reference Citation Analysis (0)] |
| 69. | Gottstein B. Hydatid Disease, Major Tropical syndromes by body system, Systemic Infections. Cambridge: Cambridge University Press 2000; . |
| 70. | Alvarado-Esquivel C, Torres-Berumen JL, Estrada-Martínez S, Liesenfeld O, Mercado-Suarez MF. Toxoplasma gondii infection and liver disease: a case-control study in a northern Mexican population. Parasit Vectors. 2011;4:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 71. | Doğan N, Kabukçuoğlu S, Vardareli E. Toxoplasmic hepatitis in an immunocompetent patient. Turkiye Parazitol Derg. 2007;31:260-263. [PubMed] |
| 72. | Webster JP. Dubey, J.P. Toxoplasmosis of Animals and Humans. Parasites Vectors. 2010;3:112. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 73. | da Silva Ramos T, de Jesus Pena HF, Dos Santos Junior AG, de Faria Santos LMJ, Cademartori BG, Oliveira S, Gennari SM, da Silva Ramos Rocha A, da Rosa Farias NA. Characterization of Toxoplasma gondii isolates from herds of sheep in southern Brazil reveals the archetypal type II genotype and new non-archetypal genotypes. Parasitol Int. 2018;67:59-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 74. | Gottstein B, Pozio E, Nöckler K. Epidemiology, diagnosis, treatment, and control of trichinellosis. Clin Microbiol Rev. 2009;22:127-145, Table of Contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 515] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 75. | Kushlan SD. Trichinosis with liver dysfunction, hypoalbuminemia, and typhoid agglutinins. JAMA. 1953;152:221-224. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 76. | Kennedy FB, Rege VB. Trichinosis. Hemiplegia and liver involvement. Arch Intern Med. 1966;117:108-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 77. | Trépo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. 2014;384:2053-2063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1004] [Cited by in RCA: 1157] [Article Influence: 105.2] [Reference Citation Analysis (0)] |
| 78. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 3802] [Article Influence: 475.3] [Reference Citation Analysis (1)] |
| 79. | Pavio N, Meng XJ, Renou C. Zoonotic hepatitis E: animal reservoirs and emerging risks. Vet Res. 2010;41:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 249] [Cited by in RCA: 260] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 80. | Center for Disease Control and Prevention. Rift Valley Fever (RVF). Available from: URL: https://www.cdc.gov/vhf/rvf/prevention/index.html. |
| 81. | Anderson A, Bijlmer H, Fournier PE, Graves S, Hartzell J, Kersh GJ, Limonard G, Marrie TJ, Massung RF, McQuiston JH, Nicholson WL, Paddock CD, Sexton DJ. Diagnosis and management of Q fever--United States, 2013: recommendations from CDC and the Q Fever Working Group. MMWR Recomm Rep. 2013;62:1-30. [PubMed] |
| 82. | Center for Disease Control and Prevention. Salmonella - Information for Healthcare Professionals and Laboratories. Available from: URL: https://www.cdc.gov/salmonella/general/technical.html. |
| 83. | Altekruse SF, Stern NJ, Fields PI, Swerdlow DL. Campylobacter jejuni--an emerging foodborne pathogen. Emerg Infect Dis. 1999;5:28-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 601] [Cited by in RCA: 564] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 84. | Center for Disease Control and Prevention. Campylobacter (Campylobacteriosis). Available from: URL: https://www.cdc.gov/campylobacter/index.html. |
| 85. | Budihal SV, Perwez K. Leptospirosis diagnosis: competancy of various laboratory tests. J Clin Diagn Res. 2014;8:199-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 86. | Charan J, Saxena D, Mulla S, Yadav P. Antibiotics for the treatment of leptospirosis: systematic review and meta-analysis of controlled trials. Int J Prev Med. 2013;4:501-510. [PubMed] |
| 87. | Parrish NM, Carroll KC. Role of the clinical mycobacteriology laboratory in diagnosis and management of tuberculosis in low-prevalence settings. J Clin Microbiol. 2011;49:772-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 88. | Center for Disease Control and Prevention. Tuberculosis (TB). Available from: URL: https://www.cdc.gov/tb/publications/factsheets/general/mbovis.html. |
| 89. | Center for Disease Control and Prevention. Brucellosis. Available from: URL: https://www.cdc.gov/brucellosis/clinicians/index.html. |
| 90. | Government of Canada. Pathogen Safety Data Sheets: Infectious Substances – Clostridium perfringens. Available from: URL: https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment/clostridium-perfringens.html. |
| 91. | Government of Canada. Pathogen Safety Data Sheets: Infectious Substances – Chlamydophila psittaci. Available from: URL: https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment/chlamydophila-psittaci.html. |
| 92. | Government of Canada. Pathogen Safety Data Sheets: Infectious Substances – Echinococcus granulosus. Available from: URL: https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment/echinococcus-granulosus-pathogen-safety-data-sheet.html. |
| 93. | Government of Canada. Pathogen Safety Data Sheets: Infectious Substances – Toxoplasma gondii. Available from: URL: https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment/toxoplasma-gondii-pathogen-safety-data-sheet.html. |
| 94. | Center for Disease Control and Prevention. Parasites - Trichinellosis (also known as Trichinosis). Available from: URL: https://www.cdc.gov/parasites/trichinellosis/. |