Published online Sep 27, 2018. doi: 10.4254/wjh.v10.i9.595
Peer-review started: March 30, 2018
First decision: May 11, 2018
Revised: May 25, 2018
Accepted: June 30, 2018
Article in press: June 30, 2018
Published online: September 27, 2018
Processing time: 181 Days and 14.5 Hours
Hepatitis C virus (HCV) chronic infection induces liver fibrosis and cirrhosis but is also responsible for a significant portion of hepatocellular carcinoma (HCC) occurrence. Since it was recognized as a causative factor of chronic hepatitis, there have been multiple efforts towards viral eradication, leading to the first-generation HCV treatment that was based on interferon (IFN)-αand its analogs, mainly PEGylated interferon-α (PEG IFNα). Sustained virological response (SVR), defined as the absence of detectable RNA of HCV in blood serum for at least 24 wk after discontinuing the treatment, was accepted as a marker of viral clearance and was achieved in approximately one-half of patients treated with PEG IFNα regimens. Further research on the molecular biology of HCV gave rise to a new generation of drugs, the so-called direct antiviral agents (DAAs). DAA regimens, as implied by their name, interfere with the HCV genome or its products and have high SVR rates, over 90%, after just 12 wk of per os treatment. Although there are no questions about their efficacy or their universality, as they lack the contraindication for advanced liver disease that marks PEG IFNα, some reports of undesired oncologic outcomes after DAA treatment raised suspicions about possible interference of this treatment in HCC development. The purpose of the present review is to investigate the validity of these concerns based on recent clinical studies, summarize the mechanisms of action of DAAs and survey the updated data on HCV-induced liver carcinogenesis.
Core tip: Inability to reach sustained virological response (SVR) and cirrhosis are independent prognostic factors for developing hepatocellular carcinoma (HCC) in hepatitis C virus (HCV) patients from the interferon (IFN) era. DAAs offer significantly better SVR rates. The first data regarding HCC occurrence after direct antiviral agent (DAA) treatment are similar to the data from patients who achieved SVR under IFN treatment. Some reports on early HCC occurrence or recurrence after DAA treatment are probably due to selection bias, as they were not reproduced in large comparative studies. DAAs can eradicate HCV, but they cannot terminate HCV-induced premalignant processes once triggered.
- Citation: Gigi E, Lagopoulos VI, Bekiari E. Hepatocellular carcinoma occurrence in DAA-treated hepatitis C virus patients: Correlated or incidental? A brief review. World J Hepatol 2018; 10(9): 595-602
- URL: https://www.wjgnet.com/1948-5182/full/v10/i9/595.htm
- DOI: https://dx.doi.org/10.4254/wjh.v10.i9.595
Hepatocellular carcinoma (HCC) accounts for approximately 5.6% of all cancers[1], making it the fifth most common cancer worldwide. There is an increasing trend in HCC incidence over the past two decades[2], so today, HCC is considered the second leading cause of cancer-related death[3]. One of the most well-known predisposing factors for HCC is chronic infection with hepatitis C virus (HCV), which is associated with a 15- to 20- fold increased risk for HCC development[4]. HCV was first recognized as a distinct clinical entity in 1989 and has since become one of the most rapidly evolving fields of hepatology. Multiple studies have reported progression of chronic HCV infection to severe fibrosis and cirrhosis in 5%-20% of the patients in a period of 5-20 years[2,3,5]. Once HCV-induced cirrhosis has been established, there is an estimated annual risk of 3%-7% of developing HCC[6]. The advent of the first anti-HCV drugs, predominantly PEGylated interferon-α (PEG IFNα), was a major breakthrough in the management of HCV infection and consequently in HCC prevention, as there was no other specific treatment at this time. Sustained virological response (SVR) with combined regimens, consisting of ribavirin and PEG IFN, can be achieved in approximately 55%[7] of patients, with some issues of tolerability and the limitation of being contraindicated in patients with decompensated cirrhosis. A meticulous study on the molecular biology and pathophysiology of HCV infection led to the invention of newer direct antiviral agents (DAAs), dramatically changing the landscape of HCV infection such that viral hepatitis C should be considered a highly curable disease. Although the eradication of HCV infection with DAAs seems to be feasible in the majority of patients, there are a measurable number of patients who will progress to HCC despite viral clearance. There are even some reports[8,9] that imply a correlation of HCC development with the use of newer oral regimes. In the present review, we attempt to clarify this puzzling topic based on recent reports from everyday clinical life, as well as reports on the pharmacological properties of the drugs used and the HCV-induced carcinogenesis sequence.
Chronic viral hepatitis, from either hepatitis B Virus (HBV) or HCV is the most significant predisposing factor for HCC. Forecasting models predict that without treatment, 14.4% of all HCV patients will develop HCC[10]. If we keep in mind that globally, 150 million individuals are estimated to have HCV infection[11], it is obvious that HCV eradication should be a major priority to decrease the incidence of HCC. SVR is currently accepted as the best tool to confirm viral eradication, and indeed, SVR has been shown to significantly reduce liver-related mortality, including the risk of HCC[3,4,12].
Interferon (IFN)-α was the first drug to gain approval for HCV infection, in the late 1980s. The immunomodifying, antiviral and antitumoral properties of IFN-α raised hopes that it would succeed both in HCV eradication and in HCC prevention, as it also inhibits liver cancer growth[13]. The low SVR rates, low compliance and high toxicity reported with IFN led to the modification of the IFN molecule and the circulation of PEG-IFN in the 2000s. This modified form of recombinant human IFN-α has better absorption, prolonged half-life and better compliance, so it came into wider use in the treatment of HCV for about a decade, mainly in combination with the antiviral drug ribavirin.
Several studies[2,5,14] have focused on the SVR rates with IFN-based regimens and the impact the SVR has on HCC occurrence. Achievement of viral clearance was associated with reduced incidence of HCC and diminishment of other liver-related events (0 in SVR patients vs 1.88 in non-SVR per 100 person-years of follow-up), as shown in an Italian multicenter study[14]. In that paper, 920 patients with histologically proven cirrhosis were treated with monotherapy with conventional IFN-α. Although the SVR rate in that era was extremely low (124/920 or 13.5%), there was still an obvious reduction in HCC occurrence in responders (5.6% vs 16%, P < 0.01). In the well-structured study of Van der Meer and colleagues, they compared long-term treatment outcomes among IFN monotherapy, IFN + ribavirin and PEG IFN + ribavirin in 5 hepatologic centers across Europe and Canada[15]. Recruited patients had advanced fibrosis (Ishak score > 4) and were followed up for a mean period of 8.4 years. SVR was achieved in 36% of the patients, and these patients had significantly lower all-cause mortality (8.9% vs 26.1%, P < 0.001) and liver-specific mortality (0.23 vs 3.20/100 pts, P < 0.001). The 10-year cumulative HCC incidence rate was 5.1% (95%CI: 1.3%-8.9%) for responders (SVR) vs 21.8% (95%CI: 16.6%-27.0%) for non-SVR patients (P < 0.001). The relatively low SVR rates shown in that study may be attributed to the advanced fibrosis of the patients, as well as the high percentage of patients (47%) with anti-hepatitis B core antigen positivity. Ogawa et al[16] conducted a prospective national multicenter study on the effect of PEG IFN + ribavirin treatment on chronic HCV infection, focusing on the oncologic outcomes, specifically on the incidence of HCC. The 1013-patient sample included cirrhotic (150) and non-cirrhotic (863) individuals, with 47 patients (4.6%) developing HCC during a mean follow-up of 3.6 years. As SVR rate of 55% was found, and not surprisingly, non-cirrhotic treatment responders had a better prognosis regarding HCC occurrence: 5-year cumulative incidence rates of HCC were 1.7% in the SVR group and 7.6% in the non-SVR group (P = 0.003), but this effect was attenuated in the cirrhotic group (18.9% vs 39.4%, P = 0.03). The authors identified platelet count < 150000/mL (HR = 4.04), failure of SVR (HR = 3.72), cirrhosis (HR = 3.22), male sex (HR = 2.98), age ≥ 60 years (HR = 2.81) and α-fetoprotein (αFP) above 10 ng/mL (HR = 2.50) as independent risk factors for HCC development. Similar results were shown in a retrospective study from Japan[17], where reaching SVR after IFN-based treatment significantly reduced the 5-year HCC incidence (2.6% vs 8.2%, P < 0.001).
Hepatoma development after HCV treatment with IFN-based regimens was the main question of a meta-analysis by Morgan et al[18] in 2013. The authors included in their analysis data from 30 observational studies, comprising in total 31528 patients from 17 countries. Most of the studies selected stratified the patients according to fibrosis level, while 8 studies referred only to patients with advanced fibrosis and/or cirrhosis. Mean SVR rates were found to be quite low, as only 10853 patients (34.4%) achieved an SVR to treatment, while 1742 patients (or 5.5% of all patients) developed HCC during follow-up. When they adjusted HCC incidence according to the follow-up period and viral clearance, they found that hepatoma in HCV-treated patients developed at a rate of 0.33% per person-year (CI: 0.22% to 0.50%) in those who achieved SVR, significantly lower than the 1.67% (CI: 1.15% to 2.42%) of non-responders.
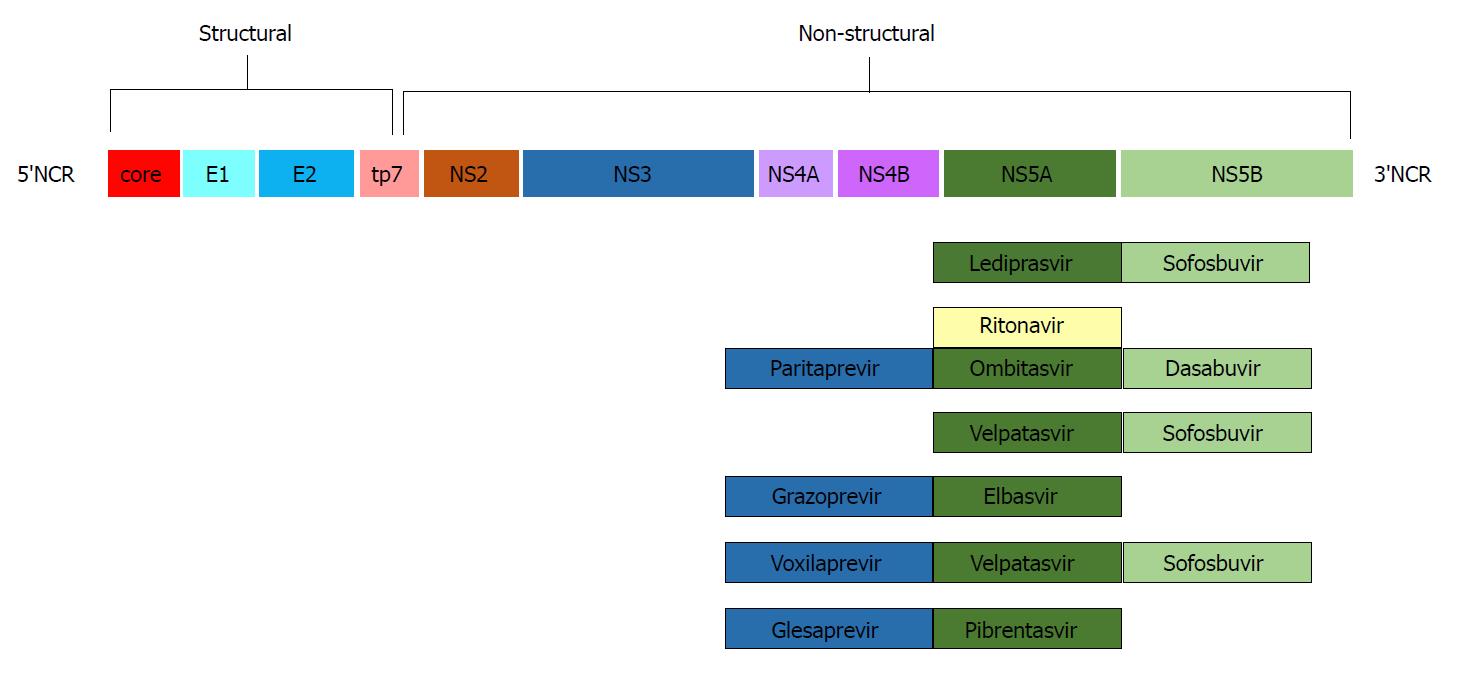
The study and analysis of the HCV genome led to the production of pharmacologic agents targeting specific regions of its nucleic RNA. The first drugs to come into commercial use were telaprevir and boceprevir, both protease inhibitors of the non-structural viral protein 3-4A (NS3-4A)[19,20]. The addition of these agents to the “traditional” PEG IFN + ribavirin regimen increased SVR rates to approximately 70% and gained FDA approval for the treatment of HCV genotype 1 in 2011. A new target area (non-structural protein 5B -NS5B) was discovered, and sofosbuvir, an NS5B polymerase inhibitor[21], was approved in 2013. Addition of sofosbuvir to the PEG IFN + ribavirin combination achieved SVR rates above 90%, and most important of all, the scheme was active against genotypes 1 and 4, while the combination of sofosbuvir and ribavirin achieved similar SVR rates in genotypes 2 and 3[22]. The advent in late 2014 of NS5A inhibitors such as ledipasvir, in combination with sofosbuvir in a once-daily oral scheme, achieved SVR rates of 94%-99% in HCV genotype 1 patients in just 12 wk[23]. Since then, more agents and combinations have come into use, and today, there are a variety of treatment options for HCV eradication, as shown in Figure 1.
Reaching SVR significantly reduces the risk of developing hepatoma, as was already known from the IFN era, so one may have expected that with the newer DAAs, such a risk would be minimized to an occasional event, but first reports from the wider clinical applications of the new treatments were worrying. Conti et al[9] published in 2016 a regional multicenter study of 344 consecutive cirrhotic patients treated with DAAs, 285 of them with no previous history of HCC. Although DAA therapy achieved sustained virological response in 91% of patients, during the 24-wk follow-up, 9 of these 285 patients (3.16%, 95%CI: 1.45-5.90) developed HCC, much higher than previous reports on IFN responders. The authors noted though that the new IFN-free oral regimens lacked the tolerability and toxicity limitations of IFN therapy, so patients with advanced liver disease who were not candidates for treatment before were receiving therapy now. Furthermore, patients were examined solely by ultrasound before the initiation of treatment, increasing the possibility of small HCC nodules escaping notice. One other study, from Spain[8], that focused on early HCC recurrence in DAA-treated HCV patients expressed similar concerns, and a few months later, the announcement of preliminary findings by Kozbial et al[24] added to the cloud of suspicion. In a way, all these reports could be considered preliminary, keeping in mind that the new oral IFN-free regimens came into wide clinical use in 2015.
Larger studies were published last year, focusing on the concerns of carcinogenesis in DAA-treated patients. A big retrospective study from 129 Veteran Health Hospitals in the United States[25] reported an annual incidence of HCC of 0.9% in patients who accomplished viral clearance after treatment with DAAs. Approximately 40% of the patients included had cirrhosis at the initiation of treatment, and approximately one-half had significant comorbidities; that may be the reason that SVR rates were relatively low (86.7%). In subgroup analysis, however, non-cirrhotic patients who achieved SVR had an even lower annual incidence of HCC, at 0.34%, while the cirrhotic responders presented an annual incidence of 1.8%, comparable to that of non-responders irrespectively of cirrhosis status (3.45%).
The same team mentioned in the IFN era led by Dr. Ogawa[26] published a retrospective multicenter analysis in 2017 regarding early HCC development in patients who responded to DAA treatment. They reported an annual incidence of de novo hepatoma of 0.4% in non-cirrhotic patients, while in the cirrhotic ones HCC developed at an annual rate of 4.9%. End-of-treatment levels of αFP above 9.0 ng/mL in the individuals who were already cirrhotic before treatment, as well as low platelet count (< 150000/mL) and advanced fibrosis in the non-cirrhotic patients, were recognized as independent predictors of de novo HCC.
As expected, research articles comparing the oncologic outcomes of IFN-based vs IFN-free regimens in HCV-infected persons were published in 2017. In the study of Nagata et al[17], 1897 patients treated for HCV over a 12-year period were analyzed, regardless of previous history of HCC. Although the IFN-free group of patients had worse baseline characteristics (advanced fibrosis/cirrhosis, older), the SVR rates were 65% for the IFN-based group and 96% for the IFN-free group (P < 0.001). The relatively high SVR rate for the IFN-based group can probably be attributed to the fact that 1/3 of the patients received DAAs in combination with PEG IFN. The 5-year incidence rates were 2.6% (SVR) vs 8.2% (non-SVR) for the IFN group (P < 0.001), and the 3-year incidence rates for the DAA-only group were 3.3% vs 5.9%, respectively (P = 0.031). In propensity score-matched analysis, no significant differences were found in HCC occurrence (P = 0.49). The authors reported that post-treatment αFP and Wisteria floribunda agglutinin-positive Mac-2 binding protein (WFA+M2BP) could be used as independent prognostic factors of HCC development in patients achieving viral clearance.
Viral clearance by DAA treatment was associated with a 71% reduction in HCC risk in the large retrospective study by Ioannou et al[27]. The 62354 patients included were stratified in three subgroups according to HCV treatment: IFN only, IFN + DAA, and DAA only. Among patients in whom treatment succeeded (SVR), HCC developed in 2.5% of the IFN, 2.1% of the IFN+DAA group and 1.4% of the DAA group, but the follow-up period was shorter for the DAA-containing groups. Conversion to HCC incidence per patient-year showed a slight increase in hepatoma development in the DAA-only group, and this was due to the high prevalence of other risk factors that these patients had, such as cirrhosis, older age, diabetes and low serum albumin.
HCV patients with more advanced disease or other significant risk factors that were previously excluded for IFN therapy but now became candidates for treatment with DAAs probably create an unavoidable selection bias. To investigate this issue further, Waziry and colleagues[28] performed a meta-analysis of HCC development following DAA therapy, including 17 studies with IFN treatment and 9 studies with DAA treatment. HCC occurrence in patient-years was estimated to be higher in DAA patients than in IFN-treated individuals because patients from the IFN-free group were followed for a shorter period and were older. Adjusted meta-regression analysis showed a relative risk of de novo HCC in DAA-treated patients vs IFN-treated of 0.67 (95%CI: 0.16-2.77, P = 0.56).
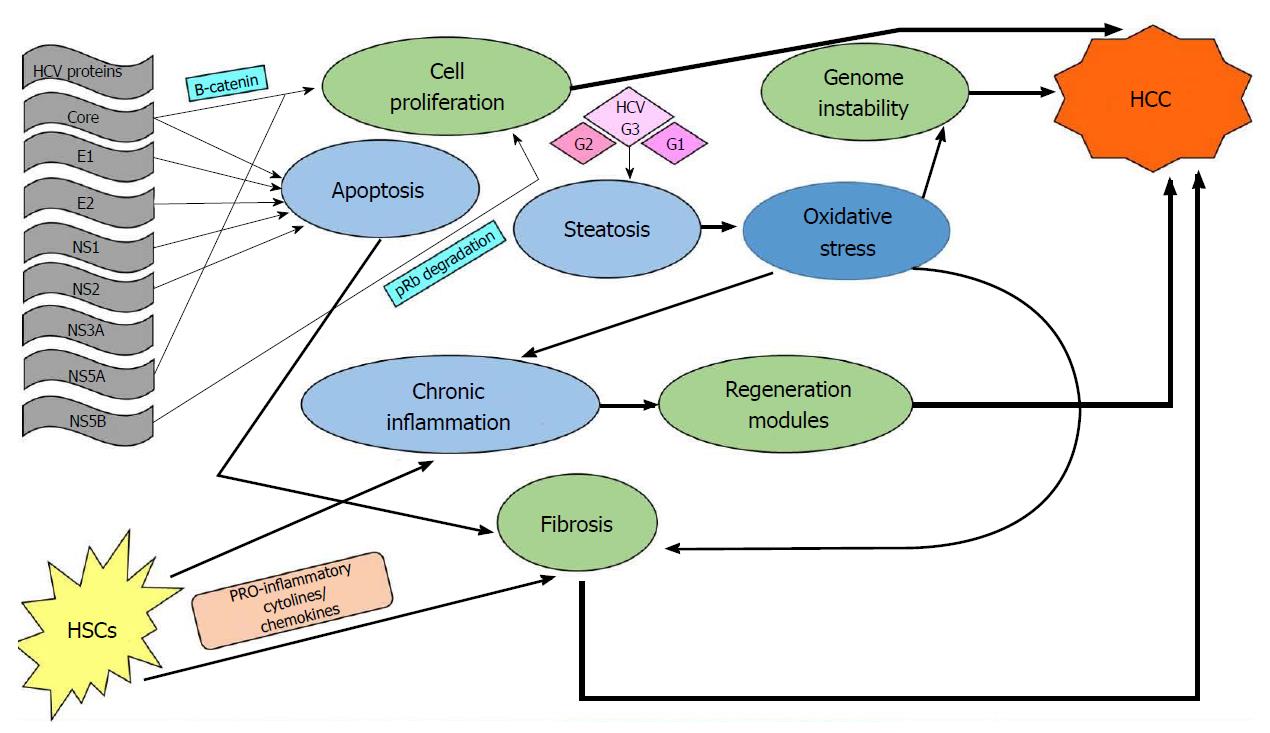
Relevant literature supports the concept that the occurrence of hepatocellular carcinoma is a multitasking process that occurs silently over years in patients with chronic HCV infection. Possible pathways include direct viral effects on the hepatic cells, immune-mediated genetic alterations, and stromal involvement via the fibrosis route. A schematic review of these pathways can be seen in Figure 2.
There is strong evidence that certain viral proteins trigger proliferating signaling in the hepatic cell, especially by the β-catenin pathway[29]. The Core and NS5A proteins are thought to play key roles in that interference, while NS5B has been found to trigger tumor suppressor protein degradation[30]. Additionally, viral proteins such as Core, E1, E2, NS1, and NS2 seem to induce the normal apoptotic pathway[31].
Immune-mediated effects of HCV infection comprise a complex pathway in liver carcinogenesis. In addition to the immediate effect of infection, which is the development of chronic inflammation and consequent regeneration[29], there is an accumulative effect on the host genome that progressively changes the hepatocellular phenotype[32]. Stromal involvement in the inflammatory process is mediated by activation of hepatic stellate cells (HSCs), which initially secrete cytokines and chemokines that further promote the inflammation/damage/regeneration cycle[30]. Activated HSCs become matrix-secreting myofibroblasts promoting liver fibrosis and therefore play a crucial role in cancer initiation[30,33]. Furthermore, certain HCV genotypes, mainly genotype 3 but also genotypes 1 and 2, induce viral steatohepatitis, probably through the action of viral Core protein[34]. Steatosis extends the oxidative stress induced by HCV infection itself[35], and once again, HSCs are activated, triggering collagen production and fibrosis[30].
From all the above, it becomes clear that there are distinct different pathways through which an HCV infection can induce HCC. It should be underlined, however, that some biologic cascades, once triggered, can go on independently of viral presence.
Nearly all studies regarding HCC incidence in HCV patients show 2 specific factors predicting unfavorable outcomes: cirrhosis and inability to reach a SVR. Which one of the two has the biggest impact on liver carcinogenesis may be suggested by the findings of Ioannou and colleagues[27]: The occurrence of hepatocellular carcinoma, regardless of treatment, was lowest in the non-cirrhotic responders (0.24), second lowest in non-cirrhotic non-responders (0.87), second highest among cirrhotic responders (1.97) and highest, as expected, in cirrhotic non-SVR patients (3.25). Based on this, one could rather safely suggest that presence of cirrhosis affects liver carcinogenesis more robustly than the HCV virus itself.
In the present review, we decided not to focus on recurrence of HCC in HCV patients treated with IFN-free regimens, for reasons associated with the oncogenic sequence mentioned above. To be more specific, the liver of a patient who has already developed a hepatoma due to HCV is probably in a diffuse precancerous state, meaning there are quite likely some other premalignant lesions, although they are not evident. This could be the reason that occurrence and recurrence of HCC after DAA therapy seem to occur quite early, presumably in the first year. Viral clearance may inhibit further direct oncogenic interference, but the fibrosis level as well as the regenerative process probably cannot be significantly reversed. Some authors believe that the sudden decrease in viral load achieved with DAAs causes immune distortion[36], deregulating the anti-tumor response and therefore releasing precancerous foci from immune surveillance. There are some data supporting this hypothesis, as DAA-induced SVR is associated with loss of intrahepatic immune activation by IFN[37], yet there is no evidence of direct or indirect oncogenic effects of direct antiviral regimens. On the other hand, Abdelaziz et al[38] conducted a retrospective analysis on HCC occurrence vs recurrence in DAA-treated patients. They found that de novo lesions had significantly better response rates to ablation. That could be interpreted as either that the recurrent tumors are less resistant or that DAA therapy somehow interferes with the malignant potential of de novo lesions.
IFN has shown antitumor properties[39], apart from its anti-inflammatory properties, that probably suppress-up to a point-the malignant growth in some patients. Whether there would be a benefit to prescribing IFN to patients who reached a sustained virologic response after IFN-free treatment, based on prognostic factors such as end-of-treatment αFP, low platelet count or low serum albumin, should be further investigated. Unfortunately, tolerability and contraindications limit the percentage of HCV patients who would benefit from such a tumor-preventive IFN maintenance.
Direct antiviral agents can radically change the landscape of HCV infection, as they achieve great SVR rates with excellent patient compliance. SVR is a well-recognized risk-reducing factor for HCC development, and early studies confirm this positive effect of DAA treatment. Although there are a few publications with unexpected and undesired oncologic outcomes after IFN-free treatment, it could be that they represent solitary incidental reports. More studies with longer follow-up are needed to investigate this topic. The new drugs seem to live up to their promise, so they deserve time to show their long-term effects in real life.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Greece
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ozaki I, Wang K S- Editor: Wang JL L- Editor: A E- Editor: Tan WW
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20516] [Article Influence: 2051.6] [Reference Citation Analysis (20)] |
| 2. | Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology. 2015;149:1471-1482.e5; quiz e17-e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 359] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 3. | D’Ambrosio R, Della Corte C, Colombo M. Hepatocellular Carcinoma in Patients with a Sustained Response to Anti-Hepatitis C Therapy. Int J Mol Sci. 2015;16:19698-19712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 4. | Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35-S50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1691] [Cited by in RCA: 1793] [Article Influence: 85.4] [Reference Citation Analysis (2)] |
| 5. | Strader DB, Wright T, Thomas DL, Seeff LB; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39:1147-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1283] [Cited by in RCA: 1232] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 6. | Roche B, Coilly A, Duclos-Vallee JC, Samuel D. The impact of treatment of hepatitis C with DAAs on the occurrence of HCC. Liver Int. 2018;38 Suppl 1:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 108] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 7. | Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL Jr, Häussinger D, Diago M, Carosi G, Dhumeaux D, Craxi A, Lin A, Hoffman J, Yu J. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4847] [Cited by in RCA: 4748] [Article Influence: 206.4] [Reference Citation Analysis (0)] |
| 8. | Reig M, Mariño Z, Perelló C, Iñarrairaegui M, Ribeiro A, Lens S, Díaz A, Vilana R, Darnell A, Varela M. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol. 2016;65:719-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 803] [Article Influence: 89.2] [Reference Citation Analysis (0)] |
| 9. | Conti F, Buonfiglioli F, Scuteri A, Crespi C, Bolondi L, Caraceni P, Foschi FG, Lenzi M, Mazzella G, Verucchi G. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol. 2016;65:727-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 699] [Article Influence: 77.7] [Reference Citation Analysis (0)] |
| 10. | Rein DB, Wittenborn JS, Weinbaum CM, Sabin M, Smith BD, Lesesne SB. Forecasting the morbidity and mortality associated with prevalent cases of pre-cirrhotic chronic hepatitis C in the United States. Dig Liver Dis. 2011;43:66-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 166] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 11. | Hoshida Y, Fuchs BC, Bardeesy N, Baumert TF, Chung RT. Pathogenesis and prevention of hepatitis C virus-induced hepatocellular carcinoma. J Hepatol. 2014;61:S79-S90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 166] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 12. | Simmons B, Saleem J, Heath K, Cooke GS, Hill A. Long-Term Treatment Outcomes of Patients Infected With Hepatitis C Virus: A Systematic Review and Meta-analysis of the Survival Benefit of Achieving a Sustained Virological Response. Clin Infect Dis. 2015;61:730-740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 212] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 13. | Yano H, Iemura A, Haramaki M, Ogasawara S, Takayama A, Akiba J, Kojiro M. Interferon alfa receptor expression and growth inhibition by interferon alfa in human liver cancer cell lines. Hepatology. 1999;29:1708-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 100] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Bruno S, Stroffolini T, Colombo M, Bollani S, Benvegnù L, Mazzella G, Ascione A, Santantonio T, Piccinino F, Andreone P, Mangia A, Gaeta GB, Persico M, Fagiuoli S, Almasio PL; Italian Association of the Study of the Liver Disease (AISF). Sustained virological response to interferon-alpha is associated with improved outcome in HCV-related cirrhosis: a retrospective study. Hepatology. 2007;45:579-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 475] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 15. | van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F, Duarte-Rojo A, Heathcote EJ, Manns MP, Kuske L. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584-2593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1165] [Cited by in RCA: 1167] [Article Influence: 89.8] [Reference Citation Analysis (0)] |
| 16. | Ogawa E, Furusyo N, Kajiwara E, Takahashi K, Nomura H, Maruyama T, Tanabe Y, Satoh T, Nakamuta M, Kotoh K, Azuma K, Dohmen K, Shimoda S, Hayashi J; Kyushu University Liver Disease Study (KULDS) Group. Efficacy of pegylated interferon alpha-2b and ribavirin treatment on the risk of hepatocellular carcinoma in patients with chronic hepatitis C: a prospective, multicenter study. J Hepatol. 2013;58:495-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 17. | Nagata H, Nakagawa M, Asahina Y, Sato A, Asano Y, Tsunoda T, Miyoshi M, Kaneko S, Otani S, Kawai-Kitahata F. Effect of interferon-based and -free therapy on early occurrence and recurrence of hepatocellular carcinoma in chronic hepatitis C. J Hepatol. 2017;67:933-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 169] [Article Influence: 21.1] [Reference Citation Analysis (1)] |
| 18. | Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158:329-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 653] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 19. | Poordad F, McCone J Jr, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N, DiNubile MJ, Sniukiene V, Brass CA, Albrecht JK, Bronowicki JP; SPRINT-2 Investigators. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1996] [Cited by in RCA: 1981] [Article Influence: 141.5] [Reference Citation Analysis (0)] |
| 20. | Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405-2416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1905] [Cited by in RCA: 1854] [Article Influence: 132.4] [Reference Citation Analysis (0)] |
| 21. | Membreno FE, Lawitz EJ. The HCV NS5B nucleoside and non-nucleoside inhibitors. Clin Liver Dis. 2011;15:611-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, Romero-Gomez M, Zarski JP, Agarwal K, Buggisch P. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889-1898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1357] [Cited by in RCA: 1365] [Article Influence: 124.1] [Reference Citation Analysis (0)] |
| 23. | Kowdley KV, Gordon SC, Reddy KR, Rossaro L, Bernstein DE, Lawitz E, Shiffman ML, Schiff E, Ghalib R, Ryan M. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370:1879-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 911] [Cited by in RCA: 928] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 24. | Kozbial K, Moser S, Schwarzer R, Laferl H, Al-Zoairy R, Stauber R, Stättermayer AF, Beinhardt S, Graziadei I, Freissmuth C. Unexpected high incidence of hepatocellular carcinoma in cirrhotic patients with sustained virologic response following interferon-free direct-acting antiviral treatment. J Hepatol. 2016;65:856-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 178] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 25. | Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of Hepatocellular Cancer in HCV Patients Treated With Direct-Acting Antiviral Agents. Gastroenterology. 2017;153:996-1005.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 685] [Article Influence: 85.6] [Reference Citation Analysis (0)] |
| 26. | Ogawa E, Furusyo N, Nomura H, Dohmen K, Higashi N, Takahashi K, Kawano A, Azuma K, Satoh T, Nakamuta M, Koyanagi T, Kato M, Shimoda S, Kajiwara E, Hayashi J; Kyushu University Liver Disease Study (KULDS) Group. Short-term risk of hepatocellular carcinoma after hepatitis C virus eradication following direct-acting anti-viral treatment. Aliment Pharmacol Ther. 2018;47:104-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 27. | Ioannou GN, Green PK, Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol. 2018;68:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 377] [Article Influence: 47.1] [Reference Citation Analysis (1)] |
| 28. | Waziry R, Hajarizadeh B, Grebely J, Amin J, Law M, Danta M, George J, Dore GJ. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: A systematic review, meta-analyses, and meta-regression. J Hepatol. 2017;67:1204-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 381] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 29. | Arzumanyan A, Reis HM, Feitelson MA. Pathogenic mechanisms in HBV- and HCV-associated hepatocellular carcinoma. Nat Rev Cancer. 2013;13:123-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 634] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 30. | Yi Z, Yuan Z. Hepatitis C Virus-Associated Cancers. Adv Exp Med Biol. 2017;1018:129-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | McGivern DR, Lemon SM. Virus-specific mechanisms of carcinogenesis in hepatitis C virus associated liver cancer. Oncogene. 2011;30:1969-1983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 171] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 32. | Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1097] [Cited by in RCA: 1104] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 33. | Thompson AI, Conroy KP, Henderson NC. Hepatic stellate cells: central modulators of hepatic carcinogenesis. BMC Gastroenterol. 2015;15:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 34. | Barba G, Harper F, Harada T, Kohara M, Goulinet S, Matsuura Y, Eder G, Schaff Z, Chapman MJ, Miyamura T. Hepatitis C virus core protein shows a cytoplasmic localization and associates to cellular lipid storage droplets. Proc Natl Acad Sci USA. 1997;94:1200-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 499] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 35. | Lonardo A, Adinolfi LE, Loria P, Carulli N, Ruggiero G, Day CP. Steatosis and hepatitis C virus: mechanisms and significance for hepatic and extrahepatic disease. Gastroenterology. 2004;126:586-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 338] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 36. | Nault JC, Colombo M. Hepatocellular carcinoma and direct acting antiviral treatments: Controversy after the revolution. J Hepatol. 2016;65:663-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 37. | Llovet JM, Villanueva A. Liver cancer: Effect of HCV clearance with direct-acting antiviral agents on HCC. Nat Rev Gastroenterol Hepatol. 2016;13:561-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 38. | Abdelaziz AO, Nabil MM, Abdelmaksoud AH, Shousha HI, Cordie AA, Hassan EM, Omran DA, Leithy R, Elbaz TM. De-novo versus recurrent hepatocellular carcinoma following direct-acting antiviral therapy for hepatitis C virus. Eur J Gastroenterol Hepatol. 2018;30:39-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Miki A, Yano Y, Kato H, Seo Y, Kuriyama M, Azuma T, Hayashi Y. Anti-tumor effect of pegylated interferon in the rat hepatocarcinogenesis model. Int J Oncol. 2008;32:603-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |