Published online Jul 27, 2018. doi: 10.4254/wjh.v10.i7.496
Peer-review started: March 12, 2018
First decision: March 29, 2018
Revised: April 6, 2018
Accepted: April 9, 2018
Article in press: April 9, 2018
Published online: July 27, 2018
Processing time: 139 Days and 1.1 Hours
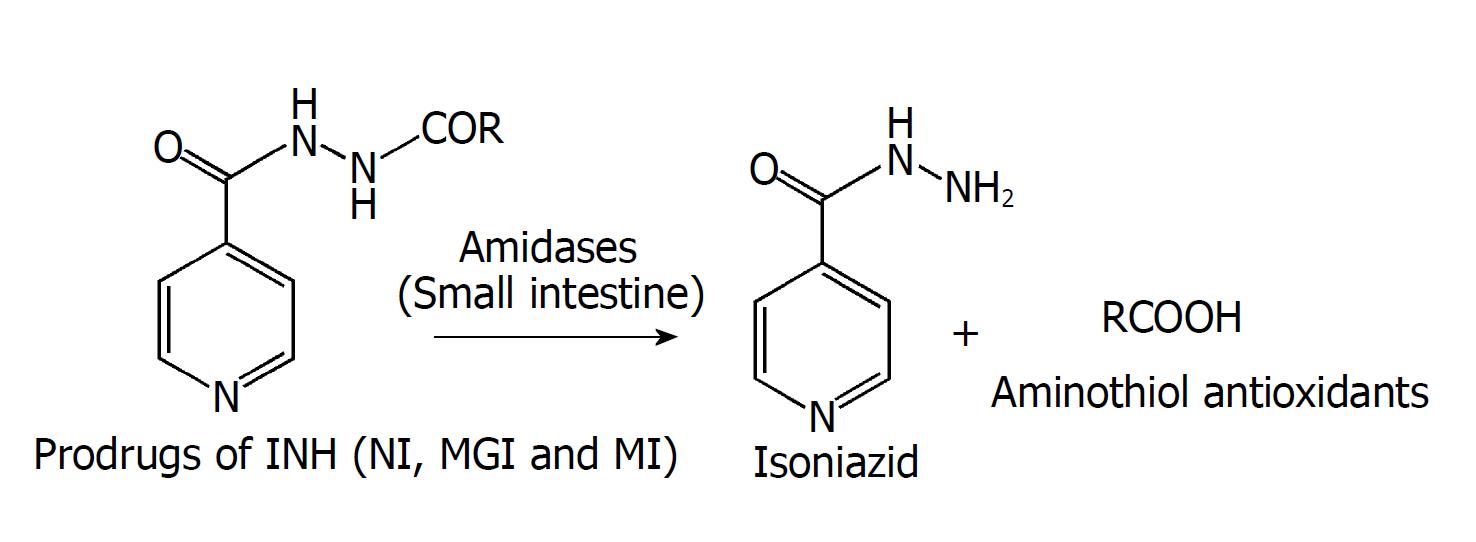
To overcome the hazardous effects on liver caused by long-term use of antitubercular agent isoniazid (INH) by developing a novel hepatoprotective prodrug strategy by conjugating INH with aminothiols as antioxidant promoities for probable synergistic effect.
INH was conjugated with N-acetyl cysteine (NAC) and N-(2)-mercaptopropionyl glycine using the Schotten-Baumann reaction and with L-methionine using Boc-anhydride through a biocleavable amide linkage. Synthesized prodrugs were characterized by spectral analysis, and in vitro and in vivo release studies were carried out using HPLC. Their hepatoprotective potential was evaluated in male Wistar rats by performing liver function tests, measuring markers of oxidative stress and carrying out histopathology studies.
Prodrugs were found to be stable in acidic (pH 1.2) and basic (pH 7.4) buffers and in rat stomach homogenates, whereas they were hydrolysed significantly (59.43%-94.93%) in intestinal homogenates over a period of 6 h. Upon oral administration of prodrug NI to rats, 52.4%-61.3% INH and 47.4%-56.8% of NAC were recovered in blood in 8-10 h. Urine and faeces samples pooled over a period of 24 h exhibited 1.3%-2.5% and 0.94%-0.9% of NAC, respectively, without any presence of intact NI or INH. Prodrugs were biologically evaluated for hepatoprotective activity. All the prodrugs were effective in abating oxidative stress and re-establishing the normal hepatic physiology. The effect of prodrug of INH with NAC in restoring the levels of the enzymes superoxide dismutase and glutathione peroxidase and abrogating liver damage was noteworthy especially.
The findings of this investigation demonstrated that the reported prodrugs can add safety and efficacy to future clinical protocols of tuberculosis treatment.
Core tip: To overcome the deleterious effects caused by long-term use of isoniazid (INH), a novel hepatoprotective prodrug strategy was developed by integrating the antioxidant property of aminothiols with INH moiety to improve its therapeutic safety. The anticipated prodrugs were synthesized using facile method of synthesis, wherein aminothiols were tethered to the INH molecule via biocleavable amide linkage. Upon hepatoprotective potential assessment, the prodrug NI displayed noteworthy effects, whereas prodrugs MGI and MI exhibited moderate effects. The findings of this study strongly suggest that concept-based design of hepatoprotective prodrugs can be applied effectively in reversing toxic effects of INH and its metabolites on liver.
- Citation: Bhilare NV, Dhaneshwar SS, Mahadik KR. Amelioration of hepatotoxicity by biocleavable aminothiol chimeras of isoniazid: Design, synthesis, kinetics and pharmacological evaluation. World J Hepatol 2018; 10(7): 496-508
- URL: https://www.wjgnet.com/1948-5182/full/v10/i7/496.htm
- DOI: https://dx.doi.org/10.4254/wjh.v10.i7.496
Tuberculosis (TB) is a worldwide pandemic and has existed for millennia. According to the Global Tuberculosis Report 2016 published by the World Health Organization, 10.4 million new TB cases (including 1.2 million among human immunodeficiency virus-positive people) occurred in 2015 and an astounding 1.4 million perished from TB that year. Overall, 10% of cases were children, whereas 90% were adults. TB disproportionately afflicts developing nations; India, Indonesia, China, Nigeria, Pakistan and South Africa were the countries that accounted for 60% of the global burden[1]. TB was one of the top 10 causes of death worldwide in 2015, despite the existence of curative chemotherapy.
Standard treatment recommended for adult respiratory TB is a 2-mo regime of isoniazid (INH), rifampicin, pyrazinamide and ethambutol, followed by INH and rifampicin for 4 mo[2]. Unfortunately, INH, the drug for the intensive phase of conventional anti-TB treatment, is highly hepatotoxic, with incidence varying between 4%-11%[3]. INH and/or its metabolites trigger systemic lupus erythematosus, peripheral neuropathy, steatosis, neurological disorders and hepatic necrosis[4]. N-arylaminoacetyl transferases (NATs) are the enzymes that metabolize INH in humans through acetylation at N2-center in the hydrazinic chain and release free radicals, which are implicated in the hepatotoxicity[4-7].
A well-established drug therapy to prevent or cure this hepatotoxicity does not exist, except discontinuing the treatment for a while and restoring it steadily later on when liver enzymes return to normal level. Such discontinuation of treatment may result in morbidity or prolonged disability. Therefore, clinical safety in long term treatment with INH is questionable and continues to be a subject of grave concern in the field of medicine[3]. In the past, considerable focus has been put on the development of INH conjugates with lower hepatotoxicity, improved hydrolytic stability, enhanced bioavailability and antitubercular activity. These include conjugation with polymers like gelatine[8], peptide[9], polyethylene-glycol[10], β-cyclodextrin conjugate[11] and antioxidants like cinnamic acids[12], curcumin[13], Schiff bases[14] and phenolic acids[15].
Resurgence of INH as a clinically safe drug appears to rely on its appropriate modification that can block acetylation by NATs and overcome toxicity. To engender these anticipated drug properties in INH, it can be structurally modified at N2-center in the hydrazinic chain[2,14]. The molecular mechanism of cytotoxicity is attributed to CYP2E1-induced oxidative stress as protein carbonyl formation and rise in reactive oxygen species occur before the beginning of hepatocyte toxicity[16,17].
Since nitrogen-centred free radicals play a critical role in instigating oxidative stress, by augmenting the hepatocellular antioxidant defence system, especially glutathione (GSH), cells can be protected against the INH-induced oxidative injury, as evidenced in previous reports[18]. Thiol-containing antioxidants have earned great interest of researchers because of the profound part played by intracellular GSH in redox regulation and antioxidant cell defence machinery. In the present study, GSH depletion is the rationale for the selection of protective amino thiol antioxidants as carriers for conjugation with INH. As precursors of GSH, sulfhydryl group-containing compounds either increase hepatic stores of reduced GSH or substitute for it in the liver[19]. Among the sulphur-containing antioxidants, N-acetyl cysteine (NAC), N-(2-mercaptopropionyl) glycine (MPG) and L-methionine (Met) are known to be nontoxic and have been used in the treatment of various disorders[20-24].
Met plays a vital role in detoxification by acting as an amino acid precursor for GSH synthesis, which protects cells from oxidative damage[25]. NAC helps in maintaining intracellular GSH levels and scavenging reactive oxygen species and its potential in attenuating INH and rifampicin-induced hepatotoxicity has already been reported[20]. Protective effects of MPG have been reported in paracetamol-induced hepatic necrosis[26].
On these grounds, a novel series of prodrugs was designed with the aim of combining antioxidant properties of aminothiols (NAC, MPG and Met) with INH to yield codrugs with lower hepatotoxicity. The hypothesis behind this concept was based on transient masking of the hydrazinic chain of INH at the N2 position to block oxidation by CYP2E1 and acetylation by NAT. Release kinetics were studied to confirm the biocleavable nature of this newly formed amide bond, and hepatoprotective efficacy was assessed.
INH was received as gift sample from Lupin Research Park (Lupin Ltd., Aurangabad, India). NAC was generously provided by Zim Laboratories Ltd (Nagpur, India). MPG and Met were purchased from Sigma Chemical Co. (St Louis, MO, United States). Precoated silica gel plates (60 F254; Merck, Kenilworth, NJ, United States) with fluorescent indicator were used for thin-layer chromatography, and ultraviolet (UV) light (254 nm) was used for spot detection. Programmable melting point apparatus (Veego, India) was used to record melting points and are uncorrected. The V530, UV-visible double-beam spectrophotometer (JASCO, Oklahoma City, OK, United States) was used to determine the λmax of the synthesized compounds.
Spectroscopic methods were used for confirmation of structures. The infrared (IR) spectrum was recorded on a JASCO V-530 FTIR in potassium bromide (anhydrous IR grade). Proton- and 13C-NMR were recorded in CDCl3 and DMSO referring chemical shifts to TMS as the internal standard using an Avance II 400 instrument (Bruker, Billerica, MA, United States) at 400 MHz, with super conducting magnet, at VIT University (Vellore, Tamilnadu, India). The mass spectra were recorded by employing an Agilent 1260 Infinity HPLC-MASS Analyzer 6460 Triple Quad LC/MS at the Poona College of Pharmacy, Food Testing Laboratory (Pune, India). The elemental analysis of synthesized prodrugs was performed on an elemental analyser (Vario Micro Cube; Elementar, Frankfurt, Germany) at the Poona College of Pharmacy.
In vitro and in vivo release studies were carried out on a JASCO PU HPLC model 2080, with UV detector UV-Vis 2070, using a Thermo C18 column (Hypersil gold, 250 mm × 4.36 mm, 5 μm). For pharmacological screening of synthesized prodrugs (CPCSEA/PCH/02/2016-17), Institutional Animal Ethical Committee-approved experimental protocols were followed and all procedures were carried out at the CPCSEA-approved animal facilities of Poona College of Pharmacy (Reg. No.100/1999/CPCSEA). The animals were bought from the National Institute of Biosciences (Pune, India).
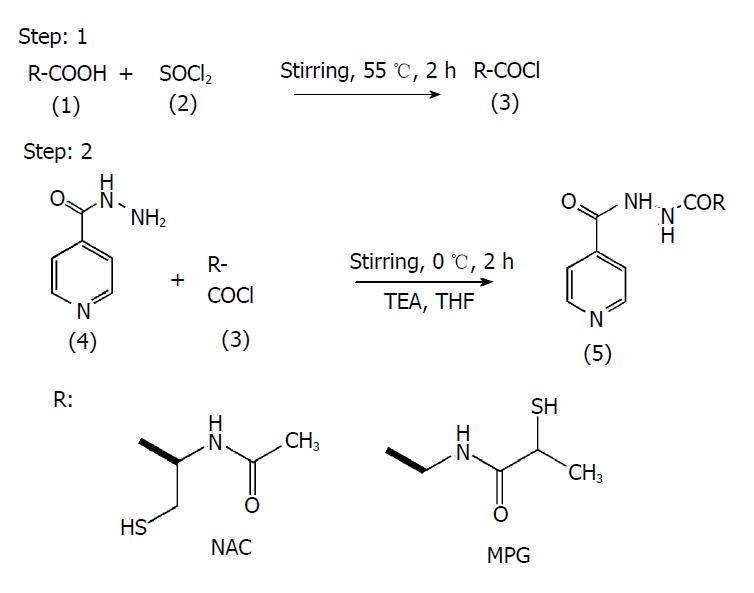
Synthesis of prodrugs of INH with NAC (NI) and MPG (MGI): To the solution of amino acid (1) (0.005 mol/L) in THF (10 mL) thionyl chloride (2) (0.005 mol/L) was added drop-wise and stirred for 2 h at 55 °C. The reaction was monitored by thin-layer chromatography using ethyl acetate:methanol:glacial acetic acid (GAA) (0.5:1.5:0.02 v/v/v). At the end of 2 h, the reaction mixture was evaporated using a rotary evaporator, and the residue of acid chloride (3) was dried under vacuum. It was then dissolved in THF and poured into a round-bottom flask which was placed in an ice bath and kept for stirring. To an ice-cold mixture of INH (4) in THF, TEA (0.5 mL) was added and the resultant solution was added drop-wise to the round-bottom flask containing acid chloride, and the temperature of the ice bath was maintained at 0 °C. At the end of 4 h, the precipitated amide (5) was separated from the mixture by filtration and was recrystallized using mixture of ethanol:water (2:1 v/v). It was further purified by preparative thin-layer chromatography using the same mobile phase mentioned above. The reaction scheme is shown in Figure 1.
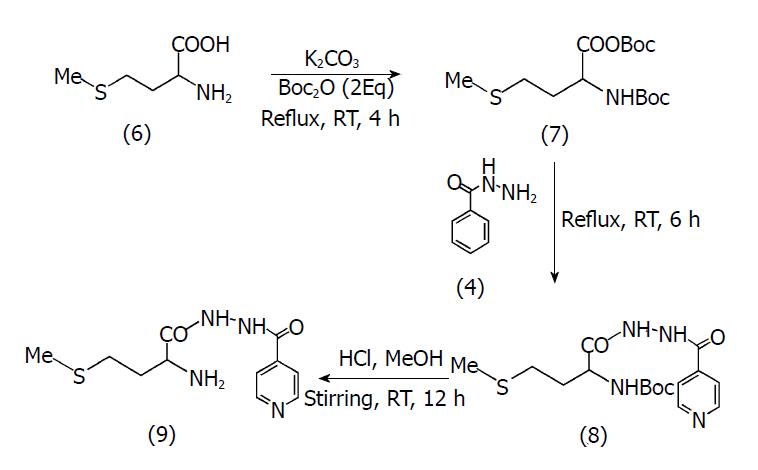
Synthesis of prodrug of INH with Met (MI): To a stirred solution of Met (6) (2 mmol) in THF (20 mL), a saturated solution of potassium carbonate (5 mL) was added. Di-tert-butyl dicarbonate (Boc2O; 2 mmoL) was then added to this solution and the resultant reaction mixture was refluxed for 4 h (7) and monitored by thin-layer chromatography using ethyl acetate:hexane:GAA (0.7:0.3:0.01 v/v/v). INH (4) (2 mmol) was added to the reaction flask and the resulting mixture was refluxed for a further 6 h. Removal of solvent yielded a pale orange paste, which was further extracted using hexane:ethyl acetate (3:2 v/v) to remove excess Boc2O and with water:DCM (1:5 v/v) to obtain a product which was dried over anhydrous magnesium sulphate.
Deprotection at the N-terminal (8) was done by addition of a solution of HCl in MeOH (10 mL) to the pale orange product obtained from the previous step. The resulting reaction mixture was stirred at room-temperature for 12 h, after which its pH was adjusted to 9 with 2 N NaOH[27]. The mixture was extracted with DCM (2 × 10 mL). The combined organics were washed with saturated aqueous brine (2 × 15 mL), dried over sodium sulphate and concentrated in vacuo, to yield the final product (9) as a pale yellow solid. Further purification was carried out using preparative thin-layer chromatography with mobile phase ethyl acetate:hexane:GAA (0.7:0.3:0.01 v/v/v). The reaction scheme is shown in Figure 2.
In vitro stability studies were performed in aqueous buffers of varied pH range and tissue homogenates of stomach and intestine of rats to ensure bioreversibility of the synthesized prodrugs. Triplicate samples were analysed and methods were validated as per ICH guidelines. Novel HPLC methods were developed for simultaneous estimation of prodrugs in the presence of their released active metabolite, INH. Percentage of hydrolysed prodrugs and release of INH was calculated using equations generated from calibration curves. Prodrugs in the presence of their hydrolysed products were estimated by simultaneous estimation method developed on reverse-phase HPLC. The method developed for estimation of NI and its hydrolysed products was comprised of water:methanol (80:20 v/v adjusted to pH 3.0 with OPA at flow rate 1 mL/min on a Thermo C18 column at 204 nm). For MGI, the mobile phase was comprised of acetonitrile:water (65:35; v/v adjusted to pH 2.5 with OPA at flow rate 1 mL/min on a Thermo C18 column at 365 nm). For the MI mobile phase, water:acetonitrile:methanol (20:20:60 v/v/v at flow rate 1mL/min on a Thermo C18 column at 205 nm) was used.
Calibration curves of prodrugs and INH were constructed in HCl buffer (pH 1.2) and phosphate buffer (pH 7.4) in the range of 10-100 μg/mL. Release study was performed in HCl buffer (pH 1.2) and stomach homogenates till 3 h and in phosphate buffer (pH 7.4) and intestinal homogenates till 6 h respectively. Prodrug (10 mg) was introduced in 100 mL of 0.05 mol/L HCl (pH 1.2) or 0.05 mol/L phosphate buffer (pH 7.4) in a beaker kept in a constant-temperature bath at 37 ± 1 °C with occasional stirring. Aliquots (5 mL) were withdrawn and replaced with fresh aqueous buffer at regular intervals of 0, 15, 30, 45, 60 min and every 30 min thereafter till 3 h for HCl buffer and 6 h for phosphate buffer, and 50 μL sample reconstituted with the mobile phase was injected in the column and analysed by HPLC.
In vivo behaviour of the orally-administered prodrug NI was investigated in Wistar rats (200-250 g; n = 3) housed in metabolic cages individually under normal conditions (at 27 ± 0.5 °C and a relative humidity of 70% ± 0.5% under natural light/dark conditions). To study the in vivo behaviour, an HPLC method was developed for simultaneous estimation of INH, NI and NAC. The same HPLC system, column and mobile phase were used for this purpose, as mentioned in the in vitro release study section above. All the kinetic studies were carried out in triplicate.
Equimolar dose of NI (Table 1) was orally administered to the 6 male Wistar rats kept in individual metabolic cages. Male Wistar rats were fasted for 24 h prior to use and were administered water ad libitum. Blood (0.5 mL) was withdrawn by retro-orbital puncture and the reading was considered as the 0-min reading. A suspension of NI in 1.0 mL of physiological saline was administered to the animals (Table 1). Blood samples were collected in EDTA-coated tubes at an interval of 15 min for the first 1 h. Then, subsequent blood collection was made on a bihourly basis till the 10th h and finally at the 24th h. These EDTA-coated tubes were centrifuged for 10 min at 5000 rpm with temperature set at 0-5 °C. The supernatant solution (0.1 mL) of centrifuged blood was added to an Eppendorf tube (1 mL capacity) and methanol (0.9 mL) was added to it for instant plasma protein precipitation. After vortexing all the solutions for 2 min, they were again centrifuged at 5000 rpm for 10 min at 0-5 °C in order to precipitate any solid matter or impurities present in the biological samples. These samples were then analysed by HPLC, using the procedure described above.
| S.N. | Compound | Dose1, mg/kg/per day |
| 1 | H | 0.9% saline (1 mL) |
| 2 | INH | 150 |
| 3 | NI | 309 |
| 4 | MGI | 311 |
| 5 | MI | 293 |
| 6 | INH + NAC | 150 + 159 |
| 7 | INH + MPG | 150 + 161 |
| 8 | INH + Met | 150 + 143 |
Urine/faeces samples were also collected at various time intervals and pooled together over a period of 24 h and analysed similarly by HPLC.
Animals: For screening of prodrugs for hepatoprotective potential, Wistar rats (male, 180-200 g) were used. Solid-bottom polypropylene cages were used to house the animals and were maintained at 24 ± 1 °C, with a relative humidity of 45%-55% and 12:12 h dark/light cycle. They were acclimatized to laboratory conditions for 1 wk. The animals had free access to food (standard chow pellets; Chakan Oil Mills, Sangli, India) and water. The doses of prodrugs were calculated on an equimolar basis to INH and are presented in Table 1. The standard and the test compounds were administered orally as a suspension in 1% sodium CMC.
Evaluation of hepatoprotective potential: Rats were randomly divided into eight groups, after an acclimatization period of 1 wk, with each group consisting of 6 animals. Doses as mentioned in the Table 1 were administered for 21 d to the respective groups. Body weights and relative liver weights of animals from prodrug-treated groups and physical mixture-treated groups were calculated at the end of study. After completion of 21 d, on the 22nd d, the animals were fasted overnight and sacrificed immediately after withdrawal of blood from the retrobulbar venous plexus. Afterwards, centrifugation of whole blood was carried out to obtain serum samples. Liver samples were dissected out and washed immediately with ice-cold saline to remove as much blood as possible. One fraction of the liver samples was excised, fixed in a 10% formalin solution and sent for histopathological analysis; another fraction was immediately stored at -80 °C for future analysis. The histopathologist was unaware of the protocols to ensure unbiased analysis.
Assessment of antioxidant parameters: Liver tissues were washed with normal saline to remove any blood or clots, and homogenized on ice in Tris-HCl (5 mmol/L containing 2 mmol/L EDTA, pH 7.4). Homogenates were centrifuged at 1000 × g for 15 min at 4 °C. Aliquoted samples of the supernatants were used immediately for the assays of superoxide dismutase (SOD) and glutathione peroxidase (GSHPx). SOD activity was estimated by the method described by Marklund and Marklund (1974). GSHPx content was determined by the method of Moron et al[28-30] (1979).
Assay of lipid peroxidation products: The extent of lipid peroxidation was assessed by estimating the concentration of thiobarbituric acid reactive product malondialdehyde (MDA) as described by Ohkawa et al[31] (1979). MDA concentrations were measured using 1,1,3,3-tetraethoxypropane as standard and expressed as micromoles per gram of tissue[31].
Estimation of alanine aminotransferase and aspartate aminotransferase: Serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were estimated with help of commercially available kits (Coral Clinical Systems, Mumbai, Maharashtra, India) using Reitman and Frankel’s colorimetric method[32].
Commercially available spectrophotometric kits (Coral Clinical Systems) were used to measure triglycerides and cholesterol levels. For determination of triglycerides in plasma, glycerol-3-phosphate-oxidase enzymatic colorimetric (GPO/PAP) method was used. CHOD/PAP method based on estimation of D4 cholestenone after enzymatic cleavage of cholesterol ester by cholesterol esterase was used for the determination of cholesterol in plasma[33].
Histopathology of rat liver was carried out at SAI Research Laboratories (Pune, India). The sections were stained by haematoxylin and eosin. Coloured photomicrographs of the sections were taken on Nikon Optical Microscope, Eclipse E-200 (resolution of 10 × 10 X), with an attached trinocular camera.
An average of six readings was calculated and data were expressed as mean ± SEM; n referred to number of animals in each group. Statistical differences between the groups were calculated by one-way ANOVA followed by Dunnett’s post-hoc test. Differences were considered at P values of < 0.001-0.05 when compared with the INH group.
The log Poct of INH was found to be -0.71 and aqueous solubility was 135 mg/mL. The partition coefficients of NI, MGI and MI were found to be 0.11, 0.27 and 0.06 respectively and their aqueous solubilities were 104 mg/mL, 94 mg/mL and 114 mg/mL respectively.
For confirming the structures of synthesized prodrugs, they were subjected to spectral analysis by IR, NMR, mass spectroscopy and elemental analysis.
Amide NI: N-{1-mercaptomethyl-2-oxo-2-[N’-(pyridine-4-carbonyl)-hydrazino]-ethyl}-acetamide, M.P.: 175-178 °C (uncorrected), Rf: 0.58 (ethyl acetate:methanol:GAA; 0.5:1.5:0.02 v/v/v), Aq. Sol. : 104 mg/mL, Log Poct: 0.11, IR (anhydrous KBr; cm-1): 3300 (NH, sec amide, str), 2545 (SH, str), 1704 (C=O, CHCONH, str), 1663 (C=O, aromatic CONH, str.), 1532 (C-N, pyridine ring, str), 1406 (CH2, bend), 751 (N-N, hydrazine, sym wag) 1HNMR (CDCl3; 400 MHz): δ 2.02 [s, 3H]-CH3O, 3.05-3.06 [d, 2H]-CH2S, 4.68-4.71 [t, 1H]-CH2-CH2-SH, 7.82-7.83 [d, 2H]-pyridine ring, 8.78-8.79 [d, 2H]-pyridine ring, 9.84 [s, 3H]-NH 13C-NMR (CDCl3; 400 MHz): 19.07, 28.85, 63.75, 121.31, 134.41, 140.51, 150.22, 163.75, 164.22, 170.22 MS: m/z ratio: 282.09 (C11H14N4O3S, predicted: 282.32). Elemental analysis: Calculated for C11H14N4O3S: C, 46.80; H, 5.00; N, 19.85; S, 11.36; Found: C, 46.77; H, 5.03; N, 19.87; S, 11.32.
Amide MGI: 2-Mercapto-N-{2-oxo-2-[N’-(pyridine-4-carbonyl)-hydrazino]-ethyl}-propionamide, M.P.: 189-191 °C (uncorrected), Rf: 0.66 (ethyl acetate:methanol:GAA; 0.5:1.5:0.02 v/v/v), Aq. Sol. : 94 mg/mL, Log Poct: 0.27, IR (anhydrous KBr; cm-1): 3583 (NH, sec amide, str), 2560 (SH, str), 1692 (C=O, CHCONH, str), 1679 (C=O, aromatic CONH, str.), 1533 (C-N, pyridine ring, str), 1401 (CH2, bend), 760 (N-N, hydrazine, sym wag) 1HNMR (CDCl3; 400MHz) δ 1.20-1.21[d, 3H]-CH3, 3.04-3.07 [1H, m]-CH-CH3, 4.04 [2H, s]-CH2CO, 7.94-7.96 [d, 2H]-pyridine ring, 8.861-8.863 [d, 2H]-pyridine ring, 10.21 [s, 3H]-NH. 13C-NMR (CDCl3; 400 MHz): 18.03, 45.81, 59.18, 122.43, 139.18, 150.03, 164.38, 170.03, 175.97 MS: m/z ratio: 283.89 (C11H14N4O3S, predicted: 282.32). Elemental analysis: Calculated for C11H14N4O3S: C, 46.80; H, 5.00; N, 19.55; S, 11.36; Found: C, 46.79; H, 5.01; N, 19.88; S, 11.34
Amide MI: Isonicotinic acid N’-(2-amino-4-methylsulfanyl-butyryl)-hydrazide, M.P.: 199-201 °C (uncorrected), Rf: 0.66 (ethyl acetate:hexane:GAA; 0.7:0.3:0.01 v/v/v), Aq. Sol.: 114 mg/mL, Log Poct: 0.06, IR (anhydrous KBr; cm-1): 3461 (NH, sec amide, str), 2830, 2911 (CH, str), 1701 (C=O, CHCONH, str), 1692 (C=O, aromatic CONH, str.), 1538 (C-N, pyridine ring, str), 1325 (S-CH3, str), 754 (N-N, hydrazine, sym wag), 661 (C-S-C, str) 1HNMR (CDCl3; 400MHz) δ 1.88-2.12[m, 2H]-CH2-CH2-S, 2.46[s, 3H]-CH3S, 2.76-2.96 [2H, t]-CH2-S, 3.85-3.88 [1H, t]-CH-NH2, 7.88-7.89 [d, 2H]-pyridine ring, 8.82-8.83 [d, 2H]-pyridine ring, 10.06 [s, 4H]-NH. 13C-NMR (CDCl3; 400MHz): 18.36, 28.77, 29.98, 58.36, 122.36, 122.56, 140.40, 150.53, 150.63, 164.57, 170.17 MS: m/z ratio: 268.19 (C11H16N4O2S, predicted: 268.34). Elemental analysis: Calculated for C11H16N4O2S: C, 49.21; H, 6.07; N, 20.85; S, 11.89; Found: C, 49.25; H, 6.04; N, 20.89; S, 11.96.
Kinetic parameters like rate constants, half-lives and order of kinetics of hydrolysis were also calculated (Table 2). No change in the peak of prodrugs was noted for the 0.05 M HCl buffer (pH 1.2) and phosphate buffer (pH 7.4). All the three prodrugs showed negligible release of INH in stomach homogenates of rats. They were readily hydrolysed in intestinal homogenates by first-order kinetics. There was gradual increase in the percentage of prodrugs hydrolysed in intestinal homogenates (59.43%-94.93%; Table 2); thus, confirming their activation by intestinal amidases (Figure 3).
| Prodrug | Incubation medium | ||||||
| HClbuffer,pH 1.2 | Phosphatebuffer,pH 7.4 | Stomach homogenates | Intestinal homogenates | ||||
| K ± SD,min-11 | t1/2,min | % Prodrug hydrolysed | % INH released | ||||
| NI | Stable | Stable | Negligible | 3.4 × 10-3 | 233.42 | 94.93 | 46.10 |
| MGI | Stable | Stable | Negligible | 3.3 × 10-3 | 217.34 | 70.11 | 33.83 |
| MI | Stable | Stable | Negligible | 5.1 × 10-3 | 181.36 | 86.55 | 44.26 |
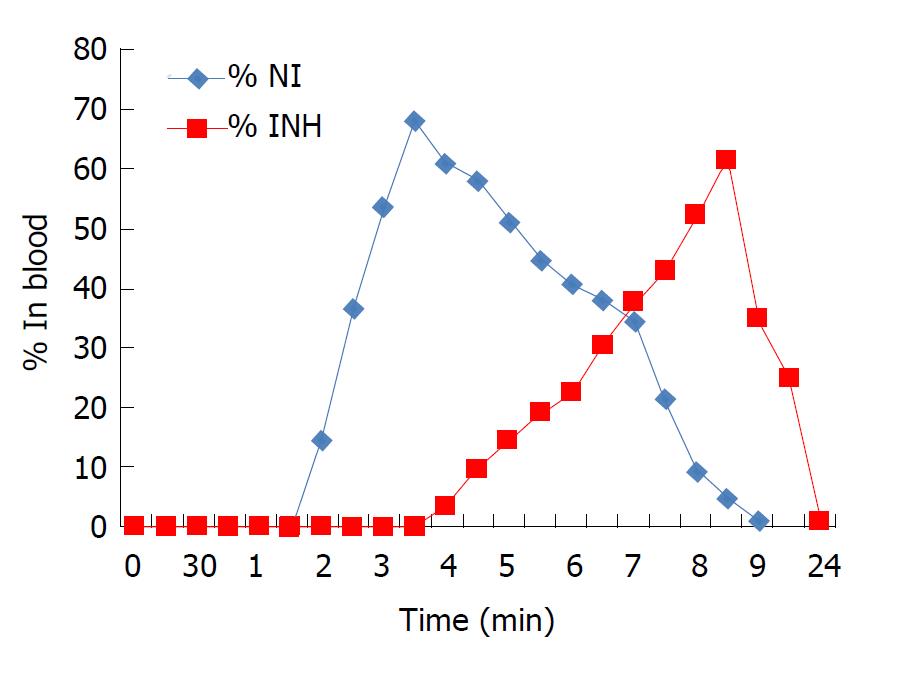
Prodrug NI was selected as a representative of the three synthesized prodrugs to investigate the in vivo behaviour. NI appeared in blood between 2-2.5 h after oral administration, indicating absorption of intact prodrug through stomach (Figure 4). There was consistent rise in the concentration of NI in blood till 4.5-5 h, indicating absorption of intact prodrug from the small intestine also. INH and NAC appeared in the blood from 4 h onwards, indicating hydrolytic activation of NI in the small intestine. The concentration of intact NI in the blood started declining from 5.5-6.5 h and completely disappeared at 7.5-9 h. The concentration of INH and NAC reached a maximum of 52.4%-61.3% at 9.5-10 h and 47.4%-56.8% at 8-9 h respectively. Intact NI was not observed in urine and faeces samples, whereas 1.3%-2.5% and 0.94%-0.9% of NAC was detected in 24 h pooled samples of urine and faeces respectively.
The body weights and relative liver weights of animals from prodrug-treated groups and physical mixture-treated groups calculated at the end of study had no statistical significance when compared to those of the INH-treated group.
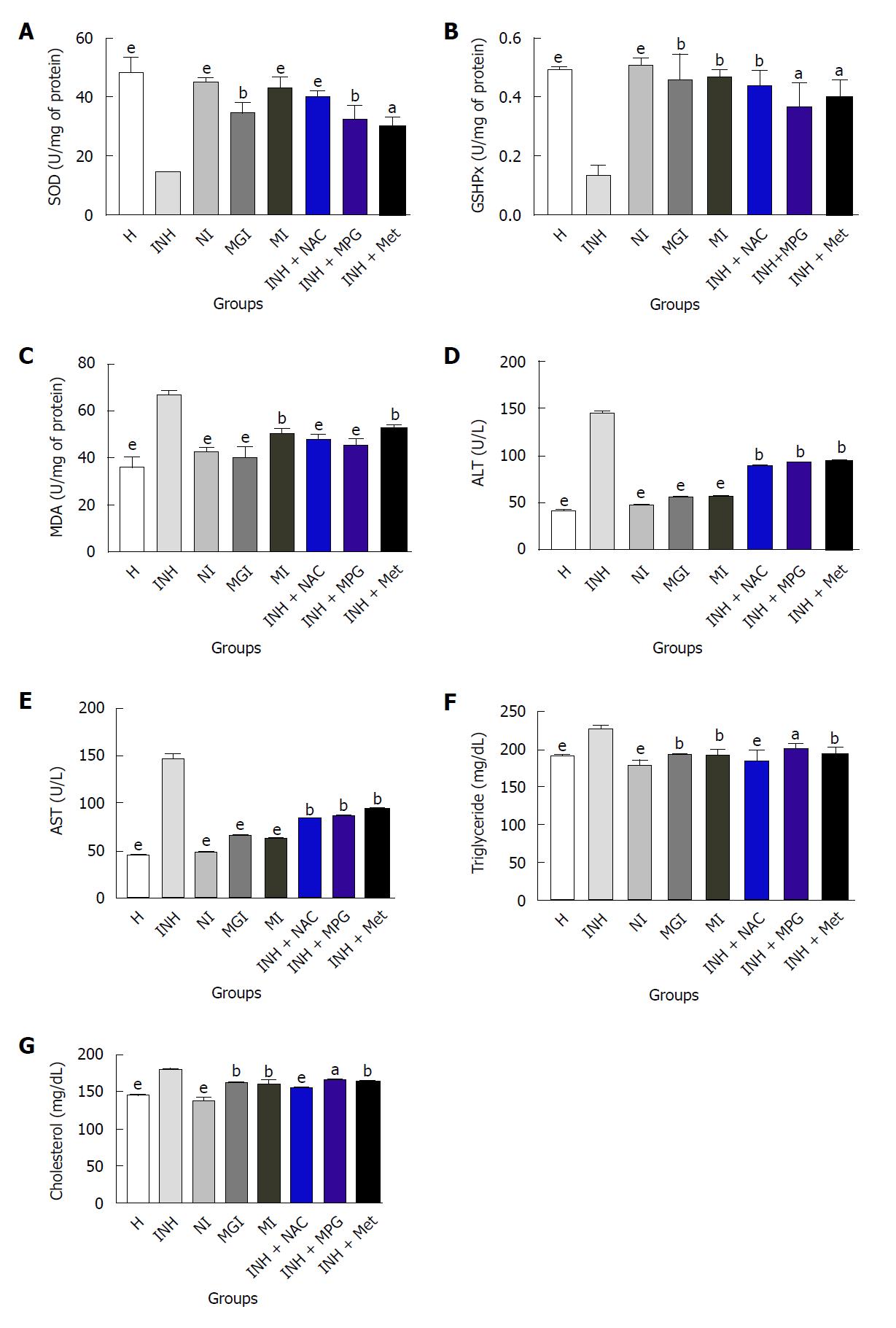
Antioxidant markers (SOD, GSHPx and MDA): The SODs are a group of closely associated enzymes that catalyse the breakdown of the superoxide anion into hydrogen peroxide and oxygen. The function of GSHPx is to reduce free hydrogen peroxide to water and reduce lipid hydroperoxides to their corresponding alcohols[34]. Elevating GSH levels aids the productivity of the GSHPx and vice versa. The process of oxidative degradation of lipids is brought about by the enzyme lipid peroxidase. In this course, free radicals steal electrons from the lipids that are present in cell membranes, causing cell damage. This process progresses through a free radical chain reaction mechanism[35,36]. Polyunsaturated fatty acids are most often affected by this reaction, as they possess multiple double bonds within which are methylene groups that contain specifically reactive hydrogen. It is a free radical-mediated destructive autocatalytic process, whereby polyunsaturated fatty acids undergo degradation to form MDA. Raised MDA levels in liver signify enhanced lipid peroxidation causing hepatic tissue damage and breakdown of antioxidant defence mechanisms to obviate the excessive free radical formation. In the group treated with INH, the level of SOD and GSHPx was significantly lower and the MDA level was significantly higher. This finding confirmed the induction of oxidative stress due to administration of INH[37,38].
Treatment with NI, MGI and MI significantly re-established the levels of SOD to 93.39%, 71.98% and 88.69% respectively and GSHPx to 100%, 91.83% and 93.87% respectively (Figure 5A and B). Physical mixture INH + NAC had significant effect, whereas the effect of INH + MPG and INH + MI on both the enzymes was moderate. MDA levels were significantly decreased by prodrugs compared to the INH-treated group (Figure 5C). The effect of NI and MGI in reducing the MDA level was better compared to that achieved with MI. Physical mixtures moderately decreased the MDA level. Thus, prodrugs were found to restore the levels of antioxidant enzymes, demonstrating their hepatoprotective potential.
ALT and AST: Raised levels of ALT and AST, bilirubinuria, bilirubinaemia, jaundice and rarely severe and occasionally fatal hepatitis often occur with normal dosing procedures of antitubercular drugs. ALT and AST levels are often used to assess hepatic damage. Hepatic injury causes necrosis or membrane damage, which permits intracellular enzymes to circulate and, hence, to be detected in serum. Elevated concentration of these enzymes in the serum is an indication of loss of functional integrity of the hepatic membrane[39,40]. Elevated ALT level, especially, is a signal of hepatocellular necrosis and has been used as a surrogate marker of liver injury[41].
In the present study, drastic elevation in ALT and AST levels (144 ± 7.70 U/L and 148 ± 7.60 U/L respectively) occurred in the INH-treated group, indicating pathological conditions in liver (Figure 5D and E). Physical mixtures as well as prodrug-treated groups displayed decline in ALT and AST levels, signifying restoration of these enzymes. The effect of prodrugs compared to physical mixtures was significantly superior; the effect of prodrug NI was especially noteworthy as it restored levels of ALT and AST to 48 ± 4.50 and 46 ± 7.30 U/L respectively. This is in agreement with the generally established observation that levels of transaminases return to normal with the healing of hepatic parenchyma and regeneration of hepatocytes.
Triglycerides and cholesterol: Previous studies have documented a strong association between hypercholesterolemia and increased free radical production[42]. Increase in cholesterol levels in the liver might be due to increased uptake of low-density lipoprotein from the blood by the tissues[43]. Earlier studies state that treatment with INH resulted in accumulation of triglycerides (TGs) in the liver of INH-treated animals. This was found to be a consequence of decreased lipoprotein synthesis, causing impaired mobilization of lipids[44]. Moreover, the levels of total cholesterol also increased, indicating that INH induces the hypercholesterolemic condition. Increased uptake of low-density lipoprotein from the blood by the tissues and inhibition of bile secretion might be the reason behind this increase in cholesterol levels[45]. Hepatotoxic agents like INH have also been reported to disrupt the membrane fluidity and thus affect the normal hepatocellular functions[45].
In groups administered with prodrugs, TG and cholesterol level decreased remarkably in comparison to the group administered with INH (Figure 5F and G). TG and cholesterol level decreased, to a lesser extent, in the groups treated with physical mixtures INH + MPG and INH + Met, whereas the effect of INH + NAC was more significant. Prodrugs provided remarkable defence against INH-induced aberrations in liver.
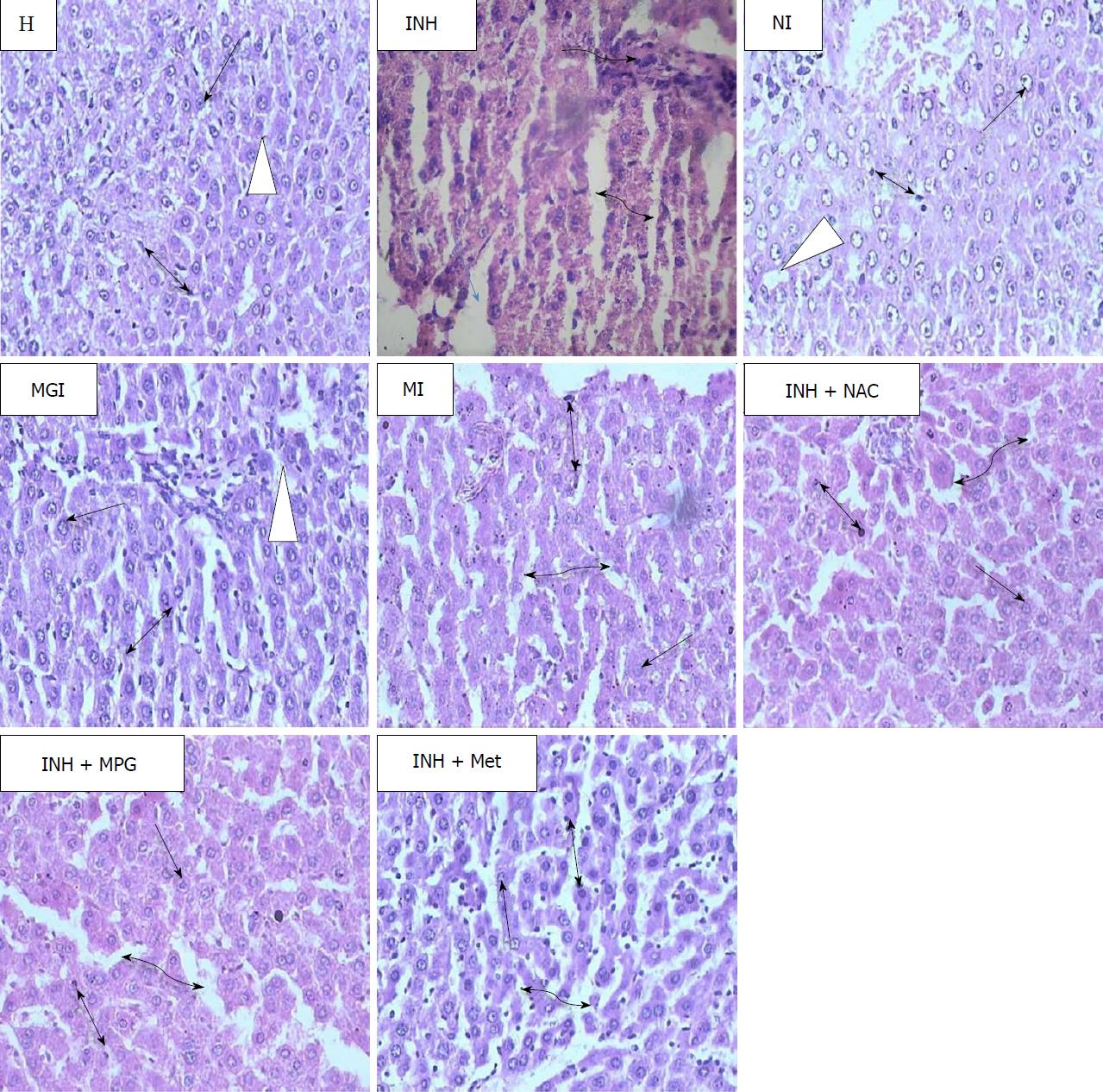
Microscopic examination of liver sections of healthy rats showed intact parenchymal cells and normal lobular architecture (Figure 6). Polygonal hepatocytes (single headed black arrow) arranged in hexagonal lobules and portal triads at vertices were also visible. Inside each lobule, adjacent blood sinusoids (white triangle) separated the hepatocytes and Kupffer cells (two headed black arrow), along with abundant eosinophilic cytoplasm being observable.
Membrane disintegration, degenerated nuclei, intense vacuolation accompanied by cytoplasmic rarefication leading to loss of the polyhedral structure is generally seen in liver sections of rats treated with INH. Blood sinusoidal dilatation (double headed curved black arrow), portal triaditis, multifocal area of necrosis (single headed blue arrow) and inflammatory cells (single headed curved black arrow) with granular swelling are also the prominent features in the histopathology of INH-damaged liver[46]. In the present study, all these pathological changes were noticed in liver sections of INH-treated rats (Figure 6).
The liver of rats treated with NI showed reversal of such pathology, with no evidence of necrosis, inflammation or fibrosis. Normal lobular structural design of the liver with well-preserved cytoplasm and blood sinusoids running in between normal hepatocytes were seen. Liver sections of groups treated with prodrug MGI and MI showed minimal inflammation, with minimal portal triaditis and mild dilation of blood sinusoids. The lobular architecture and hepatocytes were normal, and overall normal hepatic morphology with no histopathological derangements was observed.
Histological observations of rats administered with physical mixtures INH+NAC and INH+MPG displayed slight degenerative alterations in the parenchymal lining. Moderate sinusoidal dilatation was also observed. However, liver sections of the INH+ Met-treated group exhibited hepatocytic vacuolation, necrotic areas, moderate inflammation and severe blood sinusoidal dilatation.
Amide prodrugs of INH and three different sulphur containing amino acids (NI, MGI and NI) were synthesized. NI and MGI were synthesized using Schotten-Baumann reaction as described in the above section of synthesis of NI, and MGI and MI were synthesized by using the Boc-anhydride as a protecting as well as an activating group in amide formation, as described in section of synthesis of MI. Reactions were monitored by thin-layer chromatography (ethyl acetate:methanol:GAA; 0.5:1.5:0.02 v/v/v for NI and MGI and ethyl acetate:hexane:GAA; 0.7:0.3:0.01 v/v/v for MI). Partition coefficients and aqueous solubilities of standard drugs and synthesized conjugates were determined experimentally. Log Poct of INH was found to be -0.71 and aqueous solubility was 135 mg/mL. Partition coefficients of prodrugs were in the range of 0.06-0.27. The results of partition coefficients of prodrugs were in accordance with their determined aqueous solubilities (94-114 mg/mL). Increased partition coefficients might enhance the penetration and bioavailability of prodrugs.
The synthesized prodrugs were characterized by FTIR, 1H-NMR, 13C-NMR, elemental analysis and mass spectroscopy. Formation of amide prodrugs was confirmed through IR by carbonyl stretching between 1663-1692 cm-1 and NH stretching of secondary amide between 3300-3583 cm-1. Chemical shifts of protons of INH and amino acids backbones were confirmed by 1H-NMR, in which number of protons matched with the anticipated structures. 13C-NMR confirmed formation of amide linkage between one molecule of INH and one molecule of amino acid. Mass spectroscopy also exhibited molecular ion peaks in accordance with the molecular weights (268-283) of respective prodrugs. Elemental analysis results were within the permissible range that confirmed molecular weights of prodrugs.
All prodrugs when incubated with HCl buffer (pH 1.2) and stomach homogenates resisted hydrolysis for 3 h and when incubated with phosphate buffer (pH 7.4) resisted hydrolysis for 6 h, as HPLC analysis did not show any peaks of INH, aminothiols or unknown hydrolysis products except for the peaks of intact prodrugs. Prodrugs were readily hydrolysed in intestinal homogenates by first-order kinetics, confirming their activation by intestinal amidases. The rate constant values increased while half-lives of the prodrugs decreased, as shown in Table 2. The extent of hydrolysis was highest for NI (94.93%; Table 2).
In vivo pharmacokinetic properties and factors influencing it are the guiding principles for effective prodrug design and hence need to be accurately estimated. Appearance of INH and NAC in blood from 4 h onwards indicated enzymatic activation of NI in the small intestine. From these results, we hypothesize that NI must have been activated in the small intestine by enzymatic (amidase) hydrolysis. Interestingly, many unknown metabolites were detected in urine and faeces, which might be the consequence of metabolism of INH and NAC.
Results of estimations of antioxidant markers, including SOD, GSHPx and MDA, of liver function markers, including ALT and AST, and biochemical parameters, including cholesterol and TG, clearly indicated the abrogating effect of synthesized prodrugs. Histopathological observations correlated with the biochemical findings, thus confirming that prodrugs were more effective than physical mixtures in re-establishing the normal hepatic cytoarchitecture[47,48].
The ameliorative effect of prodrugs observed in this study can be accredited to the thiol-containing amino acids which were used as carriers. They can cause hepatotropic detoxification by augmenting synthesis of GSH, which is an endogenous antioxidant and is usually depleted as a consequence of increased oxidative stress[49]. They also act either by maintaining GSH in the reduced state or providing an alternative nucleophilic target and, thus, protect protein-SH groups[18].
In summary, to address the issue of INH-induced hepatotoxicity, prodrugs of INH with aminothiol antioxidants were successfully synthesized employing simple methods for amide synthesis. In vitro and in vivo release studies confirmed the bioactivation of these prodrugs. Observations obtained from hepatoprotective potential assessment demonstrated that the prodrugs had remarkable restorative effect on levels of enzymes and biochemical parameters involved in liver injury, to normal. Further confirmation was obtained from histological micrographs of liver that displayed complete reparation of hepatic tissue. The underlying mechanism of this protective action of prodrugs involved the suppression of oxidative stress, cell membrane stabilization and reinforcement of cellular antioxidant defences, as evidenced from interpretations of hepatoprotective activity. Findings of this study proved the success of concept-based prodrug design in abating the deleterious effects of INH and its metabolites on liver.
We believe that these novel prodrugs may offer a new therapeutic strategy in TB treatment by preventing complications associated with long-term use of INH. However, further studies to explore their in vivo antimycobacterial efficacy are required and are under progress in our laboratory, the results of which will be communicated in our future publications.
Tuberculosis (TB) is widely viewed as a disease of the developing world and death rates are often attributed to failure of overburdened public health systems to deliver appropriate care to infected individuals. The logistics of delivering the complex regimen and ensuring patient compliance with the full course of chemotherapy challenge the resources of the public health sector, even in the most developed countries. TB drug development has made substantial progress in the past decade. There are currently at least 10 drugs being evaluated in clinical trials. Some belong to chemical classes already employed in first- or second-line treatment regimens and are being explored for more optimized use at higher doses or in new drug combinations (oxazolidinones, rifamycins and fluoroquinolones), while others represent potential novel members of the TB drug arsenal, killing Mycobacterium tuberculosis through previously untried mechanisms of action (diarylquinolines, nitroimidazoles, pyrroles and ethylene diamines). The typical challenges of drug development are augmented in TB by the complexity of the disease, the requirement for multi-drug regimens, the relative lack of TB drug development for the past several decades, and inadequate resources being brought to bear despite the urgency of the global medical need. Yet, in the face of these challenges, none of the drugs have succeeded to reach the patients. The urgent need for innovation and persistent efforts to tap novel resources cannot be denied. A fine balance needs to be achieved between protecting novel drugs or modified derivatives of existing drugs so that resistance, side effects and toxicity could be minimized, ensuring that regimens are low-cost, safe, readily available, and adopted by healthcare systems and providers. The current treatment regimen has several drawbacks, including prolonged treatment time to completely eradicate the bacteria (sterilization). This increases the risk of toxicity associated with long-term use of antitubercular drug. Isoniazid (INH) is a major first-line drug used for the treatment of TB, although the metabolic and morphological aberrations that it causes and emergence of its resistance on wide scale have been a matter of great concern for future treatment schedules of TB. Therefore, a suitable molecular modification of INH in the form of codrugs was performed in order to resolve these issues. Antioxidant aminothiols were selected as carriers to minimize hepatotoxic effects of INH. The hepatoprotective potential of these prodrugs was investigated in Wistar rats to prove effectiveness in abrogating liver damage caused by INH.
The root cause of INH toxicity is believed to be in the metabolism of INH at the N2 centre in hydrazinic chain by the enzymes N-acetyltransferases, which are responsible for acetylation of INH. This acetylation by N-acetyltransferases in humans is under genetic control and can be divided into two categories, viz. “fast acetylators” and “slow acetylators”. The fast acetylators, in long-term treatment of INH, lead to significant lowering of drug bioavailability and consequent generation of INH resistance. Whereas in the case of slow acetylators, high levels of INH lead to serious hepatotoxicity. In both the cases, optimization of dose is a major issue. INH after metabolism in the liver produces hydrazine metabolites (nitrogen-centred free radicals). These radicals generate highly reactive oxygen species, which act as stimulators of lipid peroxidation, resulting in cell death and hepatic necrosis. Acute poisoning leads to lactic acidosis and renal failure, development of agranulocytosis, INH-induced tenosynovitis, INH-induced liver injury, and fatal INH-induced acute liver failure, to name only a few of the toxic consequences of INH. Furthermore, INH and/or its metabolites (e.g., hydrazine) are associated with causing mitochondrial injury that may lead to oxidant stress in mitochondria and destruction of energy homeostasis. This specific observation inspired us to design prodrugs of INH; transiently masking the N2 centre in the hydrazinic chain of the INH moiety by using aminothiols which could serve by protecting the liver against toxic effects of INH. Previous studies have shown that aminothiols have antioxidant potential and they attenuate liver injury induced by INH, acetaminophen, fluoride, cisplatin, carbon tetrachloride and lead overdose. But, none of the studies have been based on introducing these antioxidant promoeities [N-acetyl cysteine (NAC), N-(2-mercaptopropionyl) glycine (MPG) and L-methionine (Met)] in the INH molecule via amide linkage at the hydrazinic centre and exploring their therapeutic potential. This study is the first to explore the hepatoprotective potential of aminothiol-INH conjugates for restoration of normal hepatic physiology in INH-intoxicated rats and their possible healing mechanism. It was foreseen that these prodrugs may find utility in safer treatment of TB.
The chief objective of this work was to minimize hepatotoxic effects of the antitubercular drug INH and, thereby, improve its safety profile in the management of TB. As metabolism at the hydrazinic chain of INH is responsible for possible side effects, we thought of masking the N2 centre in the hydrazinic chain transiently. Hepatoprotective action was achieved by using aminothiols such as NAC, MPG and Met as antioxidant carriers, which act by scavenging free radicals. Signs of liver injury did not manifest in the prodrug-treated groups, which was one of the important objectives of the present study. Future research could be directed at investigating the in vivo antimycobacterial potential of these prodrugs.
Facile synthesis of target mutual prodrugs was accomplished through optimization of the Schotten-Baumann reaction and Boc-anhydride to avoid complex purification procedures. Spectral analysis was used for extensive characterization of synthesized prodrugs. Novel HPLC methods were developed and validated for simultaneous estimation of INH and aminothiols in the presence of intact prodrugs in order to study their release profiles in buffers of varied pH, rat homogenates of the gastrointestinal tract, blood, urine and faeces. For in vivo study, male Wistar rats weighing 180-220 g were fasted for 24 h. The animals were given drug solution in stipulated dose, quantity depending on the body weight of each animal. At 0 min, 30 min, 1 h, 2 h, 4 h, 6 h, 8 h, 10 hr and 24 h of treatment, 3 mL of blood was withdrawn by retro-orbital puncture into EDTA-coated tubes and centrifuged at 5000 rpm at 0-5 °C for 10 min. A 0.1 mL aliquot of the supernatant solution of centrifuged blood was added to an Eppendorf tube and 0.9 mL of methanol added to it for immediate plasma protein precipitation. The solution was vortexed for 2 min and then centrifuged at 5000 rpm for 10 min at 0-5 °C in order to precipitate solid matter present in the biological sample and other impurities. Then, 20 μL of the supernatant was injected into the HPLC instrument. Hepatoprotective potential was evaluated in male Wistar rats in a 21-d study. Doses calculated on equimolar basis were administered for 21 d to respective groups. All the animals were examined for liver function markers, antioxidant markers, biochemical parameters and liver histology. Livers of the sacrificed animals were removed and fixed in 10% buffered formalin and samples were sent for microscopic examination. Various markers like aminotransferases, superoxide dismutase, glutathione peroxidase and malondialdehyde, cholesterol and triglycerides were estimated at the end of study. Relevant statistical tests were used for analysing the data.
Activation of prodrugs by amidases in the small intestine, restoration of enzyme levels, re-establishment of the antioxidant defence system to avert the formation of excessive free radicals, regeneration of hepatocytes, maintenance of structural integrity of liver by released aminothiols and significantly notable reparation of INH-induced hepatotoxicity in Wistar rats were the promising outcomes of this study. This is the pioneer study to identify and describe the therapeutic potential of hepatoprotective prodrugs of INH in Wistar rats. These prodrugs could be explored further as an alternative to INH for treatment of TB.
In the present work, the N2 centre of the hydrazinic chain in INH was transiently masked with aminothiols, as literature review revealed that antioxidant aminothiols play a vital role in restoring the antioxidant defence system of a host after a free radical-mediated destructive autocatalytic process which occurs in INH-induced hepatotoxicity. The novel hepatoprotective prodrug strategy proved to be advantageous in terms of lowering the biochemical parameters and liver function markers, and remarkably improving the levels of enzymes involved in the antioxidant defence system. This study emphasized the effectiveness of aminothiols to maintain integrity of the liver. These prodrugs have the potential to be screened further for their effectiveness in patients who are on long-term treatment with INH.
This study proved that concept-based design of hepatoprotective prodrugs can be applied successfully in overcoming toxic effects of a drug. This work proved that an amide conjugation strategy can provide a potential synergistic effect in abrogation of INH-induced hepatotoxicity. Future research should be directed towards investigation of in vivo antimycobacterial potential and pharmacokinetic profiles in order to elucidate the exact molecular and biochemical mechanisms of these synthesized prodrugs.
The authors would like to gratefully acknowledge Lupin Research Park, Lupin Ltd. (Aurangabad, India) for providing the gift sample of isoniazid and Zim Laboratories (Nagpur, India) for providing the gift sample of N-acetylcysteine.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: India
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Akbulut S, Skrypnyk IN, Zapater P S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Yin SY
| 1. | WHO. Global Tuberculosis Report. World Health Organization Report. 2016;. |
| 2. | Hearn MJ, Cynamon MH. In vitro and in vivo activities of acylated derivatives of isoniazid against mycobacterium tuberculosis. Drug Des Discov. 2003;18:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Adhvaryu MR, Reddy N, Vakharia BC. Prevention of hepatotoxicity due to anti tuberculosis treatment: a novel integrative approach. World J Gastroenterol. 2008;14:4753-4762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (2)] |
| 4. | Scharer L, Smith JP. Serum transaminase elevations and other hepatic abnormalities in patients receiving isoniazid. Ann Intern Med. 1969;71:1113-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 92] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Nolan CM, Goldberg SV, Buskin SE. Hepatotoxicity associated with isoniazid preventive therapy: a 7-year survey from a public health tuberculosis clinic. JAMA. 1999;281:1014-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 298] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 6. | Metushi IG, Cai P, Zhu X, Nakagawa T, Uetrecht JP. A fresh look at the mechanism of isoniazid-induced hepatotoxicity. Clin Pharmacol Ther. 2011;89:911-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 7. | Boelsterli UA, Lee KK. Mechanisms of isoniazid-induced idiosyncratic liver injury: emerging role of mitochondrial stress. J Gastroenterol Hepatol. 2014;29:678-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 8. | Cassano R, Trombino S, Ferrarelli T, Cavalcanti P, Giraldi C, Lai F, Loy G, Picci N. Synthesis, characterization and in-vitro antitubercular activity of isoniazid-gelatin conjugate. J Pharm Pharmacol. 2012;64:712-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Horváti K, Mezo G, Szabó N, Hudecz F, Bosze S. Peptide conjugates of therapeutically used antitubercular isoniazid-design, synthesis and antimycobacterial effect. J Pept Sci. 2009;15:385-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Kakkar D, Tiwari AK, Chuttani K, Kumar R, Mishra K, Singh H, Mishra AK. Polyethylene-glycolylated isoniazid conjugate for reduced toxicity and sustained release. Ther Deliv. 2011;2:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Dandawate P, Vemuri K, Venkateswara Swamy K, Khan EM, Sritharan M, Padhye S. Synthesis, characterization, molecular docking and anti-tubercular activity of Plumbagin-Isoniazid Analog and its β-cyclodextrin conjugate. Bioorg Med Chem Lett. 2014;24:5070-5075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Wu ZR, Zhi DJ, Zheng LF, Li JY, Li Y, Xie QJ, Feng N, Bao YF, Gao QY, Song Y. Design and applications of bifunctional cinnamide derivatives as potential antimycobacterial agents with few hepatotoxic effects. Med Chem Res. 2015;24:161-170. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Singh C, Jodave L, Bhatt TD, Gill MS, Suresh S. Hepatoprotective agent tethered isoniazid for the treatment of drug-induced hepatotoxicity: Synthesis, biochemical and histopathological evaluation. Toxicol Rep. 2014;1:885-893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Hearn M, Cynamon M, Chen M, Coppins R, Davis J, Joo-On Kang H, Noble A, Tu-Sekine B, Terrot MS, Trombino D. Preparation and antitubercular activities in vitro and in vivo of novel Schiff bases of isoniazid. Eur J Med Chem. 2009;44:4169-4178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 15. | Bhilare NV, Dhaneshwar SS, Mahadik KR. Phenolic acid-tethered isoniazid for abrogation of drug-induced hepatotoxicity: design, synthesis, kinetics and pharmacological evaluation. Drug Deliv Transl Res. 2018;8:770-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Tafazoli S, Mashregi M, O’Brien PJ. Role of hydrazine in isoniazid-induced hepatotoxicity in a hepatocyte inflammation model. Toxicol Appl Pharmacol. 2008;229:94-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Li S, Tan HY, Wang N, Zhang ZJ, Lao L, Wong CW, Feng Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int J Mol Sci. 2015;16:26087-26124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1267] [Cited by in RCA: 1088] [Article Influence: 108.8] [Reference Citation Analysis (1)] |
| 18. | Attri S, Rana SV, Vaiphei K, Sodhi CP, Katyal R, Goel RC, Nain CK, Singh K. Isoniazid- and rifampicin-induced oxidative hepatic injury--protection by N-acetylcysteine. Hum Exp Toxicol. 2000;19:517-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 91] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Zhang JG, Lindup WE. Tiopronin protects against the nephrotoxicity of cisplatin in rat renal cortical slices in vitro. Toxicol Appl Pharmacol. 1996;141:425-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Rana SV, Attri S, Vaiphei K, Pal R, Attri A, Singh K. Role of N-acetylcysteine in rifampicin-induced hepatic injury of young rats. World J Gastroenterol. 2006;12:287-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Bhilare NV, Dhaneshwar SS, Sinha AJ, Kandhare AD, Bodhankar SL. Novel Thioester Prodrug of N-acetylcysteine for Odor Masking and Bioavailability Enhancement. Curr Drug Deliv. 2016;13:611-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Pinnen F, Cacciatore I, Cornacchia C, Sozio P, Cerasa LS, Iannitelli A, Nasuti C, Cantalamessa F, Sekar D, Gabbianelli R. Codrugs linking L-dopa and sulfur-containing antioxidants: new pharmacological tools against Parkinson’s disease. J Med Chem. 2009;52:559-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Caylak E, Aytekin M, Halifeoglu I. Antioxidant effects of methionine, alpha-lipoic acid, N-acetylcysteine and homocysteine on lead-induced oxidative stress to erythrocytes in rats. Exp Toxicol Pathol. 2008;60:289-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Bhilare NV, Dhaneshwar SS. Synthesis and evaluation of morpholinoethylester conjugate of N-acetylcysteine in ovalbumin- induced airway hyperresponsiveness in Sprague dawley rats. Lett Drug Des Discov. 2016;13:1-7. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Reed DJ, Orrenius S. The role of methionine in glutathione biosynthesis by isolated hepatocytes. Biochem Biophys Res Commun. 1977;77:1257-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 116] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Labadarios D, Davis M, Portmann B, Williams R. Paracetamol-induced hepatic necrosis in the mouse-relationship between covalent binding, hepatic glutathione depletion and the protective effect of alpha-mercaptopropionylglycine. Biochem Pharmacol. 1977;26:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Jhaumeer L, KhodaBoccus A, Hemraz U, Sunnassee S. Use of Di-tert-butyl-dicarbonate Both as a Protecting and Activating Group in the Synthesis of Dipeptides. Synth Commun. 2007;37:4191-4197. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (1)] |
| 28. | Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6497] [Cited by in RCA: 6570] [Article Influence: 128.8] [Reference Citation Analysis (1)] |
| 29. | Chanchal SK, Mahajan UB, Siddharth S, Reddy N, Goyal SN, Patil PH, Bommanahalli BP, Kundu CN, Patil CR, Ojha S. In vivo and in vitro protective effects of omeprazole against neuropathic pain. Sci Rep. 2016;6:30007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 30. | Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979;582:67-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2499] [Cited by in RCA: 2659] [Article Influence: 57.8] [Reference Citation Analysis (1)] |
| 31. | Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17627] [Cited by in RCA: 18794] [Article Influence: 408.6] [Reference Citation Analysis (0)] |
| 32. | Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5887] [Cited by in RCA: 5409] [Article Influence: 79.5] [Reference Citation Analysis (0)] |
| 33. | Sullivan DR, Kruijswijk Z, West CE, Kohlmeier M, Katan MB. Determination of serum triglycerides by an accurate enzymatic method not affected by free glycerol. Clin Chem. 1985;31:1227-1228. [PubMed] |
| 34. | Blum J, Fridovich I. Inactivation of glutathione peroxidase by superoxide radical. Arch Biochem Biophys. 1985;240:500-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 373] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 35. | Aruoma OI, Halliwell B, Hoey BM, Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med. 1989;6:593-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1324] [Cited by in RCA: 1325] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 36. | Jaya DS, Augustine J, Menon VP. Protective role of Nacetylcysteine against alcohol - and paracetamol - induced toxicity. Ind J Clin Biochem. 1994;9:64-71. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Bais B, Saiju P. Ameliorative effect of Leucas cephalotes extract on isoniazid and rifampicin induced hepatotoxicity. Asian Pac J Trop Biomed. 2014;4:S633-S638. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Saxena B, Sharma S. Food Color Induced Hepatotoxicity in Swiss Albino Rats, Rattus norvegicus. Toxicol Int. 2015;22:152-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 39. | Tian Z, Liu H, Su X, Fang Z, Dong Z, Yu C, Luo K. Role of elevated liver transaminase levels in the diagnosis of liver injury after blunt abdominal trauma. Exp Ther Med. 2012;4:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 40. | Timmins GS, Deretic V. Mechanisms of action of isoniazid. Mol Microbiol. 2006;62:1220-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 271] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 41. | Gelen V, Şengül E, Gedikli S, Atila G, Uslu H, Makav M. The protective effect of rutin and quercetin on 5-FU-induced hepatotoxicity in rats. Asian Pac J Trop Dis. 2017;7:647-653. [RCA] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 42. | Martinet W, Knaapen MW, De Meyer GR, Herman AG, Kockx MM. Oxidative DNA damage and repair in experimental atherosclerosis are reversed by dietary lipid lowering. Circ Res. 2001;88:733-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 117] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 43. | Peretti E, Karlaganis G, Lauterburg BH. Acetylation of acetylhydrazine, the toxic metabolite of isoniazid, in humans. Inhibition by concomitant administration of isoniazid. J Pharmacol Exp Ther. 1987;243:686-689. [PubMed] |
| 44. | Pal R, Rana SV, Vaiphei K, Singh K. Isoniazid-rifampicin induced lipid changes in rats. Clin Chim Acta. 2008;389:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 45. | Santhosh S, Sini TK, Anandan R, Mathew PT. Effect of chitosan supplementation on antitubercular drugs-induced hepatotoxicity in rats. Toxicology. 2006;219:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 46. | Evan Prince S, Udhaya LB, Sunitha PS, Arumugam G. Reparation of Isoniazid and Rifampicin Combinatorial Therapy-Induced Hepatotoxic Effects by Bacopa monnieri. Pharmacology. 2016;98:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 47. | Flora SJ, Dwivedi N, Deb U, Kushwaha P, Lomash V. Effects of co-exposure to arsenic and dichlorvos on glutathione metabolism, neurological, hepatic variables and tissue histopathology in rats. Toxicol Res. 2014;3:23-31. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Tousson E, Atteya Z, El-Atrash A, Jeweely OI. Abrogation by Ginkgo Byloba leaf extract on hepatic and renal toxicity induced by methotrexate in rats. Cancer Res Treat. 2014;2:44-51. |
| 49. | Grinberg L, Fibach E, Amer J, Atlas D. N-acetylcysteine amide, a novel cell-permeating thiol, restores cellular glutathione and protects human red blood cells from oxidative stress. Free Radic Biol Med. 2005;38:136-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 164] [Article Influence: 8.2] [Reference Citation Analysis (0)] |