Published online Jan 27, 2019. doi: 10.4254/wjh.v11.i1.119
Peer-review started: August 28, 2018
First decision: October 15, 2018
Revised: November 30, 2018
Accepted: December 12, 2018
Article in press: December 12, 2018
Published online: January 27, 2019
Processing time: 152 Days and 16.5 Hours
Hepatocellular carcinoma (HCC) is the second most lethal malignancy worldwide. There has been virtually no change in the survivability of HCC in spite of improvement in therapies. Surgery is considered the ideal first, curative intervention, however most patients present in advanced stages with unresectable disease. Therefore, systemic and liver-directed non-operative therapies are initially offered to downstage the disease. To ensure optimal management, a multidisciplinary team approach is often warranted. Our case highlights the benefits of a multidisciplinary approach in a young woman with multifocal, bilobar HCC.
A 36-year-old Chinese woman with untreated hepatitis B was found to have large bilobar HCC during work up for abdominal pain. Her initial serum alpha-fetoprotein was significantly elevated to 311136 ng/mL. Computed tomography scan demonstrated bulky bilobar liver masses, consistent with intermediate stage HCC, Barcelona Clinic Liver Cancer Stage B. Her case was discussed and a personalized care plan was developed at the Multidisciplinary Center for Advanced Minimally Invasive Liver Oncologic Therapies at the University of Washington. She initially underwent bilobar transarterial chemoembolization with partial response of the left lobar tumor. Salvage hypofractionated proton beam radiation therapy was delivered to the right lobe followed by two additional transarterial chemoembolizations to the left lobe with good response. Finally, to remove left lobar residual disease, she was taken to the operating room for a left hepatectomy eleven months after her initial presentation. She continues to be without evidence of disease.
Coordinating the multiple HCC treatment modalities is complex and our case highlights the benefits of a multidisciplinary approach.
Core tip: Hepatocellular carcinoma is a challenging disease that requires a personalized and multidisciplinary approach for treatment. Our case highlights a favorable patient outcome in a young woman with multifocal bilobar hepatocellular carcinoma as a result of a coordinated multimodal treatment approach utilizing catheter-based ablative techniques, external beam radiation therapy, and surgical resection.
- Citation: Labadie KP, Schaub SK, Khorsand D, Johnson G, Apisarnthanarax S, Park JO. Multidisciplinary approach for multifocal, bilobar hepatocellular carcinoma: A case report and literature review. World J Hepatol 2019; 11(1): 119-126
- URL: https://www.wjgnet.com/1948-5182/full/v11/i1/119.htm
- DOI: https://dx.doi.org/10.4254/wjh.v11.i1.119
Hepatocellular carcinoma (HCC) is the second most lethal malignancy worldwide, claiming over 745000 lives in 2012[1]. This figure is projected to rise despite new and effective antiviral drugs to eradicate hepatitis C infection and is due to increasing rates of fatty liver disease from diabetes and the obesity epidemic[2]. There has been virtually no change in the survivability of HCC over the last three decades[3]. This is especially true for intermediate and advanced HCC where the standard of care therapy with sorafenib provides only a 2% response rate and 3-mo survival advantage[4]. While checkpoint inhibition immunotherapy (e.g., nivolumab and pembrolizumab) have demonstrated significant response rates, a large proportion of HCC patients do not respond to this immunotherapy[5]. Therefore, more effective treatments for advanced cases of HCC are greatly needed.
Several modalities exist for treatment of intermediate and advanced stage HCC. Potential therapies include ablative techniques using ethanol (percutaneous ethanol injection), microwave or radiofrequency; catheter-directed transarterial chemoembolization (TACE) or radioembolization (TARE); external beam radiation therapy in the form of stereotactic body radiation therapy or proton beam therapy (PBT); systemic targeted small molecules; and immunotherapy and investigational agents. In certain cases, these therapies can be utilized simultaneously or in sequence to achieve optimal tumor control. Furthermore, this approach can be used to effectively downstage advanced HCC and make it amenable for curative liver resection (LR) or transplantation[6].
Coordinated management of the HCC patient with these different modalities has become increasingly complex. To navigate this complexity, discuss suitable therapeutic options, and generate personalized treatment plans, many centers have adopted a multidisciplinary team (MDT) approach with established "tumor boards." These teams include members from several complementary specialties including surgical, medical and radiation oncology, transplant surgery and hepatology, diagnostic and interventional radiology, pathology, physical and occupational therapy, nursing, nutrition, genetic counseling, spiritual, and palliative care. The MDT approach has been adopted for the treatment of a wide variety of malignancies, and it is in fact required for accreditation as a comprehensive cancer care center by the American College of Surgeons Commission on Cancer. Although the MDT approach has several putative benefits for patient care, there is a concern over the paucity of high quality evidence demonstrating an improvement in patient outcomes[7,8]. Furthermore, it has been associated with high operating costs.
Our patient presented with advanced, multifocal, and bilobar HCC; an extent of disease associated with a dismal prognosis. Herein, we highlight a year-long MDT approach that demonstrates how it can downstage advanced disease and lead to improved patient outcomes. We report a young patient with advanced, multifocal, bilobar, and initially unresectable HCC that benefited from a year-long MDT approach.
A 36-year-old woman originally from China was evaluated in September 2016 for management of large bilobar liver masses. Her pertinent past medical history was significant for untreated hepatitis B virus infection diagnosed in 1999 while pregnant with her first child. The rest of her past medical and surgical history was remarkable only for a previously excised benign breast mass. Her family history was positive for lymphoma in her father. She had never smoked tobacco, consumed alcohol, or used illicit drugs. Her physical examination was unremarkable with no appreciable liver masses.
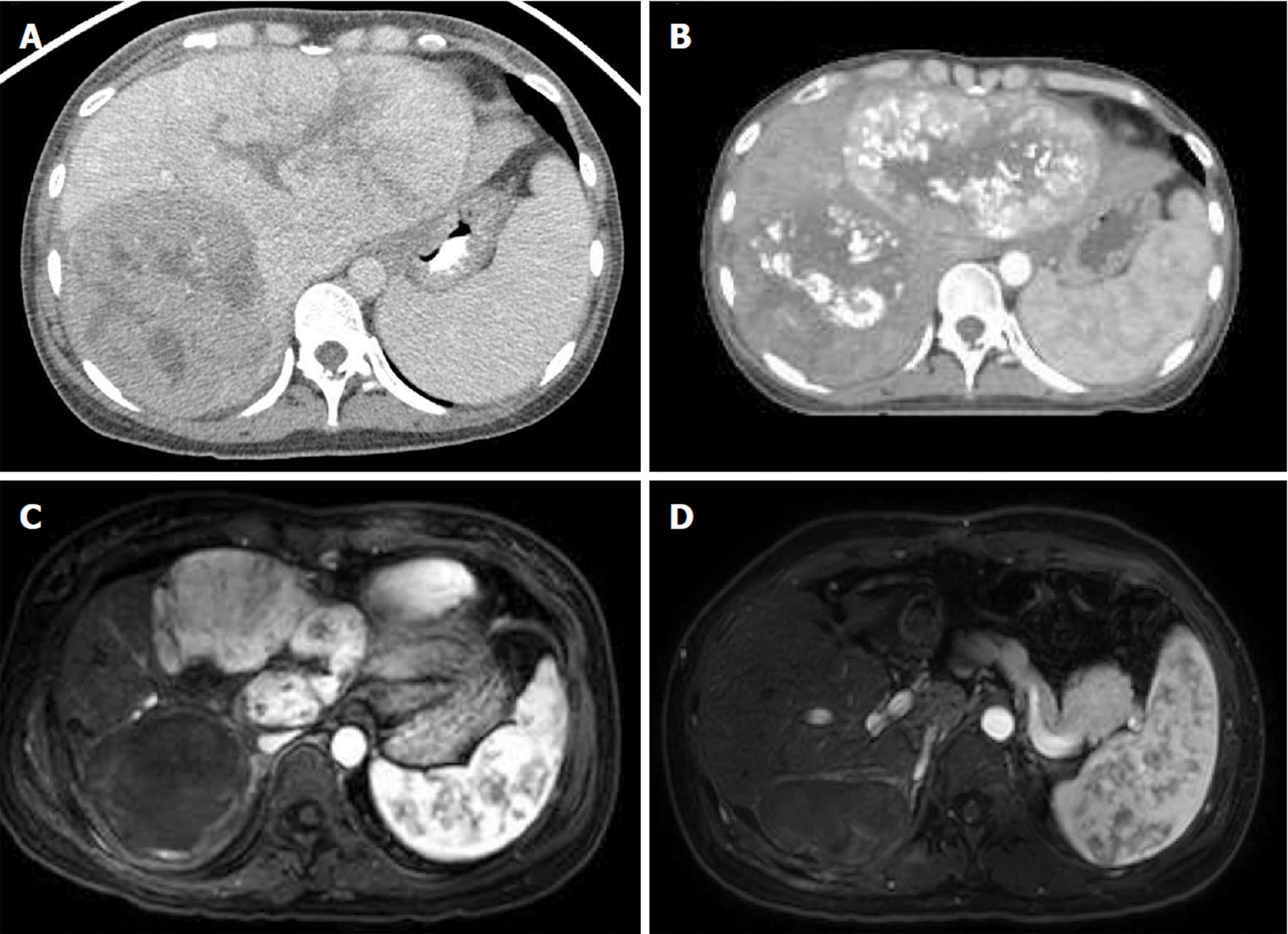
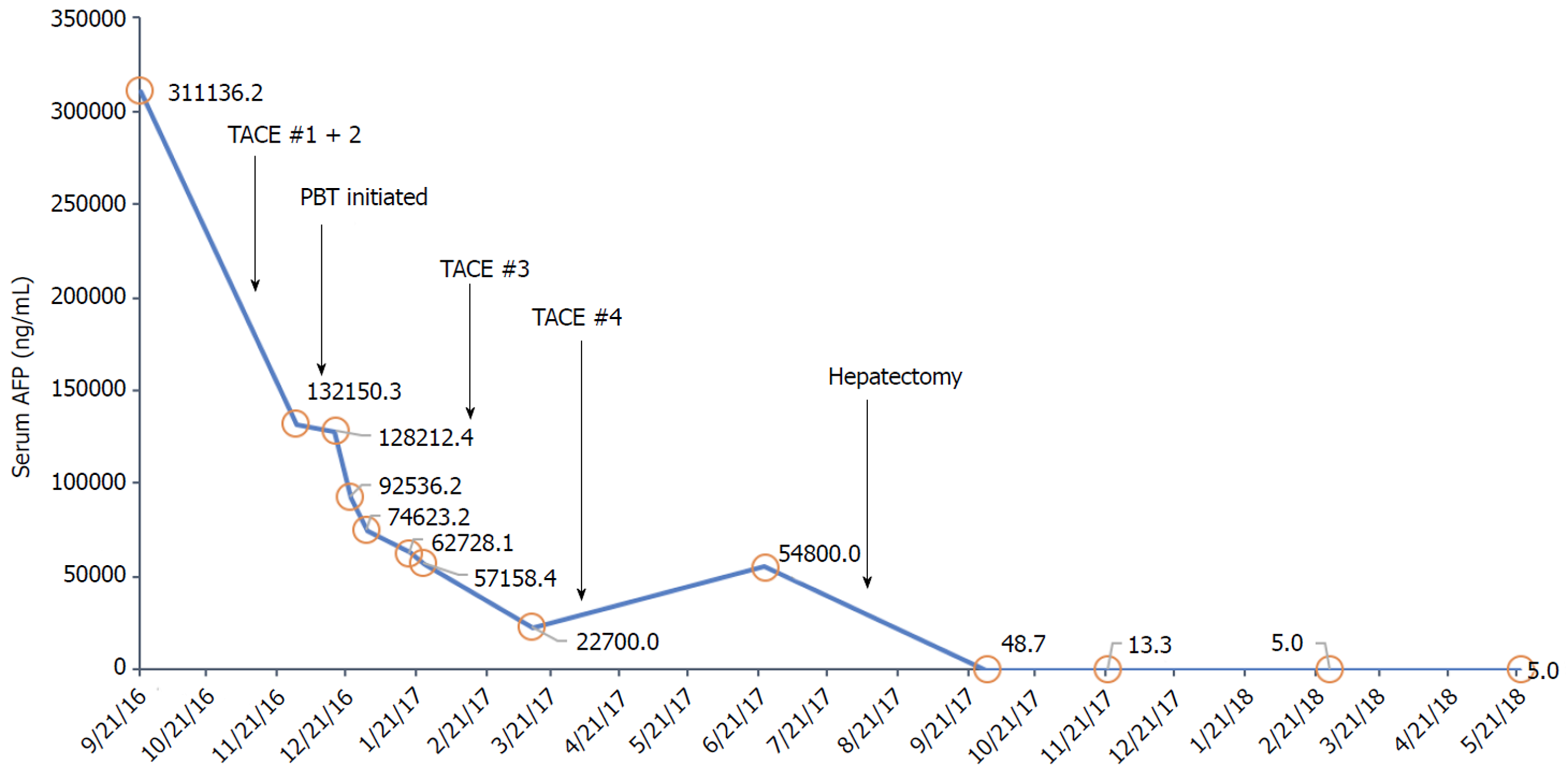
Her oncologic history began in 2015 when she first experienced right shoulder and chest pain with work-up revealing mildly elevated transaminases. No further evaluation was performed until 2016 when the pain worsened with associated weight loss and fatigue. She underwent right upper quadrant ultrasound, which demonstrated large bilobar liver masses. The serum alpha-fetoprotein (AFP) was significantly elevated to 311136 ng/mL (reference 0-8.6). A multiphase liver protocol computed tomography (CT) scan demonstrated bulky bilobar liver masses (Figure 1A), including a left lobe lesion measuring 8.1 cm x 14.4 cm x 10.7 cm with central necrosis, in addition to a multilobular right lobe lesion measuring 10.5 cm x 9.5 cm x 14.9 cm. No IVC, hepatic, or portal venous invasion or thrombosis was identified, and there was no evidence of extrahepatic disease. Her serum was positive for HBsAg with a HBV quantitative PCR of 12000 IU/mL for which she initiated entecavir therapy. Her liver function was well-preserved with a child-turcotte-pugh score of A5 and model for end-stage liver disease score of 6.
Her case was discussed at the University of Washington Multidisciplinary Center for Advanced Minimally Invasive Liver Oncologic Therapies composed of members from the surgical, medical and radiation oncology, transplant surgery and hepatology, pathology, and diagnostic and interventional radiology teams. Given her history of hepatitis B, elevated AFP, and imaging characteristics consistent with HCC, a diagnosis was made.
Intermediate stage HCC, Barcelona Clinic Liver Cancer Stage B.
She was not a candidate for orthotopic liver transplantation, LR, or any ablative therapies given the large size of the masses and their intimate relationship to the vasculature. She underwent bilobar TACE in October 2016, resulting in a decrease of the serum AFP to 132150 ng/mL, and post-treatment imaging demonstrated a partial response (Figure 1B). However, during chemoembolization of the right lobar mass, the right inferior phrenic artery was inaccessible, leaving part of the right tumor untreated.
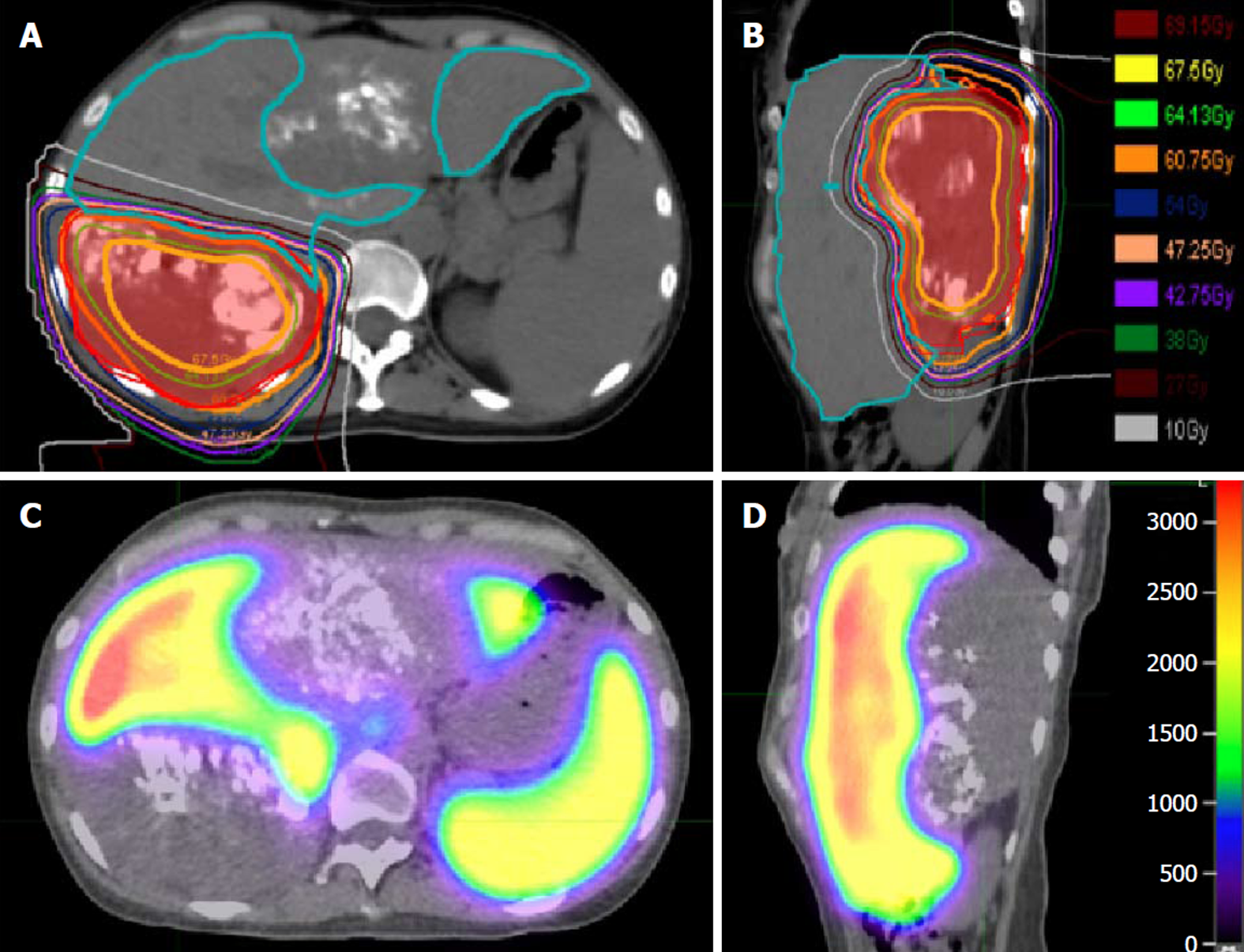
Given this and the large size of the right lobar mass, she underwent salvage hypofractionated PBT to the right lobe tumor using a simultaneously integrated boost intensity modulated proton therapy technique with pencil beam scanning in December 2016. A total dose of 60 GyE to the tumor periphery and 67.5 GyE to internal sub-volumes within the tumor were delivered in 15 fractions (Figure 2A and B). Because optimally preserving the future normal liver remnant from radiation was critically important, a pre-treatment functional liver imaging scan with technetium-99m sulfur colloid single-photon emission CT was employed to assist in functional liver avoidance radiation planning (Figure 2C and D). She tolerated radiotherapy well with maintenance of normal liver function.
Three weeks after completion of PBT, the serum AFP declined to 62728 ng/mL, with a liver protocol magnetic resonance imaging (MRI) scan demonstrating complete response of her right lobar mass but significant residual tumor in the left lobe (Figure 1C). In February 2017 and April 2017, she underwent two additional TACE sessions to the left lobe. The serum AFP continued to decrease with each treatment to a nadir of 22700 ng/mL. However, post-treatment MRI demonstrated residual disease in the left lobe. She was referred for consideration of hepatic resection.
Pre-operative MRI demonstrated an adequate future liver remnant but with an obscured middle hepatic venous outflow with drainage of the non-tumor involved anterior right lobe via a large collateral vessel connecting to the middle hepatic vein and circumscribing the previously irradiated right lobe tumor. Doppler ultrasound was performed and confirmed appropriate venous caval outflow through this collateral vessel, which drained through the right hepatic vein into the IVC. Therefore, the middle hepatic vein, the anomalous venous collateral, and the right hepatic vein all required preservation. The patient underwent formal left hepatectomy in August 2017, eleven months after initial presentation. The case was quite challenging due to the tumor’s long segment abutment of the middle hepatic vein, but LR with salvage of all aforementioned vasculature was successful. Pathology demonstrated a 11.5 cm x 10.5 cm x 7.5 cm HCC involving segments 2, 4a, and 8 with 25% tumor necrosis. The tumor was confined to the liver without vascular invasion and negative surgical margins was achieved with a final pathologic stage of ypT1bN0M0.
The serum AFP level at 16, 29, and 42 wk postoperatively was 13.3, < 5.0 and < 5.0 ng/mL, respectively (Figure 3). MRI scans performed 16, 29, and 42 wk postoperatively have been negative for any evidence of residual or recurrent disease (Figure 1D). The patient continues to do well greater than 12 mo postoperatively, with normal liver function (Na 139 mEq/L, Cr 0.63 mg/dL, bilirubin 0.7 mg/dL, albumin 4.1 g/dL, and platelet count 117000/μL) and performance status of ECOG 0. There have been no adverse or unanticipated events.
The MDT approach has been increasingly utilized for the management of a variety of diseases from diabetes to cancer. In oncology, some of the first successes were demonstrated in the management of breast cancer in the National Health Service[9]. It has been widely adopted internationally as the standard of care for newly diagnosed malignancies. Published evidence demonstrates improved accuracy of pre-operative staging[10,11], increased access to care, adherence to clinical guidelines[12,13], and satisfaction for both patients and health care providers[14-16]. In spite of this widespread adoption, there has been concern over the paucity of evidence demonstrating a significant improvement in clinical outcomes with the MDT approach[7,15]. Two recent systematic reviews published by Croke et al[8] and Pillay et al[11] demonstrated MDT approaches resulted in a significant impact on patient management, but there was little evidence to support an improvement in clinical outcomes. On an individual level, our case supports the significant patient impact of the MDT approach.
The management of HCC is increasingly complex as advances in more effective liver-directed therapies and systemic therapies become available. Optimal management of intermediate or advanced stage HCC requires thoughtful sequencing and timing of the various therapeutic modalities, which is best achieved through a tightly knit multidisciplinary team. We have found in our experience at the University of Washington Center for Advanced Minimally Invasive Liver Oncologic Therapies that multidisciplinary management of these patients is a beneficial endeavor, as seen in our presented case. With complementary multidisciplinary therapy, a combination of local, loco-regional, and systemic therapies can be successful in downstaging an advanced-stage HCC to one amenable to curative intervention with LR or orthotopic liver transplantation[6,17-19]. This has been demonstrated in prior series with multiple studies indicating the impact of downstaging prior to LR or transplantation. A prospective study by Yao et al[17] demonstrated that with careful patient selection, loco-regional therapies was successful in downstaging > 50% of patients to liver transplantation. This has similarly been examined in LR for HCC where liver-directed non-operative therapies have been successful in downstaging tumors for resection. Our present case is novel in that it highlights a patient with extensive multifocal disease involving both lobes requiring several rounds of sequential TACE and proton beam radiation therapy followed by a complex partial hepatectomy. A limitation of this approach is that it is resource intensive with complex scheduling issues that arise in routine clinical practice.
An important technique in tumor downstaging includes catheter based therapies such as TACE and TARE. These have been demonstrated to both improving overall survival[20] in addition to serving as both bridging and downstaging treatment modalities. A study published in 2009 by Lewandowski et al[21] reported successful downstaging from UNOS T3 to T2 in 61% and 37% for TARE and TACE, respectively[13]. Six of twenty-one patients in a 2012 case series who underwent TARE for palliative intent for UNOS T3 HCC were sufficiently downstaged, enabling definitive LR, definitive ablation, or orthotopic liver transplantation[22]. TACE was chosen over TARE for our patient due to the potential of inadvertent lung dose of yttrium-90 due to significant shunting.
Technological advancements have resulted in the increasing use of external beam radiation therapy in the treatment of HCC given high rates of durable local control ranging from 87%-100% at 1 to 3 years[23-26]. For our patient, radiotherapy offered a promising local treatment option for the right-sided lesion due to the large tumor size and vascular anatomy prohibitive of further catheter-based therapy or surgical resection. PBT was the preferred radiation modality due to the tumor size, and dosimetric advantage with improved normal liver parenchyma sparing from low-to-moderate doses as compared to photon-based radiation, e.g., stereotactic body radiation therapy. In radiation therapy akin to surgery, a “critical volume model” is applied to describe the minimum volume of normal liver that should be uninjured by radiation as to limit the incidence of radiation-induced liver disease. Dose and dosimetric constraint guidelines were derived from Hong et al[26] multicenter phase II clinical trial of dose-escalated proton beam radiation for unresectable primary hepatic cancers, where they demonstrated a low incidence of radiation-induced liver disease as measured by worsening Child-Turcotte-Pugh-score in 3.6% and no grade 4 or 5 radiation-related toxicities. Given the complexity of this case, we used technetium-99m sulfur colloid single-photon emission CT imaging co-registered to our radiation planning CT to assist in delineating the borders of the residual gross tumor volume, as well as a tool for more accurate global and spatial liver function to guide placement of radiation beams through less functional normal liver parenchyma[27-29].
LR continues to be a potentially curative treatment option in 15% to 20% of HCC patients with an operative mortality of less than 5% even in cirrhotic patients or those undergoing major LR, resulting in a 5-year overall survival of over 50%. The safety and outcomes of LR for HCC have improved substantially over the last three decades, which is attributed to refinements in patient evaluation and selection, the ability to manipulate the future liver remnant volume, advances in surgical and anesthetic techniques, and the enhanced peri-operative management. In this case, the patient was young and medically fit, with an ECOG performance status 0, adequate hepatic reserve in the future liver remnant, and absence of portal hypertension.
Biologic markers to predict HCC tumor biology are under development. Current surrogate markers predicting prognosis are based on tumor size, number, and vascular invasion. For HCCs, some researchers reported that larger tumor sizes exceeding the University of California at San Francisco criteria and serum AFP levels over 400 ng/mL were associated with post-resection recurrence. Other markers such as retinoic acid-induced protein 3 as well as miRNA expression profiles have been used to predict poor prognosis and assess the risk of disease recurrence after orthotopic liver transplantation. Large tumor size has traditionally been a relative contraindication to LR given the elevated risk of vascular invasion. However, HCCs over 10 cm in size without invasion amenable to LR can result in good outcomes, and therefore identification of such tumors is important[30,31]. In this case, the lack of macrovascular invasion, and good response to TACE and PBT were considered in the surgical decision making.
The primary aim of MDT approach in cancer care is to improve patient outcomes by bringing together key specialists with complementary expertise to review cases, ensure accurate diagnosis and develop optimal, individualized plans of care. They often result in significant changes in patient diagnosis and management and are generally considered beneficial for patient care. A multidisciplinary approach to our presented case clearly resulted in an improved patient outcome on an individualized level; however, recent systematic reviews have failed to demonstrate improvement in patient outcomes on a population level. Further research is needed to understand the true clinical impact of a MDT approach to cancer care.
CARE Checklist (2013) statement: The authors have read the CARE Checklist (2013), and the manuscript was prepared and revised according to the CARE Checklist (2013).
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and Hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Memeo R, Mikulic D S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Tan WW
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20515] [Article Influence: 2051.5] [Reference Citation Analysis (20)] |
| 2. | Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820-1832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 968] [Cited by in RCA: 1014] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 3. | Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1196] [Cited by in RCA: 1326] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 4. | Benson AB, Abrams TA, Ben-Josef E, Bloomston PM, Botha JF, Clary BM, Covey A, Curley SA, D'Angelica MI, Davila R, Ensminger WD, Gibbs JF, Laheru D, Malafa MP, Marrero J, Meranze SG, Mulvihill SJ, Park JO, Posey JA, Sachdev J, Salem R, Sigurdson ER, Sofocleous C, Vauthey JN, Venook AP, Goff LW, Yen Y, Zhu AX. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. J Natl Compr Canc Netw. 2009;7:350-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 422] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 5. | Harding JJ, El Dika I, Abou-Alfa GK. Immunotherapy in hepatocellular carcinoma: Primed to make a difference? Cancer. 2016;122:367-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 6. | Yao FY, Kerlan RK, Hirose R, Davern TJ, Bass NM, Feng S, Peters M, Terrault N, Freise CE, Ascher NL, Roberts JP. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology. 2008;48:819-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 409] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 7. | Fleissig A, Jenkins V, Catt S, Fallowfield L. Multidisciplinary teams in cancer care: are they effective in the UK? Lancet Oncol. 2006;7:935-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 426] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 8. | Croke JM, El-Sayed S. Multidisciplinary management of cancer patients: chasing a shadow or real value? An overview of the literature. Curr Oncol. 2012;19:e232-e238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Blamey RW. The British Association of Surgical Oncology Guidelines for surgeons in the management of symptomatic breast disease in the UK (1998 revision). BASO Breast Specialty Group. Eur J Surg Oncol. 1998;24:464-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Sundi D, Cohen JE, Cole AP, Neuman BP, Cooper J, Faisal FA, Ross AE, Schaeffer EM. Establishment of a new prostate cancer multidisciplinary clinic: Format and initial experience. Prostate. 2015;75:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Pillay B, Wootten AC, Crowe H, Corcoran N, Tran B, Bowden P, Crowe J, Costello AJ. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: A systematic review of the literature. Cancer Treat Rev. 2016;42:56-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 451] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 12. | Kelly SL, Jackson JE, Hickey BE, Szallasi FG, Bond CA. Multidisciplinary clinic care improves adherence to best practice in head and neck cancer. Am J Otolaryngol. 2013;34:57-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 13. | Stephens MR, Lewis WG, Brewster AE, Lord I, Blackshaw GR, Hodzovic I, Thomas GV, Roberts SA, Crosby TD, Gent C, Allison MC, Shute K. Multidisciplinary team management is associated with improved outcomes after surgery for esophageal cancer. Dis Esophagus. 2006;19:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 210] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 14. | Chang TT, Sawhney R, Monto A, Davoren JB, Kirkland JG, Stewart L, Corvera CU. Implementation of a multidisciplinary treatment team for hepatocellular cancer at a Veterans Affairs Medical Center improves survival. HPB (Oxford). 2008;10:405-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Taylor C, Munro AJ, Glynne-Jones R, Griffith C, Trevatt P, Richards M, Ramirez AJ. Multidisciplinary team working in cancer: what is the evidence? BMJ. 2010;340:c951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 304] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 16. | Siddique O, Yoo ER, Perumpail RB, Perumpail BJ, Liu A, Cholankeril G, Ahmed A. The importance of a multidisciplinary approach to hepatocellular carcinoma. J Multidiscip Healthc. 2017;10:95-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Yao FY, Hirose R, LaBerge JM, Davern TJ, Bass NM, Kerlan RK, Merriman R, Feng S, Freise CE, Ascher NL, Roberts JP. A prospective study on downstaging of hepatocellular carcinoma prior to liver transplantation. Liver Transpl. 2005;11:1505-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 221] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 18. | Shi XJ, Jin X, Wang MQ, Wei LX, Ye HY, Liang YR, Luo Y, Dong JH. Effect of resection following downstaging of unresectable hepatocelluar carcinoma by transcatheter arterial chemoembolization. Chin Med J (Engl). 2012;125:197-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 19. | Kim Y, Stahl CC, Makramalla A, Olowokure OO, Ristagno RL, Dhar VK, Schoech MR, Chadalavada S, Latif T, Kharofa J, Bari K, Shah SA. Downstaging therapy followed by liver transplantation for hepatocellular carcinoma beyond Milan criteria. Surgery. 2017;162:1250-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2207] [Cited by in RCA: 2271] [Article Influence: 103.2] [Reference Citation Analysis (0)] |
| 21. | Lewandowski RJ, Kulik LM, Riaz A, Senthilnathan S, Mulcahy MF, Ryu RK, Ibrahim SM, Sato KT, Baker T, Miller FH, Omary R, Abecassis M, Salem R. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant. 2009;9:1920-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 437] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 22. | Iñarrairaegui M, Pardo F, Bilbao JI, Rotellar F, Benito A, D'Avola D, Herrero JI, Rodriguez M, Martí P, Zozaya G, Dominguez I, Quiroga J, Sangro B. Response to radioembolization with yttrium-90 resin microspheres may allow surgical treatment with curative intent and prolonged survival in previously unresectable hepatocellular carcinoma. Eur J Surg Oncol. 2012;38:594-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 23. | Bujold A, Massey CA, Kim JJ, Brierley J, Cho C, Wong RK, Dinniwell RE, Kassam Z, Ringash J, Cummings B, Sykes J, Sherman M, Knox JJ, Dawson LA. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol. 2013;31:1631-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 593] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 24. | Cárdenes HR, Price TR, Perkins SM, Maluccio M, Kwo P, Breen TE, Henderson MA, Schefter TE, Tudor K, Deluca J, Johnstone PA. Phase I feasibility trial of stereotactic body radiation therapy for primary hepatocellular carcinoma. Clin Transl Oncol. 2010;12:218-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 219] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 25. | Lasley FD, Mannina EM, Johnson CS, Perkins SM, Althouse S, Maluccio M, Kwo P, Cárdenes H. Treatment variables related to liver toxicity in patients with hepatocellular carcinoma, Child-Pugh class A and B enrolled in a phase 1-2 trial of stereotactic body radiation therapy. Pract Radiat Oncol. 2015;5:e443-e449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 139] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 26. | Hong TS, Wo JY, Yeap BY, Ben-Josef E, McDonnell EI, Blaszkowsky LS, Kwak EL, Allen JN, Clark JW, Goyal L, Murphy JE, Javle MM, Wolfgang JA, Drapek LC, Arellano RS, Mamon HJ, Mullen JT, Yoon SS, Tanabe KK, Ferrone CR, Ryan DP, DeLaney TF, Crane CH, Zhu AX. Multi-Institutional Phase II Study of High-Dose Hypofractionated Proton Beam Therapy in Patients With Localized, Unresectable Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. J Clin Oncol. 2016;34:460-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 332] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 27. | Yeung RH, Chapman TR, Bowen SR, Apisarnthanarax S. Proton beam therapy for hepatocellular carcinoma. Expert Rev Anticancer Ther. 2017;17:911-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Bowen SR, Chapman TR, Borgman J, Miyaoka RS, Kinahan PE, Liou IW, Sandison GA, Vesselle HJ, Nyflot MJ, Apisarnthanarax S. Measuring total liver function on sulfur colloid SPECT/CT for improved risk stratification and outcome prediction of hepatocellular carcinoma patients. EJNMMI Res. 2016;6:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Bowen SR, Saini J, Chapman TR, Miyaoka RS, Kinahan PE, Sandison GA, Wong T, Vesselle HJ, Nyflot MJ, Apisarnthanarax S. Differential hepatic avoidance radiation therapy: Proof of concept in hepatocellular carcinoma patients. Radiother Oncol. 2015;115:203-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Ng KK, Vauthey JN, Pawlik TM, Lauwers GY, Regimbeau JM, Belghiti J, Ikai I, Yamaoka Y, Curley SA, Nagorney DM, Ng IO, Fan ST, Poon RT; International Cooperative Study Group on Hepatocellular Carcinoma. Is hepatic resection for large or multinodular hepatocellular carcinoma justified? Results from a multi-institutional database. Ann Surg Oncol. 2005;12:364-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 188] [Article Influence: 9.4] [Reference Citation Analysis (0)] |