Published online Jul 27, 2018. doi: 10.4254/wjh.v10.i7.517
Peer-review started: March 29, 2018
First decision: April 23, 2018
Revised: April 25, 2018
Accepted: May 30, 2018
Article in press: May 31, 2018
Published online: July 27, 2018
Processing time: 120 Days and 20.2 Hours
Primary hepatic gastrinoma is a rare disease, with fewer than 40 cases reported in the medical literature. Because it is located in an organ in which metastases are common, its diagnosis is difficult. We report a case of a 19 years old male patient with a history of gastric ulcers since the age of nine. Following gastric surgery, an antrectomy and a vagotomy, there was some alleviation of symptoms. Subsequently, the patient reported various intermittent episodes of diarrhea, diffuse abdominal pain, and vomiting. The patient underwent tomography, which revealed the presence of a hepatic mass measuring 19.5 cm × 12.5 cm × 17 cm. Primary hepatic gastrinoma was diagnosed based on laboratory examinations that indicated hypergastrinemia and a positron emission tomography/magnetic resonance study with somatostatin analogue that confirmed the liver as the primary site. After hepatic trisegmentectomy (II, III, IV, V, VIII), the patient’s symptoms improved. The case is notable for the presence of a rare tumor with uncommon dimensions.
Core tip: Primary hepatic gastrinoma is a very rare disease. Due to its location in an organ in which metastases are common, its diagnosis is difficult. We report a case of a 19 years old male patient with a history of gastric ulcers since the age of nine. Hypergastrinemia and a PET/MR study with somatostatin analogue confirmed that the liver was the primary site. After hepatic trisegmentectomy, the patient’s symptoms improved. The case is notable for the presence of a rare tumor with uncommon dimensions.
- Citation: Pipek LZ, Jardim YJ, Mesquita GHA, Nii F, Medeiros KAA, Carvalho BJ, Martines DR, Iuamoto LR, Waisberg DR, D’Albuquerque LAC, Meyer A, Andraus W. Large primary hepatic gastrinoma in young patient treated with trisegmentectomy: A case report and review of the literature. World J Hepatol 2018; 10(7): 517-522
- URL: https://www.wjgnet.com/1948-5182/full/v10/i7/517.htm
- DOI: https://dx.doi.org/10.4254/wjh.v10.i7.517
Zollinger-Ellison syndrome[1] is characterized by gastric hypersecretion, resulting in peptic disease and diarrhea[2]. Diagnosis is confirmed by gastrin levels of over 1000 pg/mg. This syndrome was first described by Zollinger and Ellison[2] in 1955. With an incidence of around 0.5 to 2 per million, the majority of patients are diagnosed between 20 and 50 years of age, with a higher prevalence among men. The majority of gastrinomas are sporadic, and 20%-30% are associated with type 1 endocrine neoplasias, NEM-1[3].
The classical clinical presentation of the syndrome is the presence of abdominal pain (75%) and chronic diarrhea (73%). Half of patients present with burning as a result of gastroesophageal reflux. There is still a significant population (17%-25%) that presents with weight loss and gastrointestinal bleeding[4]. Over 90% of cases of gastrinomas are present in the so-called “gastrinoma triangle”, delimited by the cystic duct, the junction between the second and third portions of the duodenum, and the junction between the colon and the pancreatic body. Occasionally, gastrinomas have also been described in other areas, including lymph nodes, biliary tree, ovaries, kidneys, heart, gallbladder, omentum, and liver[5-7]. This article describes a case of primary hepatic gastrinoma in a 19 years old hypergastremic patient who underwent hepatic trisegmentectomy to remove the tumor.
A male patient, 19 years of age, was referred as a result of intermittent abdominal pain over the previous 10 years. He reported an attack of diffuse abdominal pain for 1 month at the age of nine. At that time, a gastric ulcer was diagnosed, and due to the failure of clinical treatment, he underwent antrectomy with vagotomy in another service. In the years following the surgery, the patient presented sporadic episodes of non-specific diffuse abdominal pain associated with nausea and vomiting. Diarrhea was also present, but dyspeptic characteristics were not. During that time, emergency treatment was sought with regularity, with improvement following clinical treatment. A loss of around 16 kg of body weight was reported over the year prior to admission to our service (from 68 to 52 kg).
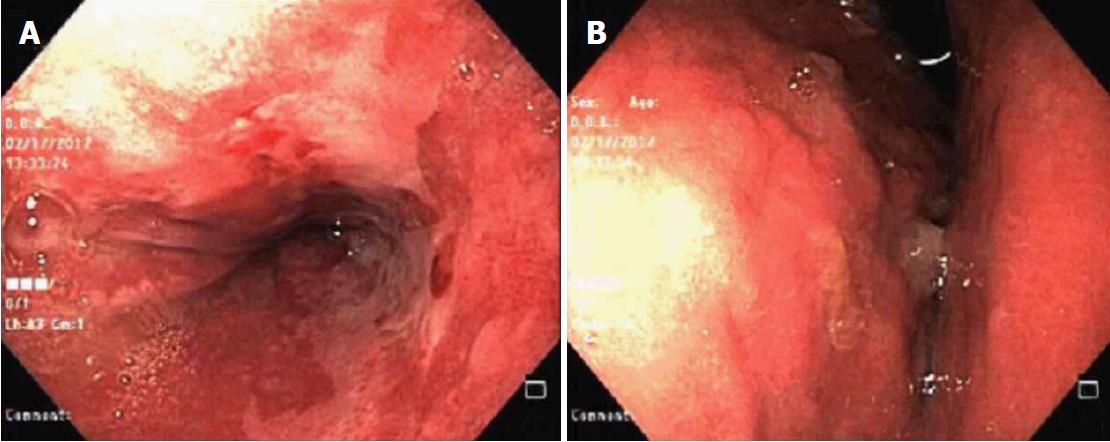
Upper digestive endoscopy was carried out. In the third distal, close to the esophageal transition, the mucosa was pearlescent and thickened, which was associated with confluent erosions larger than 5 mm. This covered 100% of the circumference of the organ, with ulcerations and fibrin (intense erosive distal esophagitis-Los Angeles Class D). Furthermore, an image compatible with extrinsic compression of the stomach was revealed (Figure 1).
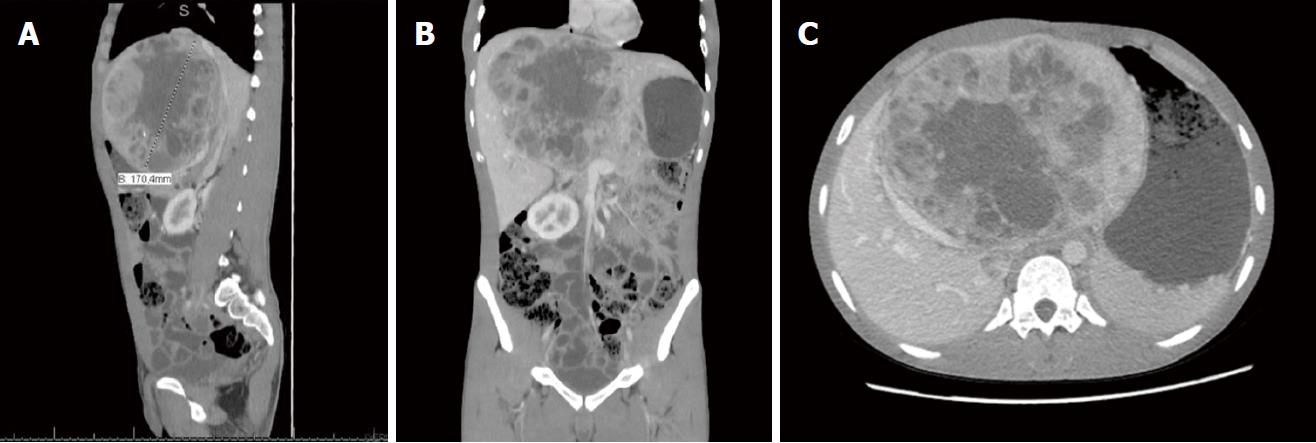
Computed tomography (CT) and magnetic resonance (Figure 2) were carried out, showing increased dimensions of the liver, as the result of a voluminous expansive hypervascularized lesion with necrotic central areas. Involvement in the portal vein was identified, and the mass mainly occupied the central portion of the liver and hilum, affecting segments I, II, III, IV, VIII and measuring 19.5 cm × 12.5 cm × 17.0 cm.
A biopsy was carried out with the following immuno-histochemical characteristics: cytokeratin 7 negative, cytokeratin 20 negative, chromogranin positive, synaptophysin positive, CD99 positive, Hep par 1 negative, polyclonal CEA positive (standard cytoplasma and membrane), and Ki 67 positive (around 5%). It was concluded that the profile was compatible with a well-defined neuroendocrine tumor (NET Grade 2). The diagnosis was confirmed by the discovery of gastrin hypersecretion, via chemiluminescence, with a resulting value of 6709.0 pg/mL.
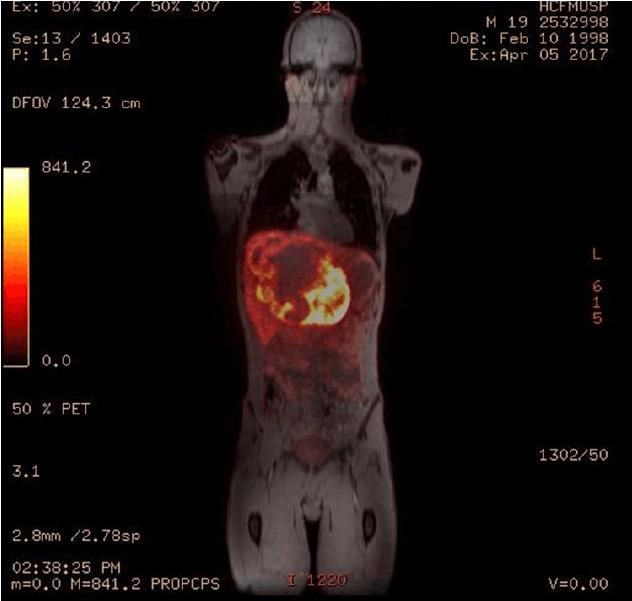
Considering that the liver is a common location for metastasis, it was important to define whether or not the tumor was primary. To do this, positron emission tomography associated with magnetic resonance was carried out - PET-MR - with somatostatin analogue (Figure 3). The examination was carried out following the administration of 2.7 mCi of Galium-68-DOTA-Octreotate (68Ga-DOTATATE) intravenously. The examination showed a relatively well-defined expansive lesion on the liver, with lobulated contours, situated in the left lobe. Heterogeneity in T2 and an anomalously high concentration of radiopharmacus was noted. It was heterogeneously distributed, with central and peripheral areas with hypoconcentration of radiotracer (which can correspond to necrosis/liquefaction), measuring around 18.1 cm × 11.3 cm at the largest axes. Contact and constriction of the inferior vena cava, which was permeable, were sustained. There were no signs of invasion of the head or body of the pancreas.
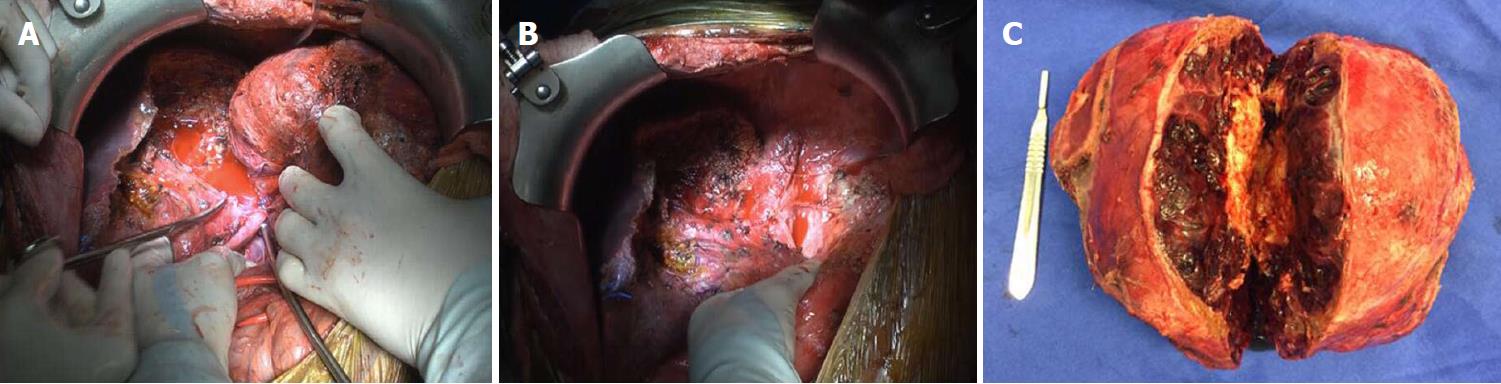
From these examinations it was possible to confirm the diagnosis of primary hepatic gastrinoma and to recommend surgical resection. Prior to the surgical procedure, there were a number of intracavity adher–ences. Hepatic trisegmentation (II, III, IV, V, VIII) was carried out (Figure 4), identifying a large volume mass, mainly occupying the left lobe of the liver. Segments V and VIII were most affected, with atrophy in segment II, as well as compensatory hypertrophy in segments VI and VII. The remaining liver maintained approximately 80% of the volume of a functional liver. The patient was readmitted in the early postoperative phase with abdominal pains and leaking of bile through the drain due to a biliary fistula. The patient was submitted to a surgical procedure with a good outcome. Six months after the resection, the patient was well, had no relapses, and had a gastrin level of 40.5 pg/mL. At 1 year after the surgery, exam showed a gastrin level of 89.2 pg/mL. The normal range of gastrin is 13 to 115 pg/mL.
Zollinger-Ellison syndrome (ZES) is predominantly sporadic, although in 20%-30% of cases its origin is type 1 multiple endocrine neoplasia (MEN1). MEN 1 is a genetic disease that occurs in the gene MEN1, leading to the formation of various neoplasias; pancreatic endocrine tumors, pituitary adenomas, and hyperplasia of the parathyroid are the most prevalent. Despite the evidence of ulcers in this patient at the age of nine, ZES should be considered, principally because of the patient’s history and hypergastrinemia. There was no report of the occurrence of MEN1 together with primary hepatic gastrinoma, including in our case.
Gastropancreatic neuroendocrine neoplasias, which can cause ZES, are classified according to their prognosis. Well-defined neuroendocrine tumors have a clinical course that is much less aggressive and can be subclassified as G1 (Ki-67 < 3) and G2 (Ki-67 of 3%-20%), according to the Ki-67 index that assesses the level of differentiation of cells in tissue. Poorly defined tumors are in category G3, presenting Ki-67 > 20%. Histopathological analysis of the patient’s tumor revealed a Ki-67 of 5% (G2)[8]. The most common gastrinomas, duodenal and pancreatic, present a mean diameter of 1 and 3 cm, respectively[9]. The tumor in this case had dimensions of 19.5 cm × 12.5 cm × 17.0 cm.
Primary ectopic gastrinomas are neuroendocrine tumors located outside of the triangle of gastrinoma and are responsible for less than 10% of cases. One specific site in which they occur is the liver, with only 26 cases described in the literature in a review from 2012[10]. In recent years, there were a few more cases described, but the total number is still very low. According to a recent study[11], in ZES patients, primary gastrinomas of the liver were the second most common extrapancreatic, extraintestinal site for a primary gastrinoma and may metastasize to regional lymph nodes. Compared with typical ZES patients, the epidemiology of primary hepatic gastrinoma is a little different. The patients are generally affected much younger, with a predominance of males and no association with MEN 1, as it was in our case[12].
This rare hepatic gastrinoma is slow growing, with around 65% of cases malignant and 30%-40% of those cases being metastatic at initial presentation. This relatively high rate of metastases arises from the difficulty of diagnosis, especially in palliative care and hepatic metastases[13]. Despite this, our patient did not present metastases in radiological examinations.
A clinical presentation of ZES similar to gastroesophageal reflux can lead to treatment with proton-pump inhibitors. This medication masks the symptoms and alters gastrin levels, prolonging the time necessary to identify the real cause of the disease. A study[14] showed that prior to widespread use of proton-pump inhibitors (before 1985), the rate of metastases was only 19%. Ten years later, this number had risen to 55%. Prior to the advent of proton-pump inhibitors, partial and full gastrectomies were common to reduce the incidence of ulcers. In the report of this case, the fact that the patient had previously undergone antrectomy with vagotomy meant that his symptoms were less apparent than they would otherwise have been.
A second factor that can complicate diagnosis is related to the morphofunctional characteristics of the liver. It is known that the liver is a common site of metastasis for a variety of tumors, and classifying the neoplasia as primarily hepatic requires some examinations to confirm the absence of other primary sites. One of the examinations currently used is PEC/CT with 68 gallium-dotatate. This radiopharmacum is associated with computerized tomography and is a somatostatin analogue, which allows the identification of tumors[15]. The sensitivity of the examination was 93% (95%CI: ± 2%) and the specificity was 91% (95%CI: 82%-97%), as identified in a meta-analysis[9]. Once the neuroendocrine tumors present somatostatin receptors, it is possible to confirm whether the hepatic gastrinoma is primary or the result of metastasis[16,17]. In this case, the existence of a single focus in the liver combined with a high level of gastrin highly suggested it as a primary site. Is important to emphasize the importance of the follow up of gastrin levels to ensure that there is not a small small duodenal primary gastrinoma that could not have been detected in the PET/CT and surgery[11].
Despite few reported cases, surgical resection should be the treatment of choice and, furthermore, the only chance of a cure. The rate of success in these cases is 86%, and 60% present early postoperative eugastrinemia, 40% after five years. In the event that surgery is not possible, such as in cases of diffuse metastasis or comorbidities, it is possible to follow conventional treatment: Ablation by radiofrequency, chemotherapy (doxorubicin, streptozocin, 5-fluorouracil), interferon, and transplantation[13]. A study with 160 patients with gastrinoma showed that 15-year disease-related survival was 98% for operated and 74% for unoperated (P = 0.0002)[18].
In conclusion, primary hepatic gastrinomas are extremely rare tumors that cause Zollinger-Ellison syndrome. Due to their clinical presentation and the liver being a significant site of metastases, diagnosis of the disease is time consuming and difficult. Following diagnosis, the treatment of choice is surgery, and, in cases where there are no metastases, the prognosis is good. Therefore, it is important to diagnose properly the primary gastrinoma.
Primary hepatic gastrinoma in a 19 years old hypergastremic patient who underwent hepatic trisegmentectomy to remove the tumor.
Primary hepatic gastrinoma.
Gastrointestinal tumors, MEN 1.
Gastrin levels were 6709.0 pg/mL before surgery. After the procedure it was 40.5 pg/mL.
Computed tomography (CT) showed a voluminous heterogeneous hepatic lesion, with a neoplasic aspect and dimensions 19.5 cm × 12.5 cm × 17.0 cm.
Histopathological analysis of the patient’s tumor revealed a Ki-67 of 5% (G2).
Hepatic trisegmentectomy.
Gastrinoma: A gastrinoma is a tumor that secretes an excess of gastrin, leading to ulceration in the duodenum, stomach, and the small intestine.
Primary hepatic gastrinoma is a very difficult to diagnose. Meticulous examination is necessary for appropriate diagnosis and treatment.
The authors are thankful to Justin Axel-Berg for the English corrections.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): D, D
Grade E (Poor): 0
P- Reviewer: Gencdal G, Jensen RT, Kaya M, Luo GH, Ozenirler S, Singh S, Tsuchiya A S- Editor: Ji FF L- Editor: Filipodia E- Editor: Tan WW
| 1. | Ellison EC, Johnson JA. The Zollinger-Ellison syndrome: a comprehensive review of historical, scientific, and clinical considerations. Curr Probl Surg. 2009;46:13-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Zollinger RM, Ellison EH. Primary peptic ulcerations of the jejunum associated with islet cell tumors of the pancreas. Ann Surg. 1955;142:709-723; discussion, 724-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 942] [Cited by in RCA: 696] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 3. | Bergsland E. Zollinger-Ellison syndrome (gastrinoma): Clinical manifestations and diagnosis. Available from: https://www.uptodate.com/contents/zollinger-ellison-syndrome-gastrinoma-clinical-manifestations-and-diagnosis?source=search_resultsearch=gastrinomaselectedTitle=1~90. |
| 4. | Roy PK, Venzon DJ, Shojamanesh H, Abou-Saif A, Peghini P, Doppman JL, Gibril F, Jensen RT. Zollinger-Ellison syndrome. Clinical presentation in 261 patients. Medicine (Baltimore). 2000;79:379-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 225] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 5. | Smyrniotis V, Kehagias D, Kostopanagiotou G, Tripolitsioti P, Paphitis A. Primary hepatic gastrinoma. Am J Gastroenterol. 1999;94:3380-3382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Thompson NW, Vinik AI, Eckhauser FE, Strodel WE. Extrapancreatic gastrinomas. Surgery. 1985;98:1113-1120. [PubMed] |
| 7. | Norton JA, Alexander HR, Fraker DL, Venzon DJ, Gibril F, Jensen RT. Possible primary lymph node gastrinoma: occurrence, natural history, and predictive factors: a prospective study. Ann Surg. 2003;237:650-657; discussion 657-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Klimstra DS, Yang Z. Pathology classification, and grading of neoplasms arising in the digestive system. Available from: https://www.uptodate.com/contents/pathology-classification-and-grading-of-neuroendocrine-tumors-arising-in-the-digestive-system?source=search_resultsearch=gastrinoma%20ki-67selectedTitle=1~150. |
| 9. | Ogawa S, Wada M, Fukushima M, Shimeno N, Inoue S, Chung H, Fujita M, Suginoshita Y, Okada A, Inokuma T. Case of primary hepatic gastrinoma: Diagnostic usefulness of the selective arterial calcium injection test. Hepatol Res. 2015;45:823-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Harvey A, Pasieka JL, Al-Bisher H, Dixon E. Primary hepatic gastrinoma causing zollinger-ellison syndrome: a rare and challenging diagnosis. Cancers (Basel). 2012;4:130-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Incidence and Prognosis of Primary Gastrinomas in the Hepatobiliary Tract. JAMA Surg. 2018;153:e175083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Moriura S, Ikeda S, Hirai M, Naiki K, Fujioka T, Yokochi K, Gotou S. Hepatic gastrinoma. Cancer. 1993;72:1547-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Tsalis K, Vrakas G, Vradelis S, Dimoulas A, Pilavaki M, Papaemmanouil S, Micheli A, Lazarides C, Kartalis G. Primary hepatic gastrinoma: Report of a case and review of literature. World J Gastrointest Pathophysiol. 2011;2:26-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Evans JT, Nickles S, Hoffman BJ. Primary hepatic gastrinoma: an unusual case of zollinger-ellison syndrome. Gastroenterol Hepatol (NY). 2010;6:53-56. [PubMed] |
| 15. | Treglia G, Castaldi P, Rindi G, Giordano A, Rufini V. Diagnostic performance of Gallium-68 somatostatin receptor PET and PET/CT in patients with thoracic and gastroenteropancreatic neuroendocrine tumours: a meta-analysis. Endocrine. 2012;42:80-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 188] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 16. | Mojtahedi A, Thamake S, Tworowska I, Ranganathan D, Delpassand ES. The value of (68)Ga-DOTATATE PET/CT in diagnosis and management of neuroendocrine tumors compared to current FDA approved imaging modalities: a review of literature. Am J Nucl Med Mol Imaging. 2014;4:426-434. [PubMed] |
| 17. | Deppen SA, Liu E, Blume JD, Clanton J, Shi C, Jones-Jackson LB, Lakhani V, Baum RP, Berlin J, Smith GT. Safety and Efficacy of 68Ga-DOTATATE PET/CT for Diagnosis, Staging, and Treatment Management of Neuroendocrine Tumors. J Nucl Med. 2016;57:708-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 169] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 18. | Norton JA, Fraker DL, Alexander HR, Gibril F, Liewehr DJ, Venzon DJ, Jensen RT. Surgery increases survival in patients with gastrinoma. Ann Surg. 2006;244:410-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 140] [Article Influence: 7.4] [Reference Citation Analysis (0)] |