Published online Feb 27, 2018. doi: 10.4254/wjh.v10.i2.277
Peer-review started: December 8, 2017
First decision: December 18, 2017
Revised: February 1, 2018
Accepted: February 23, 2018
Article in press: February 23, 2018
Published online: February 27, 2018
Processing time: 86 Days and 15.4 Hours
To stably correct tyrosinaemia in proliferating livers of fumarylacetoacetate-hydrolase knockout (Fah-/-) mice by homologous-recombination-mediated targeted addition of the Fah gene.
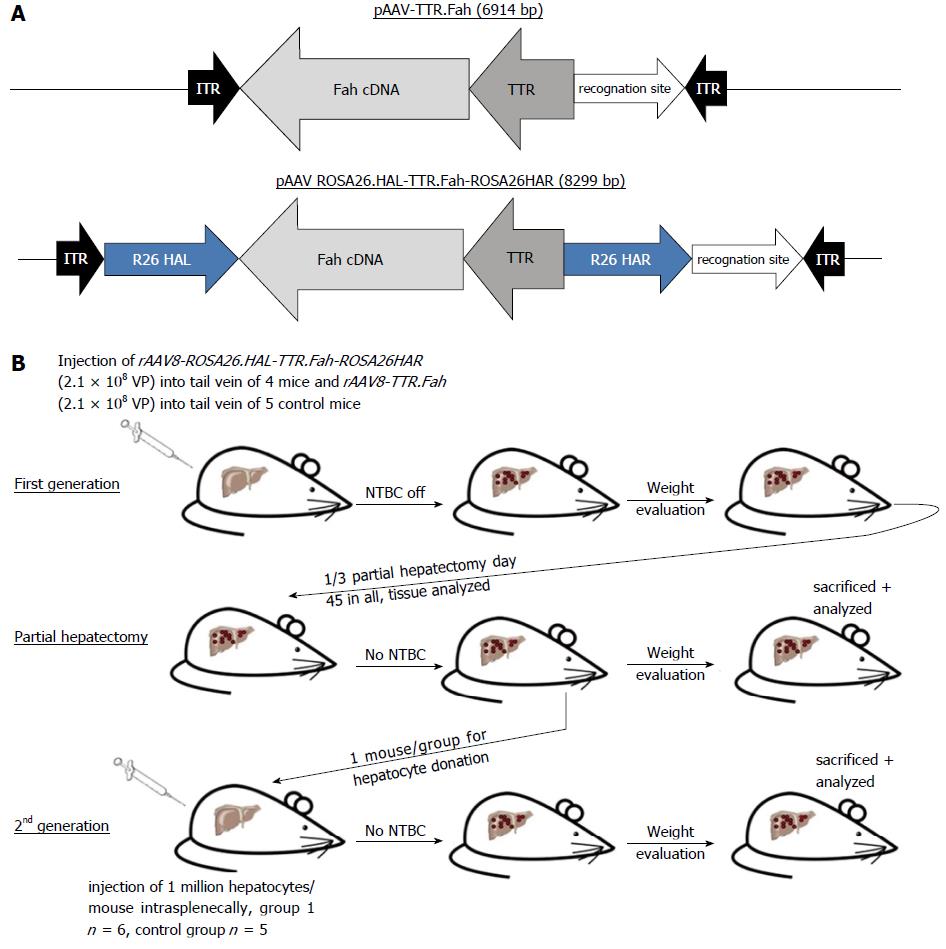
C57BL/6 Fah∆exon5 mice served as an animal model for human tyrosinaemia type 1 in our study. The vector was created by amplifying human Fah cDNA including the TTR promoter from a lentivirus plasmid as described. The Fah expression cassette was flanked by homologous arms (620 bp and 749 bp long) of the Rosa26 gene locus. Mice were injected with 2.1 × 108 VP of this vector (rAAV8-ROSA26.HAL-TTR.Fah-ROSA26.HAR) via the tail vein. Mice in the control group were injected with 2.1 × 108 VP of a similar vector but missing the homologous arms (rAAV8-TTR.Fah). Primary hepatocytes from Fah-/- recipient mice, treated with our vectors, were isolated and 1 × 106 hepatocytes were transplanted into secondary Fah-/- recipient mice by injection into the spleen. Upon either vector application or hepatocyte transplantation NTBC treatment was stopped in recipient mice.
Here, we report successful HR-mediated genome editing by integration of a Fah gene expression cassette into the “safe harbour locus” Rosa26 by recombinant AAV8. Both groups of mice showed long-term survival, weight gain and FAH positive clusters as determined by immunohistochemistry analysis of liver sections in the absence of NTBC treatment. In the group of C57BL/6 Fah∆exon5 mice, which have been transplanted with hepatocytes from a mouse injected with rAAV8-ROSA26.HAL-TTR.Fah-ROSA26.HAR 156 d before, 6 out of 6 mice showed long-term survival, weight gain and FAH positive clusters without need for NTBC treatment. In contrast only 1 out 5 mice, who received hepatocytes from rAAV8-TTR.Fah treated mice, survived and showed few and smaller FAH positive clusters. These results demonstrate that homologous recombination-mediated Fah gene transfer corrects the phenotype in a mouse model of human tyrosinaemia type 1 (Fah-/- mice) and is long lasting in a proliferating state of the liver as shown by withdrawal of NTBC treatment and serial transplantation of isolated hepatocytes from primary Fah-/- recipient mice into secondary Fah-/- recipient mice. This long term therapeutic efficacy is clearly superior to our control mice treated with episomal rAAV8 gene therapy approach.
HR-mediated rAAV8 gene therapy provides targeted transgene integration and phenotypic correction in Fah-/- mice with superior long-term efficacy compared to episomal rAAV8 therapy in proliferating livers.
Core tip: Recombinant adeno-associated virus (rAAV) has been explored for gene delivery in various murine models of hereditary liver disease, but in young children transgene expression from AAV-epigenomes diminishes over time. We thus explored, whether homologous recombination-mediated targeted gene addition of the fumarylacetoacetate hydrolase (Fah) gene would stably correct tyrosinaemia in rapidly proliferating livers of Fah-/- mice. Here, we report successful homologous recombination-mediated genome editing of a Fah gene expression cassette at the Rosa26 locus by rAAV8. We demonstrate that this approach corrects the phenotype and is long lasting in a proliferating state of the liver, as shown by serial transplantation.
- Citation: Junge N, Yuan Q, Vu TH, Krooss S, Bednarski C, Balakrishnan A, Cathomen T, Manns MP, Baumann U, Sharma AD, Ott M. Homologous recombination mediates stable Fah gene integration and phenotypic correction in tyrosinaemia mouse-model. World J Hepatol 2018; 10(2): 277-286
- URL: https://www.wjgnet.com/1948-5182/full/v10/i2/277.htm
- DOI: https://dx.doi.org/10.4254/wjh.v10.i2.277
Therapy for many liver-based metabolic diseases (LBMD) is limited to supportive measures and may entail significant side effects, such as organ failure, metabolic crisis, malignancy and impairment of quality of life. Until now, the only established curative treatment is liver organ transplantation (LTX). Although LTX for LBMDs has excellent long-term outcomes, the procedure is associated with significant morbidity and mortality and dependent on limited donor organ availability. Gene therapy could provide a minimally invasive therapeutic alternative to whole organ transplantation.
Recombinant adeno-associated viruses (rAAV) have evolved as promising vehicles for gene therapy to date and shown to produce long-term therapeutic effects in many mouse models of inherited liver diseases as well as in patients with haemophilia B[1-3]. AAV of serotype 8 has been shown to target mainly hepatocytes in the liver and is considered to be safe for clinical application[3-6]. Recombinant AAVs express the transgenes from epigenomic circular DNA with only rare genomic integration events[7]. Insertional mutagenesis resulting from random vector integrations has been observed in only one study[8] and these results remain to be confirmed by other studies[9]. Notably, AAV gene therapy in 77 dogs did not cause tumour formation during an observation period of up to 10 years[10]. Nathwani et al[11] presented a study in non-human primates with no signs of insertional mutagenesis 5 years after AAV application. Further, serotype 8 shows lower seroprevalence of preformed antibodies in humans than other AAV serotypes[3,12] thus minimizing risk of significant immune response.
Epigenomic expression of the therapeutic transgene from rAAV is thought to gradually decline in tissues with high cell turnover. Therapeutic efficacy of AAV-mediated gene transfer would thus decrease in growing livers of newborns or in diseases with intrinsic stimuli causing hepatocyte turnover. In some studies, gene correction by homologous recombination of rAAV transduced therapeutic genes was shown to result in long-term cellular persistence. Although the feasibility of in vivo gene correction in mice has been demonstrated in several models, superior therapeutic efficacy of gene therapy by gene addition mediated by homologous recombination remains to be demonstrated. Therefore, we examined whether the application of a Fah expression cassette flanked by homologous arms for the ROSA 26 Locus improves the efficacy and persistence of Fah gene delivery by integration at the Rosa26 gene locus through homologous recombination in a mouse model of human tyrosinaemia type 1. We used C57BL/6 Fah∆exon5 mice, which served as an animal model for human tyrosinaemia type 1[13]. Liver physiology and function in these animals can be maintained by providing water that is supplemented with the drug NTBC [2-(2-nitro-4-fluoromethylbenzoyl)-1,3-cyclohexanedione]. Control mice die 20-45 d after deprivation of NTBC due to liver failure. In the absence of NTBC, gene corrected hepatocytes proliferate and repopulate the liver.
All mouse experiments were granted permission and were performed according to the guidelines of the Hannover Medical School, Germany and the local government. Mice were kept on standard laboratory chow and free access to drinking water. They were housed in a restricted access room with controlled temperature and a light/dark cycle. We used C57BL/6 Fah∆exon5 mice, which served as an animal model for human tyrosinaemia type 1[13]. Tyrosinaemia type 1 is caused by genetic alterations of the gene coding for FAH. The mutated Fah gene produces an unstable protein, which results in deficiency of fumarylacetoacetate hydrolase activity. The mice were provided with water supplemented with 1 mg/100 mL of NTBC [2-(2-nitro-4-(fluoromethyl) benzoyl) cyclohexane-1,3-dione] before performing experiments. Surgery was done under general anaesthesia with 2% isoflurane and 2 litres/min oxygen flow.
For cloning of the rAAV8-ROSA26.HAL-TTR.Fah-ROSA26.HAR plasmid, 620 and 749 bp Rosa26 gene locus homologous arms flanking the Fah expression cassette were subcloned into a pBlue-Script II plasmid. The entire transgene was further subcloned into the AAV backbone plasmid for virus generation. For the Fah expression cassette, we amplified hFah cDNA, including the TTR promoter, from a lentivirus plasmid described earlier from our group[14] by PCR (Phusion® High-Fidelity PCR Kit, Thermo scientific).
For cloning the rAAV8-TTR.Fah expression cassette, we created a similar plasmid with the same transgene cassette but not flanked by the homologous arms.
The adeno-associated virus serotype 8 (AAV8) vectors (Figure 1A), rAAV8-ROSA26.HAL-TTR.Fah-ROSA26HAR and rAAV8-TTR.Fah, were prepared as described previously[15]. The titre was determined by qRT-PCR using primers spanning the region of the TTR promoter, as published before[16].
Mice were injected with 2.1 × 108 VP rAAV8-ROSA26.HAL-TTR.Fah-ROSA26HAR via the tail vein. Mice in the control group were injected with 2.1 × 108 VP rAAV8-TTR.Fah. Viruses were diluted in sorbitol to a total volume of 220 μL for injection. Non-treated control mice were injected with 0.9% sodium chloride. We used one control mouse group (n = 3) for the first generation experiment. Subsequently, the mice were monitored and weighed daily until they reached stable conditions or gained body weight. After 45 to 47 d, a 1/3 hepatectomy was conducted to analyse the presence of FAH protein-positive cell clusters. Tissues were fixed in 4% paraformaldehyde or snap frozen for subsequent analyses.
Primary hepatocytes from primary Fah-/- recipient mice were isolated with the two-step collagenase (Roche) perfusion method, as described previously[4]. Hepatocytes (1 × 106) were transplanted into secondary Fah-/- recipient mice by injection into the spleen. Control mice were injected with sodium chloride into the spleen. We used one control mouse group (n = 3) for the second generation experiment.
Tissues were embedded in paraffin (ROTH) and cut in 2-μm-thick slices. Immunohistochemistry was carried out as described previously[17]. Briefly, after deparaffinization and blocking for endogenous H2O2, the slides were incubated in 1 x target retrieval solution (Dako) at 98 °C for 20 min. For FAH (primary antibody, Abcam, ab81087) staining, tissues were blocked with the Avidin/Biotin blocking kit (Vector laboratories). Goat serum (Abcam) or rabbit serum (Abcam) was then used for blocking. Biotinylated goat anti-rabbit and rabbit anti-goat secondary antibodies (Vectastain, Vector laboratories) were used. Colour development was conducted using AEC substrate chromogen (Dako). Counterstaining was performed using haematoxylin (Merck Millipore, Germany).
Genomic liver DNA was extracted from snap-frozen liver tissue with the DNeasy Blood and Tissue Kit (Qiagen) according to the protocol of the vendor. Two primers were designed, A and B. A was located in the Rosa26 locus of recipient mouse 5’ to the donor gene. B was located in the Fah sequence of the donor DNA. Primer sequences were A: 5’-GGAGAGAGGCATTCATGGGAGTGGAAAGTTAAGC-3’ and B: 5’-GCAGCATGGTCCAGTACATGTGCTTAAAGTTAGACC-3’. The expected length of the PCR amplicon was 1107 bp. PCR amplification was conducted with the Phusion® PCR Kit (New England BioLabs), and 200 ng of liver genomic DNA was used. The amplification was carried out under the following conditions: one cycle for 190 s at 98 °C, followed by 50 cycles for 10 s at 98 °C and 90 s at 72 °C, finished by one cycle for 10 min at 72 °C. The PCR product was analysed utilizing gel electrophoresis on a 1% agarose gel (Biozym) for 50 min at 90 V.
RNA was isolated from snap frozen liver tissue of sacrificed mice. RNA was isolated with RNeasy® mini Kit (Qiagen) and QIAshredder® according to manufacturer instructions. After DNase treatment cDNA writing was performed (iScript™ reverse transcriptase supermix, BIO-RAD). SYBR green qRT-PCR (Qiagen QuantiTect Sybr green®) was performed at Stratagene Mx3000P (Aligent) with following primer (forward primer AGAATGCGCTGTTGCCAAA, reverse primer GGAAGCTCGGCCATGGTAT) spanning exon 5-6 and beta actin as housekeeping gene.
We confirmed the correct design of our plasmids (Figure 1A) by sequencing and by evaluating FAH-Expression in Hepa1.6 cells by RT qPCR. For our experiments we used Fah-/- mice that contain a disruptive insertion in exon 5 of the Fah gene[13].
We prepared a high titre AAV8 vector suspension using the aforementioned AAV vector plasmids.
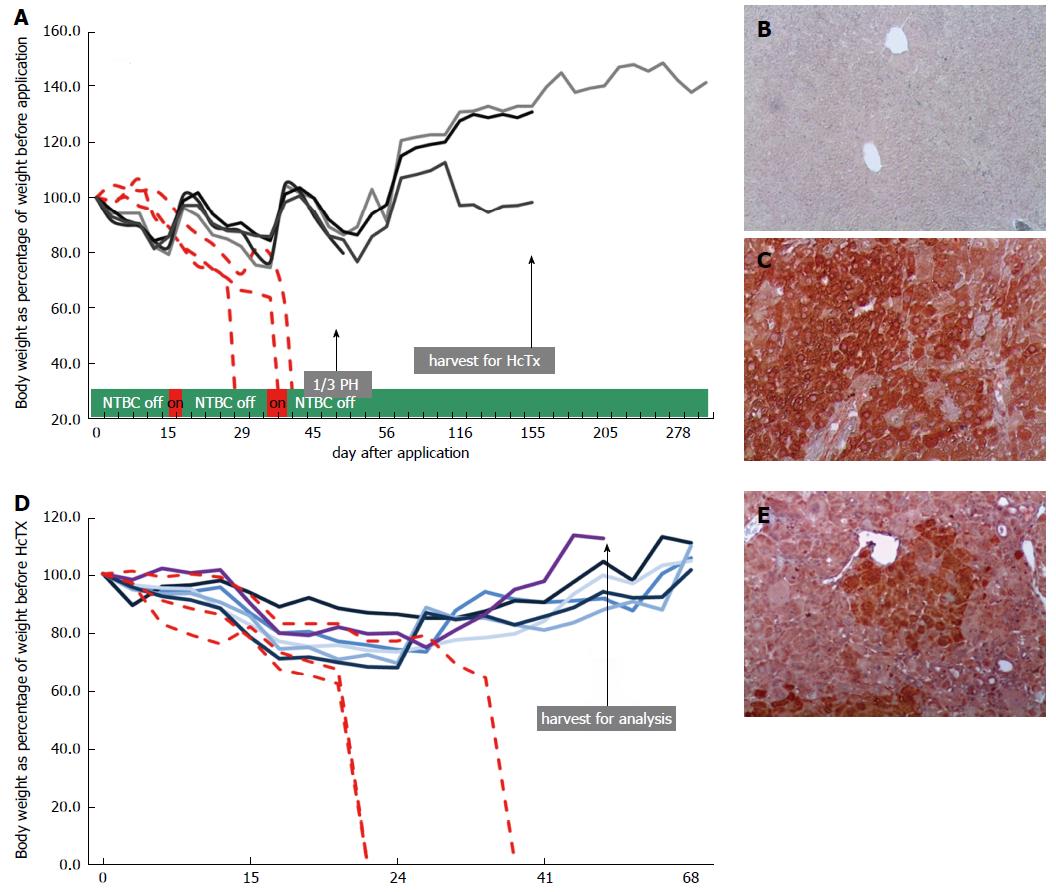
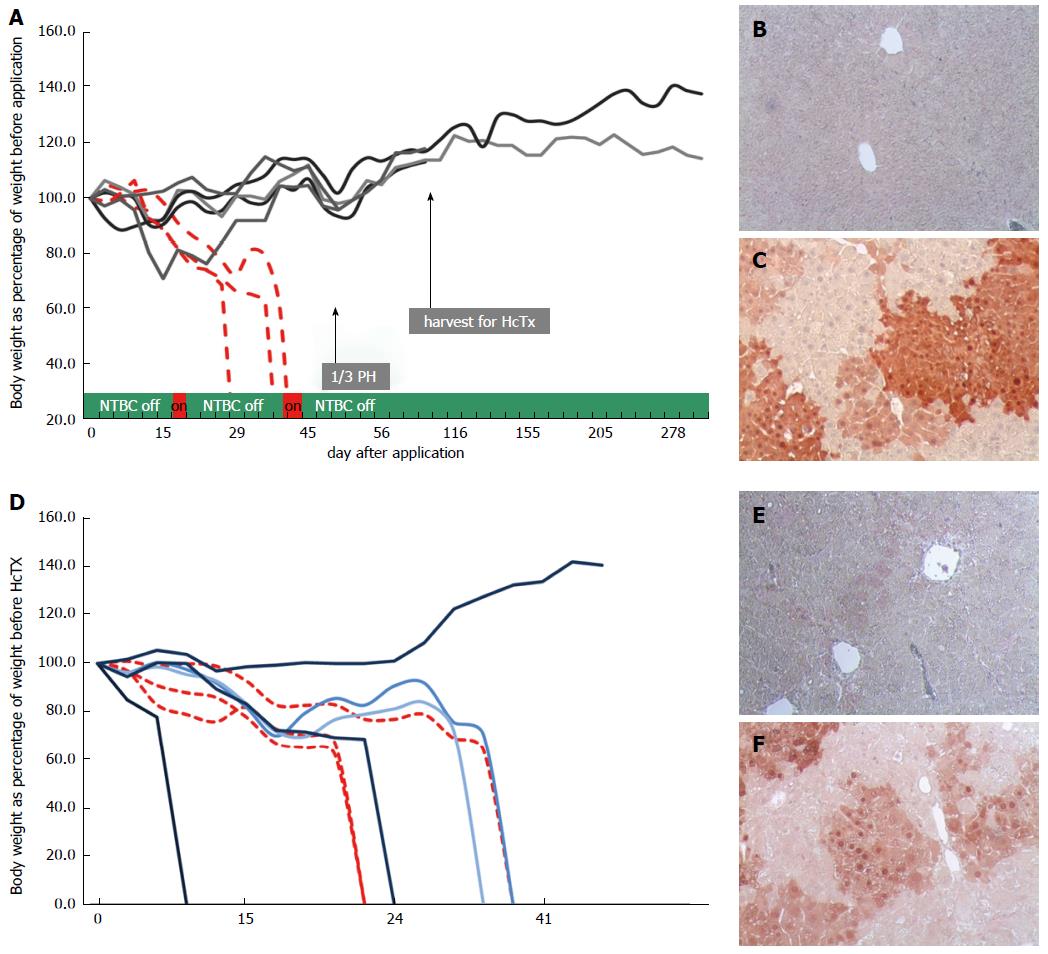
Next, we injected 4 mice with rAAV8-ROSA26.HAL-TTR.Fah-ROSA26HAR via the tail vein (Figure 1B). To stimulate the proliferation of FAH-expressing hepatocytes, protective NTBC-treatment was discontinued immediately after injection. Whereas control mice (injected with saline) died before 45 d, all mice injected with rAAV8-ROSA26.HAL-TTR.Fah-ROSA26HAR survived beyond 45 d after injection (Figure 2A). On the 45th-47th days, 1/3 of the liver was removed and analysed for the presence of FAH cell clusters by immunohistochemistry. All animals injected with rAAV8-ROSA26.HAL-TTR.Fah-ROSA26HAR showed robust repopulation of the liver as indicated by survival, weight gain (Figure 2A) and multiple large FAH protein positive cell clusters in immunohistochemistry analyses (Figure 2C). Importantly, these mice survived without NTBC until the end of the study (day 288; Figure 2A).
Due to high selection pressure for gene corrected hepatocytes in the Fah-/- model, phenotypic correction of the enzyme deficiency as result of diluted, but still sufficient, FAH protein expression from epigenomic AAV DNA could not be excluded in the first generation. To test whether homologous sequences facilitated targeted integration and increased therapeutic efficacy, we isolated primary hepatocytes from one recipient mouse after recovery from partial hepatectomy and transplanted 1 x 106 cells each into the spleens of the secondary Fah-/- recipient mice (Figure 1B). All recipient animals (6/6) that were transplanted with hepatocytes from repopulated Fah-/- mouse showed liver repopulation and survived long-term in the absence of NTBC (Figure 2D and E).
To establish unequivocally that homologous recombination is indeed capable of long-term stable correction of Fah deficiency and superior to non-homologous, episomal gene therapy, we generated a control group with five mice, who were injected with rAAV8-TTR.Fah. All five primary recipient mice survived with weight gain (Figure 3A) and showed clusters of FAH-positive cells at partial hepatectomy on day 45 (Figure 3C). To show inferiority of this episomal approach we further increased the proliferation conditions by transplanting hepatocytes (1 × 106 cells for each recipient) from one first generation recipient mouse into 5 secondary Fah-/- recipient mice in this group also. Only one of the five secondary recipient mice (hepatocyte recipients) survived NTBC withdrawal and showed few and small FAH-positive cell clusters (Figure 3D and E). Hence, these results suggest that in the absence of homologous arms, the observed FAH-positive clusters in the primary recipient Fah-/- mice mostly resulted from epigenomic AAVs or an unexplained mechanism of integration/anchorage on cellular DNA, which was lost upon transplantation into secondary Fah-/- recipient mice.
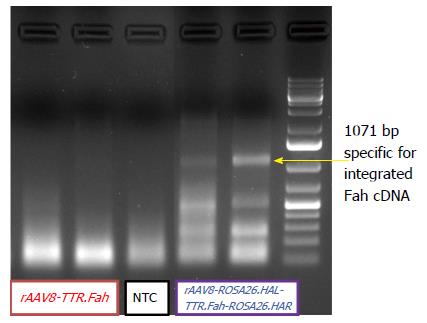
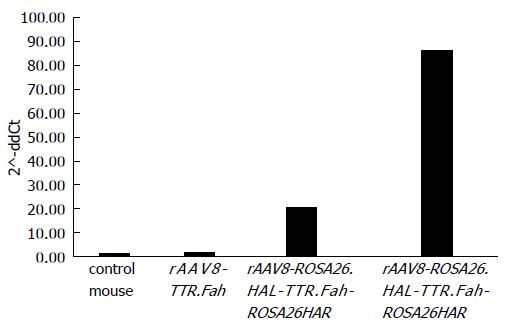
So far, our results revealed that mice injected with rAAV8-ROSA26.HAL-TTR.Fah-ROSA26HAR had robust liver repopulation and improved survival after secondary transplantation. However, it is important to prove that homologous arms facilitated targeted integration/gene addition of Fah cDNA into the Rosa26 locus. We therefore examined targeted integration by genomic PCR amplifying portions of the Rosa26 gene locus and the Fah transgene cassette. Indeed, we found an expected band of 1071 bp in mice injected with rAAV8-ROSA26.HAL-TTR.Fah-ROSA26HAR (Figure 4) but not in mice injected with rAAV8-TTR.Fah. Our data thus indicate that homologous arms facilitated targeted integration at a frequency sufficient for increased therapeutic outcome and phenotypic correction in Fah-/- mice. This is further confirmed by Sybr green qRT-PCR results. These showed a clearly higher expression of FAH in mice treated with rAAV8-ROSA26.HAL-TTR.Fah-ROSA26HAR compared to mice treated with rAAV8-TTR.Fah alone (Figure 5).
In summary, we can conclude that in the first generation we could not detect a difference for survival, weight gain and FAH positive cell cluster between mice injected with rAAV8-ROSA26.HAL-TTR.Fah-ROSA26HAR or rAAV8-TTR.Fah but in secondary generation (recipients of 1 x 106 hepatocytes from first generation) we could detect a clear improved survival for the group with homologous arms in the vector. In this group 6 out of 6 mice survived and in the other group 1 out 5 mice survived. Furthermore the detection FAH positive cell clusters showed the same distribution.
In in vitro and in vivo studies[18,19], the AAV vector is used as the vector of choice for gene correction approaches by homologous recombination; one important reason is its single-stranded nature. Reports on gene correction or gene addition by homologous recombination for liver-based metabolic diseases are rare and have shown correction frequencies[20] too low for phenotypic correction, except for the study of Paulk et al[19]. However, they used a mouse model with a point mutation for Fah gene; therefore, their approach was a gene correction. Here, we provide proof of concept for in vivo targeted gene addition mediated by homologous recombination in a liver-based metabolic disease. Our findings demonstrate that in a state of extensive hepatocyte proliferation, targeted integration by homologous recombination was superior to gene therapy based on episomal AAV gene therapy.
Primary recipient mice that were injected with rAAV8-ROSA26.HAL-TTR.Fah-ROSA26HAR or rAAV8-TTR.Fah survived and showed phenotypic rescue after NTBC withdrawal. Notably, livers of mice from both groups showed clear FAH-positive cell clusters in immunohistochemistry. We determined the presence of FAH positive areas in the both groups of primary recipients. We did not find significant differences in FAH positivity indicating similar number of FAH positive hepatocytes in both groups of mice. So far, cell clusters have always been explained by clonal expansion of corrected hepatocytes, which would implicate the necessity of vector integration. In the tyrosinemia mouse model Fah corrected hepatocytes have a strong selective advantage so they grow clonally, form nodules and can repopulate the entire liver at least[21,22]. Therefore, it is reasonable that a small number of hepatocytes with random integrations or another unexplained mechanism such as of integration/anchorage on cellular DNA proliferate preferentially and repopulate the diseased liver, leading to FAH-positive cell clusters. A human liver contains approximately 300 billion hepatocytes, which means, in case of 10% transduction efficiency with an integration rate of 0.1%, a single individual will have approximately 30 million hepatocytes with at least one integration event[23]. Therefore, one can assume that the phenotypic correction in these mice can be explained by the selective proliferation advantage of a small number of hepatocytes with successfully integrated Fah cassettes. A spontaneous reversion of the genetic defect, as the underlying cause for phenotypic correction and FAH-positive cell clusters, as described in humans[24], is not possible in the Fahexon5 mouse model[25].
Therefore we increased the proliferation conditions by hepatocyte transplantation from one first generation recipient per group into secondary Fah-/- recipient mice (1 x 106 hepatocytes for each secondary recipient mouse). In this experiment, the advantage of homologous recombination became clearly visible, since phenotypic correction could be achieved in all mice (6/6). In the rAAV8-TTR.Fah group, only 1/5 mice survived. In accordance with these results, 6/6 mice co-injected with rAAV8-ROSA26.HAL-TTR.Fah-ROSA26HAR showed clear FAH-positive cell clusters in livers, whereas only 1/5 mice injected with rAAV8-R26.Fah had FAH-positive clusters. Furthermore Sybr green qRT-PCR showed higher FAH expression in liver tissue of ROSA26.HAL-TTR.Fah-ROSA26HAR-mice than in rAAV8-R26.Fah-mice.
Partial hepatectomy and serial transplantation together are supposed to have triggered at least 30 rounds of cell doubling for the hepatocytes[26], nevertheless we could not find any tumour formation in any of our mice. This is in line with other studies showing a good safety profile for rAAV8 gene therapy[5]. Our proof of concept approach demonstrated that the targeted integration/addition of a therapeutic gene allows for safer (compared to random integration) and more efficient (compared to epigenomic) gene therapy, especially for gene therapy of liver-based metabolic diseases in paediatric patients, since the Rosa26 locus exists in mice[27,28] as well as in humans[29]. In contrast to the assumption that homologous recombination alone is not sufficient for a long-lasting phenotypic correction of a liver-based metabolic disease, we could show the opposite with this study, at least for diseases with selection advantage for corrected hepatocytes, like tyrosinaemia type 1. Further potential target diseases with selection advantage could be Wilson disease or bile-acid transporter defects. Continuing studies should evaluate the efficiency of this approach in liver-based metabolic diseases without selection advantage such as Crigler Najjar Syndrome.
In summary, we demonstrate that targeted in vivo integration of a Fah expression cassette mediated by homologous arms is a highly efficient approach to stably correct a metabolic liver disease in an FAH mouse model with extensive hepatocyte proliferation. Since many metabolic disorders must already be treated in children with fast-dividing hepatocytes, targeted transgene integration is an important step to safe and long-lasting gene therapy in the developing liver.
We describe an important proof of concept in the field of AAV gene therapy for liver based metabolic diseases (LBMD). First gene therapy studies in humans are done (Hemophilia B) or very ready to start (Crigler-Najjar Syndrome); even an EMA approved drug for AAV gene therapy (Glybera) exists already. But all these approaches have a major weakness, the missing permanence of the gene therapy effect, especially in young children. But they are the main target group for gene therapy in LBMD, since early therapy could avoid irreversible damage to the organs of the patient. In these patients the advantage of recombinant AAV gene therapy, the almost missing integration into the host genome turns into a disadvantage since donor cDNA will be lost during cell turn over.
Targeted integration into safe harbors like the ROSA26 locus could overcome the problem of diminishing donor-cDNA in rAAV gene therapy. There are studies, showing proof of concept for targeted integration with nucleases like zinc fingers or CRISP/CAS9, but these approaches contain also new potential sources of side effects. However in our study only natural appearing cellular repair mechanism has been used to generate a targeted integration.
Up to know it was assumed that the efficiency of gene addition by targeted integration into a safe harbor mediated by homologous recombination would be to low for phenotypic correction of liver based metabolic diseases (LBMD) in growing livers. But we could show in a disease model for LBMD with selection advantage of corrected hepatocytes that this is not the case. This could be transferred to other diseases like the group of familial intrahepatic cholestasis or Wilson disease or even to diseases with less selection advantage.
C57BL/6 Fah∆exon5 mice served as an animal model for human tyrosinaemia type 1 in our study. We treated these mice with a rAAV Vector containing human Fah cDNA, a liver specific promotor (TTR) and homologous arms for ROSA26 locus. We compared this group to mice treated with a vector without homologous arms. Hepatocyte proliferation was induced by partial hepatectomy and serial hepatocyte transplantation. Survival of mice without NTBC and existence of FAH positive cell cluster at immunohistochemistry staining on liver tissue of the mice were the main endpoints.
We could show for the first time proof of concept for phenotypic correction of a LBMD in a mouse model under conditions of extensive hepatocyte proliferation with rAAV mediated gene addition by targeted integration at a safe harbor without the use of nucleases or gene repair. Further studies have to show if this concept is transferable to LBMD with less section advantage of corrected hepatocytes.
Our study shows that phenotypic correction of a LBMD by rAAV gene therapy under conditions of extensive hepatocyte proliferation is possible with homologous recombination (HR) alone and does not necessarily have the need for nucleases. In conclusion we showed that HR-mediated rAAV8 gene therapy provides targeted transgene integration and phenotypic correction in Fah-/- mice with superior long-term efficacy compared to episomal rAAV8 therapy in proliferating livers. In opposite to approaches with the aim of point mutation repair on genes of LBMD our system with gene addition into a safe harbour can be easily transferred to other LBMDs and is not mutation specific.
Our results are an important step into the solution of a main clinical problem for gene therapy of LBMD, since mostly this therapy is mandatory in growing children, where episomal gene therapy is not lasting. In opposite to studies with nucleases our study focus on a natural mechanism for targeted integration which avoids potential side effects of nucleases. A very important question for following studies would be if these results could also be observed in LBMD with less selection advantage for corrected hepatocytes (e.g., Crigler-Najjar Syndrom).
We would like to thank Sabine Brandes and Nico Jäschke for support and Rebirth, SFB 738 and the “Deutsche Forschungsgemeinschaft” (Gerok-Grant) for financial support.
ARRIVE guideline statement: This study was performed according to the ARRIVE guidelines.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gheita TAA, Guo ZS, Marin JJG S- Editor: Cui LJ L- Editor: A E- Editor: Li RF
| 1. | Nathwani AC, Reiss UM, Tuddenham EG, Rosales C, Chowdary P, McIntosh J, Della Peruta M, Lheriteau E, Patel N, Raj D. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med. 2014;371:1994-2004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 987] [Article Influence: 89.7] [Reference Citation Analysis (0)] |
| 2. | Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC, Chowdary P, Riddell A, Pie AJ, Harrington C. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med. 2011;365:2357-2365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1319] [Cited by in RCA: 1422] [Article Influence: 101.6] [Reference Citation Analysis (0)] |
| 3. | Junge N, Mingozzi F, Ott M, Baumann U. Adeno-associated virus vector-based gene therapy for monogenetic metabolic diseases of the liver. J Pediatr Gastroenterol Nutr. 2015;60:433-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Pañeda A, Vanrell L, Mauleon I, Crettaz JS, Berraondo P, Timmermans EJ, Beattie SG, Twisk J, van Deventer S, Prieto J. Effect of adeno-associated virus serotype and genomic structure on liver transduction and biodistribution in mice of both genders. Hum Gene Ther. 2009;20:908-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Hasbrouck NC, High KA. AAV-mediated gene transfer for the treatment of hemophilia B: problems and prospects. Gene Ther. 2008;15:870-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | High KA, Aubourg P. rAAV human trial experience. Methods Mol Biol. 2011;807:429-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Inagaki K, Piao C, Kotchey NM, Wu X, Nakai H. Frequency and spectrum of genomic integration of recombinant adeno-associated virus serotype 8 vector in neonatal mouse liver. J Virol. 2008;82:9513-9524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Donsante A, Miller DG, Li Y, Vogler C, Brunt EM, Russell DW, Sands MS. AAV vector integration sites in mouse hepatocellular carcinoma. Science. 2007;317:477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 502] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 9. | Bell P, Wang L, Lebherz C, Flieder DB, Bove MS, Wu D, Gao GP, Wilson JM, Wivel NA. No evidence for tumorigenesis of AAV vectors in a large-scale study in mice. Mol Ther. 2005;12:299-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 99] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Niemeyer GP, Herzog RW, Mount J, Arruda VR, Tillson DM, Hathcock J, van Ginkel FW, High KA, Lothrop CD Jr. Long-term correction of inhibitor-prone hemophilia B dogs treated with liver-directed AAV2-mediated factor IX gene therapy. Blood. 2009;113:797-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 217] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 11. | Nathwani AC, Gray JT, McIntosh J, Ng CY, Zhou J, Spence Y, Cochrane M, Gray E, Tuddenham EG, Davidoff AM. Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates. Blood. 2007;109:1414-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 216] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 12. | Calcedo R, Vandenberghe LH, Gao G, Lin J, Wilson JM. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis. 2009;199:381-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 618] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 13. | Grompe M, al-Dhalimy M, Finegold M, Ou CN, Burlingame T, Kennaway NG, Soriano P. Loss of fumarylacetoacetate hydrolase is responsible for the neonatal hepatic dysfunction phenotype of lethal albino mice. Genes Dev. 1993;7:2298-2307. [PubMed] |
| 14. | Rittelmeyer I, Rothe M, Brugman MH, Iken M, Schambach A, Manns MP, Baum C, Modlich U, Ott M. Hepatic lentiviral gene transfer is associated with clonal selection, but not with tumor formation in serially transplanted rodents. Hepatology. 2013;58:397-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Yuan Q, Loya K, Rani B, Möbus S, Balakrishnan A, Lamle J, Cathomen T, Vogel A, Manns MP, Ott M. MicroRNA-221 overexpression accelerates hepatocyte proliferation during liver regeneration. Hepatology. 2013;57:299-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 16. | Sharma AD, Narain N, Händel EM, Iken M, Singhal N, Cathomen T, Manns MP, Schöler HR, Ott M, Cantz T. MicroRNA-221 regulates FAS-induced fulminant liver failure. Hepatology. 2011;53:1651-1661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Song G, Pacher M, Balakrishnan A, Yuan Q, Tsay HC, Yang D, Reetz J, Brandes S, Dai Z, Pützer BM. Direct Reprogramming of Hepatic Myofibroblasts into Hepatocytes In Vivo Attenuates Liver Fibrosis. Cell Stem Cell. 2016;18:797-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 164] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 18. | Inoue N, Dong R, Hirata RK, Russell DW. Introduction of single base substitutions at homologous chromosomal sequences by adeno-associated virus vectors. Mol Ther. 2001;3:526-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Paulk NK, Wursthorn K, Wang Z, Finegold MJ, Kay MA, Grompe M. Adeno-associated virus gene repair corrects a mouse model of hereditary tyrosinemia in vivo. Hepatology. 2010;51:1200-1208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 20. | Miller DG, Wang PR, Petek LM, Hirata RK, Sands MS, Russell DW. Gene targeting in vivo by adeno-associated virus vectors. Nat Biotechnol. 2006;24:1022-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Manning K, Al-Dhalimy M, Finegold M, Grompe M. In vivo suppressor mutations correct a murine model of hereditary tyrosinemia type I. Proc Natl Acad Sci USA. 1999;96:11928-11933. [PubMed] |
| 22. | Overturf K, Al-Dhalimy M, Manning K, Ou CN, Finegold M, Grompe M. Ex vivo hepatic gene therapy of a mouse model of Hereditary Tyrosinemia Type I. Hum Gene Ther. 1998;9:295-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Valdmanis PN, Lisowski L, Kay MA. rAAV-mediated tumorigenesis: still unresolved after an AAV assault. Mol Ther. 2012;20:2014-2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Kvittingen EA, Rootwelt H, Brandtzaeg P, Bergan A, Berger R. Hereditary tyrosinemia type I. Self-induced correction of the fumarylacetoacetase defect. J Clin Invest. 1993;91:1816-1821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 72] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Overturf K, Al-Dhalimy M, Tanguay R, Brantly M, Ou CN, Finegold M, Grompe M. Hepatocytes corrected by gene therapy are selected in vivo in a murine model of hereditary tyrosinaemia type I. Nat Genet. 1996;12:266-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 412] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 26. | Montini E, Held PK, Noll M, Morcinek N, Al-Dhalimy M, Finegold M, Yant SR, Kay MA, Grompe M. In vivo correction of murine tyrosinemia type I by DNA-mediated transposition. Mol Ther. 2002;6:759-769. [PubMed] |
| 27. | Zambrowicz BP, Imamoto A, Fiering S, Herzenberg LA, Kerr WG, Soriano P. Disruption of overlapping transcripts in the ROSA beta geo 26 gene trap strain leads to widespread expression of beta-galactosidase in mouse embryos and hematopoietic cells. Proc Natl Acad Sci USA. 1997;94:3789-3794. [PubMed] |
| 28. | Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3944] [Cited by in RCA: 4206] [Article Influence: 161.8] [Reference Citation Analysis (0)] |
| 29. | Irion S, Luche H, Gadue P, Fehling HJ, Kennedy M, Keller G. Identification and targeting of the ROSA26 locus in human embryonic stem cells. Nat Biotechnol. 2007;25:1477-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 233] [Article Influence: 12.9] [Reference Citation Analysis (0)] |