Published online May 27, 2018. doi: 10.4254/wjh.v10.i5.409
Peer-review started: February 21, 2018
First decision: March 15, 2018
Revised: March 20, 2018
Accepted: April 15, 2018
Article in press: April 16, 2018
Published online: May 27, 2018
Processing time: 96 Days and 8 Hours
To build a regional database of chronic patients to define the clinical epidemiology of hepatitis B virus (HBV)-infected patients in the Tuscan public health care system.
This study used a cross-sectional cohort design. We evaluated chronic viral hepatitis patients with HBV referred to the outpatient services of 16 hospital units. Information in the case report forms included main demographic data, blood chemistry data, viral hepatitis markers, instrumental evaluations, and eligibility for treatment or ongoing therapy and liver transplantation.
Of 4015 chronic viral hepatitis patients, 1096 (27.3%) were HBV infected. The case report form was correctly completed for only 833 patients (64% males, 36% females; mean age 50.1 ± 15.4). Of these HBV-infected patients, 73% were Caucasian, 21% Asian, 4% Central African, 1% North African and 1% American. Stratifying patients by age and nationality, we found that 21.7% of HBV-infected patients were aged < 34 years (only 2.8% were Italian). The most represented routes of transmission were nosocomial/dental procedures (23%), mother-to-child (17%) and sexual transmission (12%). The most represented HBV genotypes were D (72%) and A (14%). Of the patients, 24.7% of patients were HBeAg positive, and 75.3% were HBeAg negative. Of the HBV patients 7% were anti-HDV positive. In the whole cohort, 26.9% were cirrhotic (35.8% aged < 45 years), and 47% were eligible for or currently undergoing treatment, of whom 41.9 % were cirrhotic.
Only 27.3% of chronic viral hepatitis patients were HBV infected. Our results provide evidence of HBV infection in people aged < 34 years, especially in the foreign population not protected by vaccination. In our cohort of patients, liver cirrhosis was also found in young adults.
Core tip: Although the introduction of a vaccine against hepatitis B virus (HBV) has been highly effective in reducing the incidence and prevalence of HBV infection in many countries, an estimated 257 million people worldwide are chronically infected. In 2015, WHO published the guidelines for prevention, care and treatment of HBV infected people promoting treatment based on noninvasive assessment. This real-world large-scale cohort study provides appropriate planning for public health programs, as well as the specific characteristics of patients, thus contributing to the successful, efficient translation of new knowledge to management and treatment of HBV patients to eradicate HBV.
- Citation: Stasi C, Silvestri C, Berni R, Brunetto MR, Zignego AL, Orsini C, Milani S, Ricciardi L, De Luca A, Blanc P, Nencioni C, Aquilini D, Bartoloni A, Bresci G, Marchi S, Filipponi F, Colombatto P, Forte P, Galli A, Luchi S, Chigiotti S, Nerli A, Corti G, Sacco R, Carrai P, Ricchiuti A, Giusti M, Almi P, Cozzi A, Carloppi S, Laffi G, Voller F, Cipriani F. Clinical epidemiology of chronic viral hepatitis B: A Tuscany real-world large-scale cohort study. World J Hepatol 2018; 10(5): 409-416
- URL: https://www.wjgnet.com/1948-5182/full/v10/i5/409.htm
- DOI: https://dx.doi.org/10.4254/wjh.v10.i5.409
While the introduction of an effective vaccine has been highly effective in reducing the incidence and prevalence of hepatitis B virus (HBV) infection in many countries, an estimated 257 million people worldwide are chronically infected[1].
HBV prevalence is > 8% in parts of sub-Saharan Africa. An intermediate prevalence (2%-8%) is present in some regions of the eastern Mediterranean, Central Asia, Southeast Asia, China, parts of South America and some European countries (Albania, Bulgaria, Romania, Turkey and Italy). There is a low prevalence (< 2%) in parts of North America and in some European countries (Belgium, the Czech Republic, Denmark, France), as well as Australia and New Zealand[2].
High levels of viremia, or the infection contracted at a young age (affecting mainly males) are associated with an increased risk of death or of developing hepatocellular carcinoma[3]. In 2015, hepatitis B resulted in 887000 deaths, mostly from complications (including cirrhosis and hepatocellular carcinoma)[1].
In 2015, WHO published guidelines for the prevention, care and treatment of persons with chronic HBV infection. These promote the use of simple, noninvasive tests to assess liver disease stage and eligibility for treatment. For adult patients in countries with limited resources, the test for ratio of aspartate aminotransferase to platelets (APRI) is recommended as a noninvasive diagnostic to assess the presence of cirrhosis (APRI score > 2), while transient elastography (e.g., fibroscan) or fibrotest are recommended in countries where these tests are available and cost constraints are not significant. These guidelines are based on a public health approach to the use of antiviral drugs for the treatment of chronic HBV. Such an approach considers the feasibility and effectiveness of treatment, even in countries with limited resources-for example, in areas where diagnostic methods such as HBV DNA and liver biopsy are unable to be used[4].
According to the latest Italian recommendations[5], treatment with nucleoside or nucleotide analogues is indicated in the following instances: HBeAg-positive patients with moderate or severe fibrosis who have failed seroconversion after a cycle of Peg-IFN, for whom treatment with Peg-IFN is not indicated at diagnosis; in HBeAg-negative patients with moderate or severe fibrosis who have already been treated with Peg-IFN without success or those for whom treatment with PegIFN is not indicated at diagnosis; in HBeAg-positive or HBeAg-negative patients who (for family, work or social reasons) are unable to practice or do not accept Peg-IFN therapy; in HBeAg-positive or HBeAg-negative patients with compensated or decompensated cirrhosis, regardless of the likelihood of seroconversion, serum HBV DNA levels and ALT values.
The aim of this study was to build a regional database of patients diagnosed with chronic HBV infection in specialised outpatient services in our region to define the clinical epidemiology of HBV-infected patients in the Tuscan public health care system.
This prospective observational study is fully in line with the objectives of the National Plan for the Prevention of Viral Hepatitis, in particular the Address Line 1 (LI 1)-Epidemiology, which seeks to “define the epidemiology of viral hepatitis B and C and to strengthen surveillance systems”. Objective 3 is of particular focus: It outlines the importance of the quality of reporting system data and the monitoring of HBV and HCV as acute or chronic infections[6].
All patients with HBV-related chronic liver disease referred to the hepatology outpatient services of 16 hospital units from January 1, 2015, to December 31, 2015, and who agreed to participate in the study, were evaluated. These 16 hospital units were: Hepatology Unit, University Hospital of Pisa, Pisa, Italy; Centre for Systemic Manifestations of Hepatitis Viruses (MaSVE), Internal Medicine and Liver Unit, Department of Experimental and Clinical Medicine, Careggi University Hospital, Florence, Italy; Gastroenterology Research Unit, Department of Experimental and Clinical Biomedical Sciences Mario Serio, Careggi University Hospital, Florence, Italy; Infectious Disease Unit, Hospital of Lucca, Italy; Infectious Diseases Unit, Siena University Hospital, Siena, Italy; Infectious Disease Unit, S. Maria Annunziata Hospital, Florence, Italy; Infectious Disease Unit, Hospital of Grosseto, Italy; Infectious Disease Unit, Hospital of Prato, Italy; Infectious and Tropical Diseases Unit, Careggi University Hospital, Florence, Italy; Gastroenterology and Metabolic Disorders, Cisanello University Hospital, Pisa, Italy; Gastroenterology Unit, Department of Translational Research and New Technologies in Medicine and Surgery, Cisanello University Hospital of Pisa, Pisa, Italy; Liver Surgery and Transplantation Unit, Cisanello University Hospital, Pisa, Italy; Internal Medicine Unit, San Jacopo Hospital, Pistoia, Italy; Infectious Diseases and Hepatology Unit, University Hospital of Siena, Siena, Italy; Gastroenterology Unit, San Giuseppe Hospital, Empoli, Italy; and Internal Medicine and Liver Unit, Careggi University Hospital, Florence, Italy.
The Regional Health Agency of Tuscany provided all units with a computerised clinical database to collect the main socio-demographic, clinical and treatment data for all patients with chronic HCV and HBV infection admitted at each centre. A record was created for each patient containing his or her main personal data, blood chemistry data, viral hepatitis markers, HCV-RNA and HBV-DNA viral load, histology and instrumental evaluation (FibroScan®, liver ultrasound), previous treatment (with outcome), eligibility for treatment, or therapy already in progress, and liver transplantation details.
Liver ultrasound and elastography are among the main diagnostic procedures used in patients with chronic hepatitis. Liver ultrasound allows clinicians to diagnose and monitor chronic liver diseases, including the diagnosis of liver tumours and the identification of signs of portal hypertension. Liver surface irregularities detected by ultrasound techniques define the presence of micro- and macronodules and are considered the most sensitive and reproducible signs of liver cirrhosis[7]. Elastography characterises the properties and mechanical response of tissues, measuring their stiffness[8]. Elastography is currently used to determine clinical priorities based on the estimated fibrosis stage. In HBV naïve patients with normal ALT, the cut-offs used were < 6 kPa (no significant fibrosis), 6-9 kPa (grey area), and > 9 kPa (severe fibrosis/cirrhosis). In HBV-naïve patients with elevated ALT (but < 5 × ULN), the cut offs used were < 6 kPa (no significant fibrosis), 6-12 kPa (grey area), and > 12 kPa (severe fibrosis/cirrhosis)[9].
To estimate the number of patients with more severe liver disease, we considered those patients with a Child-Pugh score (used only to assess the severity of liver cirrhosis), and/or with a liver stiffness either > 9 kPa (normal ALT) or > 12 kPa (elevated ALT)[9] at elastography, and/or with a histological diagnosis of cirrhosis (METAVIR score equal to F4 or Ishak equal to S5-6), and/or with a nodular structure of the liver identified by ultrasound examination. Duplicate cases were excluded.
The demographic data were appropriately encrypted in the database. The software not only allowed the research team to include guided and controlled information using a combo-box but also to export files ready to be sent.
The exported file (containing the “anonymized” data) was sent from the system to the Regional Health Agency of Tuscany via a secure channel (SSL). After logging in and connecting to the Agency’s web page, the user was then able to upload the file.
The research topic was described to the patients. The local Ethics Committee approved the study. Each participant gave written informed consent prior to the study, in accordance with the principles of the Declaration of Helsinki (Sixth Revision, Seoul 2008).
All data were validated, checking for out-of-range values and values that were logically inconsistent were handled. All results are expressed as mean ± SD. The numerical comparison of continuous data was performed using the Student’s t-test for unpaired and for paired samples with Bonferroni correction, after checking similar variances in the groups by Levine’s test for equality of variances. To avoid the potential bias related to the missing data, the analysis of the variables was conducted only on the completed fields.
Statistical significance was set at a value of P < 0.05. Statistical analysis was obtained using statistical software Stata 12 (College Station, TX, United States).
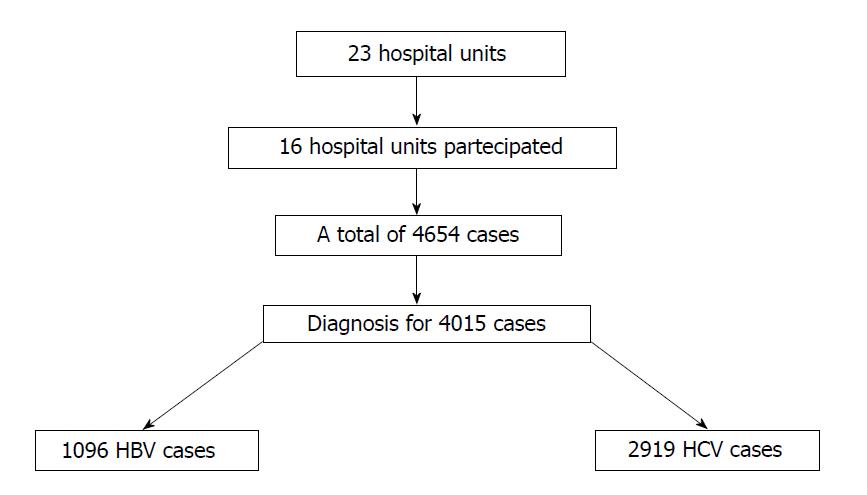
A total of 23 hepatology units were invited to participate in the study. Data were obtained from 16 hospital units for a total of 4654 cases of chronic HBV and HCV infections. Diagnoses were specified for 4015 (86.3%) patients, of whom 1096 (27.3%) were HBV infected (Figure 1). However, the case report form was correctly completed for only 833 patients (64% males, 36% females; mean age 50.1 ± 15.4). For HBV patients, 73% were Caucasian, 21% Asian, 4% Central African, 1% North African and 1% American. Of these HBV-infected patients, 106 (12.7%) were referred to the clinics for the first time during the period in question. Table 1 shows the patients stratified by age and gender.
| Age group | Males | Females | Total |
| ≤ 34 | 54 (18.3) | 46 (27.9) | 100 (21.7) |
| 35-44 | 60 (20.3) | 26 (15.7) | 86 (18.7) |
| 45-54 | 68 (23.1) | 33 (20.0) | 101 (22.0) |
| 55-64 | 58 (19.7) | 27 (16.4) | 85 (18.5) |
| 65-74 | 36 (12.2) | 22 (13.3) | 58 (12.6) |
| > 75 | 19 (6.4) | 11 (6.7) | 30 (6.5) |
| Total | 295 (100) | 165 (100) | 460 (100) |
| Missing | 479 |
Analysis of the calendar year of diagnosis and of the first access to specialised services reveals that about 98.1% of these cases occurred after 1990.
Stratifying patients by age and nationality, we found that 21.7% of HBV-infected patients were aged < 34 years (only 2.8% were Italian). Table 2 shows the most represented routes of transmission were nosocomial/dental procedures (23%), mother-to-child (17%) and sexual transmission (12%).
| Routes of transmission | Distribution |
| Intravenous drug users | 15 (3.5) |
| Coagulation factors/blood transfusions | 26 (6.0) |
| Sexual transmission | 53 (12.3) |
| Piercing and tattooing | 9 (2.1) |
| Vertical transmission | 73 (16.9) |
| Nosocomial/dental cure | 101 (23.4) |
| Undefined | 255 (59.0) |
The most represented HBV genotypes were D (72%) and A (14%), shown in Table 3, seen in 135 HBV patients.
| Genotypes | Distribution |
| A | 19 (14.1) |
| B | 4 (3.0) |
| C | 4 (3.0) |
| D | 97 (71.9) |
| E | 5 (3.6) |
| F | 6 (4.4) |
| Total | 135 (100) |
The total population displayed the following parameters: mean ALT = 59.84 ± 108.49 IU/L; mean AST = 48.20 ± 87.02 IU/L; mean Platelets = 195.23 ± 72.13 cells/L. The number of patients with PLT < 100000 was 31. In the population, 24.7% of HBV patients were HBeAg positive, 75.3% were HBeAg negative, and 7% were anti-HDV positive.
In non-treated HBeAg and anti HBeAg positive patients, the HBV DNA levels were 7.26 × 107 ± 1.71 × 108 and 8358838 ± 2.71 × 107 UI/mL, respectively.
In non-treated HBeAg and anti-HBeAg-positive patients, the ALT levels were 42.05 ± 29.70 U/L and 33.96 ± 33.49 U/L, respectively.
A total of 89 HBV patients underwent liver biopsy. Of these, 66 also had elastography, with 45% of total liver biopsies performed before 2007 (i.e., before liver elastography was used in clinical practice). Of 89 patients underwent liver biopsy, 70% were evaluated with an Ishak score (supplementary material), providing a better definition of liver fibrosis compared to METAVIR score. Twenty-three patients underwent liver biopsy alone, while 283 underwent elastography, with the latter performed exclusively after 2008.
Ultrasound examination revealed liver structure alteration in 29.4% of cases, and the presence of a nodular structure in 12%.
In the whole cohort, 26.9% of patients were cirrhotic (35.8% were aged < 45 years). The Child-Pugh score was specified in 156 patients. Most cirrhotic patients were classified as Child-Pugh Class A (n = 150, 96.2%), with an early stage severity of liver cirrhosis, with 4 classified as Child B (2.5%) and 2 as Child C (n = 1.3%). One hundred and six patients displayed signs of portal hypertension, which mostly occurred in those < 45 years (26%). Eight patients received liver transplantations. Forty-seven percent of patients were either eligible for or currently undergoing treatment, of whom 41.9% were cirrhotic. Dividing the cohort into treated (n = 162) and untreated HBeAg-positive patients (n = 182), we found significant differences between liver stiffness values (7.83 ± 3.56 vs 5.3 ± 2.3, P = 0.02). Similar differences were observed (7.84 ± 5.05 vs 4.82 ± 1.88, P < 0.001) when dividing the cohort into treated (n = 163) and untreated anti-HBeAg-positive patients (n = 181).
To our knowledge, this study involves the largest cohort of HBV patients in Italy. HBV infection was responsible for 27.3% of cases of chronic liver disease, whereas a previous study by Sagnelli et al[10] found an HBV prevalence of 20.2%.
We also found HBV-infected patients in those aged < 34 who would normally be covered by vaccination. Analysis of the nationality of HBV-infected patients highlights the prevalence of infection in the non-Italian population (only 2.8% of Italians were infected). Similar data was found in a previous study conducted in an industrialised city in the Tuscany region[11]. Further efforts are needed to improve vaccination coverage for non-infected and non-vaccinated immigrants who are susceptible to infection.
According to national data on risk factors associated with the HBV infection in the Integrated Epidemiological System of Viral Acute Hepatitis (SEIVA), nosocomial/dental procedures (23%) are some of the most represented risk factors for HBV transmission, followed by mother-to-child (17%) and sexual transmission (12%). Recently, cosmetic treatment with percutaneous exposure (28% of patients), sexual exposure (24.7%), and dental therapy (11.5%) have been recognized as the main risk factors in acute viral hepatitis B in Italy. The risk of maternal-infant transmission is related to the mother’s HBV replicative status (90% correlation with HBeAg-positive mothers compared to 10%-20% for HBeAg-negative mothers)[12]. Many studies[13-16] have highlighted that pregnant women in particular with high levels of HBV DNA (more elevated in HBeAg positive patients compared to HBeAg-negative patients) have an increased risk of transmitting infection. As recently shown by Sagnelli et al[17], the screening of pregnant women to detect circulating HBsAg and prophylaxis procedures for new-born babies, the universal vaccination against HBV infection introduced in 1991, and national media campaigns against HIV infection are considered the main reasons for the significant reduction in this type of transmission. Heterosexual (and, to a greater extent, homosexual) activity remain significant means of transmission. A study of Zuccaro et al[14] for an HBV cohort of 103 patients in 15 Italian hospital units showed a high prevalence of sexual transmission. In a study by Hahné et al[15], HBsAg prevalence estimates in men who have sex with men (MSM) ranged from < 1% to 4% in 3 of the 34 countries. This prevalence was 22 times higher than that of the general population for countries with available data[15].
As reported by Liaw et al[16], HBV genotype D is predominant in Mediterranean countries. In line with this finding, our study found that the most represented HBV genotype were D (72%), followed by A (14%). As concerns the potential bias of missing data, the statistical advantage of data that are missing completely at random is that the estimated parameters are not biased by the absence of the data.
To our knowledge, this is one of the few Italian studies to consider both infection rates and liver fibrosis stage based on international guidelines for a large cohort of patients.
We found a higher prevalence of HBeAg positive patients (24.7%) compared to Sagnelli et al[10] (7.2%) and a lower anti-HDV prevalence (7% vs 11.9%). It is well established that ongoing HBV replication or the presence of HBeAg may accelerate the progression of liver fibrosis. In a study of the natural course of HBV infection during the HBeAg-positive phase, Chu et al[18] showed that the annual incidence of cirrhosis was 0.5%, and the cumulative probability of cirrhosis after 17 years was 12.6%. Hence, age at anti-HBe seroconversion and hepatitis relapse were independent risk factors for cirrhosis. In particular, deferred HBeAg seroconversion (patients > 40 years) were associated with an increased risk of cirrhosis[18]. Moreover, HDV coinfection is associated with an accelerated progression towards liver cirrhosis, decompensation in existing cases of cirrhosis, and an increased risk of hepatocellular carcinoma[19].
In our cohort, almost all untreated patients were represented by inactive HBV carriers (HBV DNA < 2000 IU/mL), and by patients in an immune-tolerant phase (HBV DNA > 20000 IU/mL) with liver stiffness measurements < 6 kPa and normal ALT. These results are in agreement with national guidelines for treatment[5]. A study of 361 patients with inactive HBeAg-negative chronic hepatitis B patients demonstrated that liver fibrosis progression is rare given serum HBV DNA < 20000 IU/mL and normal ALT[21]. Brunetto et al[20] demonstrated that after a mean follow-up of six years, anti-HBe positive chronic hepatitis B progressed to cirrhosis in 45% of patients, with end-stage complications occurring in 24% of those presenting with cirrhosis. These outcomes seem to be associated with older age and persistent viral replication or hepatitis exacerbations in chronic hepatitis or cirrhotic patients. A recent study by Olivieri et al[22] showed that HBeAg-negative HBsAg-carriers with baseline HBV-DNA ≤ 20000 IU/mL, and normal transaminases were associated with transition to inactive carrier status in 43% of low-viremic active carriers, and to occult HBV infection in 20% of inactive carrier, within five years.
We found that almost all patients with liver stiffness > 6 kPa were treated. There are several instances of evidence showing that antiviral therapy stops the progression of liver fibrosis and induces fibrosis regression. A recent study by Stasi et al[23] conducted in anti-HBeAg-positive patients before and during antiviral treatment using liver stiffness measurements with transient elastography at different time points to assess changes in liver fibrosis showed a statistically significant reduction in stiffness values at 18 and 24 mo from those observed prior to therapy.
In the whole cohort, 26.9% were cirrhotic patients; it should be stressed here that 35.8% of these were aged < 45 years. The majority of cirrhotic patients showed signs of portal hypertension (43%). This data was different to that of Sagnelli et al[10] for a cohort of 513 HBV patients, 24% of whom had liver cirrhosis.
In conclusion, our data showed that 27.3% of chronic viral hepatitis patients were HBV infected. Evidence of HBV infection in people aged < 34 years was apparent, especially in the foreign population not protected by vaccination. The main routes of transmission were consolidated by data reported in several other studies. In our cohort of patients, we found an elevated prevalence of HBeAg-positive patients. In line with other Italian data, we found a high prevalence of liver cirrhosis in young adults. Antiviral treatment seems to be fully in line with national guidelines. Further efforts are necessary to increase vaccine coverage in immigrant populations.
While the introduction of an effective vaccine has been highly effective in reducing the incidence and prevalence of hepatitis B virus (HBV) infection in many countries, an estimated 257 million people worldwide are still chronically infected. In 2015, WHO published guidelines for the prevention, care and treatment of persons with chronic HBV infection. These promote the use of simple, noninvasive tests to assess liver disease stage and eligibility for treatment.
Few data are currently available concerning the real-word large-scale staging and treatment of HBV patients followed in specialised outpatient services. The knowledge of the specific characteristics of patients could lead to appropriate planning for public health programs, thus contributing to the successful, efficient translation of this knowledge to management and treatment of HBV patients to eradicate HBV.
The main objective of this study was to build a regional database of patients diagnosed with chronic HBV infection in specialised outpatient services in our region to define the clinical epidemiology of HBV-infected patients in the Tuscan public health care system.
This prospective observational study is fully in line with the objectives of the National Plan for the Prevention of Viral Hepatitis, in particular with those outlining the importance of the quality of reporting system data and of the monitoring of HBV and hepatitis C virus as acute or chronic infections. This study used a cross-sectional cohort design to evaluate all patients with HBV-related chronic liver disease referred to the hepatology outpatient services of 16 hospital units from January 1, 2015, to December 31, 2015. A computerised clinical database was used to collect the main socio-demographic, clinical and treatment data for all patients with chronic HBV infection admitted at each centre. The exported file (containing the “anonymized” data) was then sent to the Regional Health Agency of Tuscany via a secure channel (SSL).
The results of this study demonstrated that 27.3% of chronic viral hepatitis patients were HBV infected. Of these HBV-infected patients, 73% were Caucasian, 21% Asian, 4% Central African, 1% North African and 1% American. Stratifying patients by age and nationality, we found that 21.7% of HBV-infected patients were aged < 34 years. Among these only 2.8% were Italian. The most represented routes of transmission were nosocomial/dental procedures (23%), mother-to-child (17%) and sexual transmission (12%). The most represented HBV genotypes were D (72%) and A (14%). Of the patients, 24.7% were HBeAg positive, and 75.3% were HBeAg negative. Of the HBV patients, 7% were anti-HDV positive. In the whole cohort, 26.9% were cirrhotic (35.8% aged < 45 years), and 47% were eligible for or currently undergoing treatment, of whom 41.9% were cirrhotic.
The informatization of clinical data and the real-word large-scale staging and treatment of these kinds of patients would allow health planners to assign specific healthcare resources to targeted populations. This observational research may provide a public health opportunity to screen and treat specific groups of patients. In particular, to eradicate HBV infection further efforts are necessary to increase vaccine coverage in immigrant populations.
Based on the data collected in the course of this investigation, it is necessary to implement a series of preventive measures and strengthen the entire care process to eliminate, or at least reduce, risk factors for HBV infection and progression toward advanced liver fibrosis stages and to adopt care protocols suitable for immigrant populations.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gong ZJ, Zhao HT S- Editor: Wang XJ L- Editor: Filipodia E- Editor: Tan WW
| 1. | World Health Organization. Hepatitis B. Fact sheet. Reviewed July 2017. Available from: http://www.who.int/mediacentre/factsheets/fs204/en/. |
| 2. | Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1806] [Cited by in RCA: 1998] [Article Influence: 199.8] [Reference Citation Analysis (4)] |
| 3. | Taylor BC, Yuan JM, Shamliyan TA, Shaukat A, Kane RL, Wilt TJ. Clinical outcomes in adults with chronic hepatitis B in association with patient and viral characteristics: A systematic review of evidence. Hepatology. 2009;49:S85-S95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | World Health Organization. Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. Available from: http://www.who.int/hiv/pub/hepatitis/hepatitis-b-guidelines/en/. |
| 5. | Brunetto MR, Bruno R, Di Marco V, Taliani G, Gaeta GB. Terapia della Epatite Cronica B: aggiornamento 2015 delle raccomandazioni Italiane. Available from: http://www.webaisf.org/media/36572/raccomandazioni_hbv_aisf-simit_2016.pdf. |
| 6. | Ministero della Salute. Piano Nazionale per la prevenzione delle epatiti virali da virus B e C. Available from: https://www.salute.gov.it/imgs/C_17_pubblicazioni_2437_allegato.pdf. |
| 7. | Stasi C, Milani S. Evolving strategies for liver fibrosis staging: Non-invasive assessment. World J Gastroenterol. 2017;23:191-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Stasi C, Milani S. Non-invasive assessment of liver fibrosis: Between prediction/prevention of outcomes and cost-effectiveness. World J Gastroenterol. 2016;22:1711-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | European Association for Study of Liver. Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63:237-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1177] [Cited by in RCA: 1332] [Article Influence: 133.2] [Reference Citation Analysis (0)] |
| 10. | Sagnelli E, Stroffolini T, Sagnelli C, Smedile A, Morisco F, Furlan C, Babudieri S, Brancaccio G, Coppola N, Gaeta GB. Epidemiological and clinical scenario of chronic liver diseases in Italy: Data from a multicenter nationwide survey. Dig Liver Dis. 2016;48:1066-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Stasi C, Silvestri C, Bravi S, Aquilini D, Casprini P, Epifani C, Voller F, Cipriani F. Hepatitis B and C epidemiology in an urban cohort in Tuscany (Italy). Clin Res Hepatol Gastroenterol. 2015;39:e13-e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Navabakhsh B, Mehrabi N, Estakhri A, Mohamadnejad M, Poustchi H. Hepatitis B Virus Infection during Pregnancy: Transmission and Prevention. Middle East J Dig Dis. 2011;3:92-102. [PubMed] |
| 13. | Zhang Z, Chen C, Li Z, Wu YH, Xiao XM. Individualized management of pregnant women with high hepatitis B virus DNA levels. World J Gastroenterol. 2014;20:12056-12061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Zuccaro O, Romanò L, Mele A, Mariano A, Clementi M, Tosti ME, Taliani G, Galli C, Zanetti AR, Spada E; Study Group. Clinical, epidemiological and virological features of acute hepatitis B in Italy. Infection. 2015;43:431-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Hahné SJ, Veldhuijzen IK, Wiessing L, Lim TA, Salminen M, Laar Mv. Infection with hepatitis B and C virus in Europe: a systematic review of prevalence and cost-effectiveness of screening. BMC Infect Dis. 2013;13:181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 172] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 16. | Liaw YF, Brunetto MR, Hadziyannis S. The natural history of chronic HBV infection and geographical differences. Antivir Ther. 2010;15 Suppl 3:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 17. | Sagnelli E, Sagnelli C, Pisaturo M, Macera M, Coppola N. Epidemiology of acute and chronic hepatitis B and delta over the last 5 decades in Italy. World J Gastroenterol. 2014;20:7635-7643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 91] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 18. | Chu CM, Hung SJ, Lin J, Tai DI, Liaw YF. Natural history of hepatitis B e antigen to antibody seroconversion in patients with normal serum aminotransferase levels. Am J Med. 2004;116:829-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 229] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 19. | Alvarado-Mora MV, Locarnini S, Rizzetto M, Pinho JR. An update on HDV: virology, pathogenesis and treatment. Antivir Ther. 2013;18:541-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 20. | Brunetto MR, Oliveri F, Coco B, Leandro G, Colombatto P, Gorin JM, Bonino F. Outcome of anti-HBe positive chronic hepatitis B in alpha-interferon treated and untreated patients: a long term cohort study. J Hepatol. 2002;36:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 251] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 21. | Wong GL, Chan HL, Yu Z, Chan HY, Tse CH, Wong VW. Liver fibrosis progression is uncommon in patients with inactive chronic hepatitis B: a prospective cohort study with paired transient elastography examination. J Gastroenterol Hepatol. 2013;28:1842-1848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Oliveri F, Surace L, Cavallone D, Colombatto P, Ricco G, Salvati N, Coco B, Romagnoli V, Gattai R, Salvati A. Long-term outcome of inactive and active, low viraemic HBeAg-negative-hepatitis B virus infection: Benign course towards HBsAg clearance. Liver Int. 2017;37:1622-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Stasi C, Salomoni E, Arena U, Corti G, Montalto P, Bartalesi F, Marra F, Laffi G, Milani S, Zignego AL. Non-invasive assessment of liver fibrosis in patients with HBV-related chronic liver disease undergoing antiviral treatment: A preliminary study. Eur J Pharmacol. 2017;806:105-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |