Published online Dec 27, 2018. doi: 10.4254/wjh.v10.i12.944
Peer-review started: August 6, 2018
First decision: August 24, 2018
Revised: September 7, 2018
Accepted: October 17, 2018
Article in press: October 18, 2018
Published online: December 27, 2018
Processing time: 145 Days and 13.7 Hours
To evaluate the impact of sepsis and non-communicable diseases (NCDs) on the outcome of decompensated chronic liver disease (CLD) patients.
In this cross-sectional study, medical records of patients with CLD admitted to the Gastroenterology unit at the Aga Khan University Hospital were reviewed. Patients older than 18 years with decompensation of CLD (i.e., jaundice, ascites, encephalopathy, and/or upper gastrointestinal bleed) as the primary reason for admission were included, while those who were admitted for reasons other than decompensation of CLD were excluded. Each patient was followed for 6 wk after index admission to assess mortality, prolonged hospital stay (> 5 d), and early readmission (within 7 d).
A total of 399 patients were enrolled. The mean age was 54.3 ± 11.7 years and 64.6% (n = 258) were male. Six-week mortality was 13% (n = 52). Prolonged hospital stay and readmission were present in 18% (n = 72) and 7% (n = 28) of patients, respectively. NCDs were found in 47.4% (n = 189) of patients. Acute kidney injury, sepsis, and non-ST elevation myocardial infarction were found in 41% (n = 165), 17.5% (n = 70), and 1.75% (n = 7) of patients, respectively. Upon multivariate analysis, acute kidney injury, non-ST elevation myocardial infarction, sepsis, and coagulopathy were found to be statistically significant predictors of mortality. While chronic kidney disease (CKD), low albumin, and high Model for End-Stage Liver Disease (MELD)-Na score were found to be statistically significant predictors of morbidity. Addition of sepsis in conventional MELD score predicted mortality even better than MELD-Na (area under receiver operating characteristic: 0.735 vs 0.686; P < 0.001). Among NCDs, CKD was found to increase morbidity independently.
Addition of sepsis improved the predictability of MELD score as a prognostic marker for mortality in patients with CLD. Presence of CKD increases the morbidity of patients with CLD.
Core tip: Chronic liver disease is one of the leading causes of mortality. Child-Pugh and Model for End-Stage Liver Disease scores have been designed to predict the outcome in cirrhotic patients. Infection and renal insufficiency can worsen the outcome in cirrhotic patients. Myocardial infarction, sepsis,, and coagulopathy are associated with poor outcomes in patients with cirrhosis. The addition of sepsis can improve the predictability of the Model for End-Stage Liver Disease score as a prognostic marker for mortality in hospitalized patients with liver cirrhosis. Presence of chronic kidney disease increased the morbidity of cirrhotic patients. There is no direct impact of non-communicable disease over mortality in hospitalized patients with liver cirrhosis.
- Citation: Qazi Arisar FA, Abid S, Shaikh PA, Awan S. Impact of sepsis and non-communicable diseases on prognostic models to predict the outcome of hospitalized chronic liver disease patients. World J Hepatol 2018; 10(12): 944-955
- URL: https://www.wjgnet.com/1948-5182/full/v10/i12/944.htm
- DOI: https://dx.doi.org/10.4254/wjh.v10.i12.944
The massive global burden of chronic liver disease (CLD) has been well documented[1], with more than one million deaths per year worldwide[2] making it the 14th leading cause of death globally[3]. Many prognostic models have been developed over the years to help classify the severity of liver disease and direct the aggressiveness of medical care. The Child-Pugh Turcotte (CTP) Score and the Model for End-Stage Liver Disease (MELD) score are two of the most commonly used scoring systems worldwide[4-6].
Child and Turcotte proposed the CTP score initially using nutritional status, the presence of ascites, hepatic encephalopathy, total bilirubin, and albumin as parameters to determine mortality risk in patients undergoing portosystemic shunt surgery. Later, Pugh et al[4] modified it to its current version by replacing nutritional status with prothrombin time or international normalized ratio (INR), making it the most widely used scoring system for estimation of prognosis in CLD patients. However, the subjectivity of variables (ascites, encephalopathy) as well as inter-laboratory variability limited the accuracy of the CTP score, with the waitlist mortality for liver transplantation continuing to rise[7].
MELD score was introduced primarily to determine the survival of patients undergoing transjugular intrahepatic portosystemic shunt placement[5]. MELD score incorporates total bilirubin, creatinine, and INR. It has not only become the mainstay for prioritizing patients for liver transplant, but also for predicting mortality in non-transplant surgical procedures, alcoholic hepatitis, and acute variceal hemorrhage[6,8]. However, there are still several comorbidities such as hepatocellular carcinoma, hepatopulmonary syndrome, and portopulmonary hypertension (HTN) that can affect the prognosis of CLD patients and that are not taken into account by the MELD score[9,10].
Several studies have been done on the MELD score with proposals made for revision of the scoring system to include other factors to improve the predictive accuracy of the score. Some of these include modified CTP, MELD-Na, Reweighted MELD and the Refit MELD, which have been shown to be superior to the current CTP and MELD scores[11-14]. Such studies have led several leading researchers to investigate other variables and scoring systems to better predict mortality in patients hospitalized for decompensated CLD.
Acute kidney injury (AKI) is a devastating complication that is frequently progressive and independently associated with mortality in a stage-dependent fashion in CLD patients[15]. Bacterial infections are common in liver disease, especially in decompensated patients. Infections increase the mortality four-fold in patients with end-stage liver disease[16]. Inflammation stemming from infections plays a key role in the outcome of cirrhosis. The presence of systemic inflammatory response syndrome with or without infection is a major predictor of prognosis in CLD patients[17].
There is an alarming rise in the prevalence of non-communicable diseases (NCDs) worldwide. Diabetes, HTN, cardiovascular diseases, chronic obstructive pulmonary diseases, and cancers have emerged as leading causes of mortality globally[18]. In a recent cohort of the Asian population, diabetes was found to impact mortality in cirrhotic patients[19]. However, the effect of other NCDs on the outcomes of liver disease has not been elucidated.
Charlson et al[20] proposed a comorbidity index to predict all-cause mortality on the basis of a number of comorbid conditions. Seventeen different diseases, each allocated a score from one to six, are incorporated in the Charlson comorbidity index. The total sum gives the total burden of comorbidities in that patient[20]. Since the 1980s, the score has been extensively used to estimate mortality in a different subset of cohorts including liver disease patients[21,22]. However, the severity of liver disease was not incorporated in those studies.
The aim of this study is to evaluate the impact of NCDs on the outcome of patients admitted for decompensated CLD. We also aimed to construct a model over and above the existing scoring system.
This study employed a cross-sectional design. Medical records of CLD patients who were admitted to the Gastroenterology unit at the Aga Khan University Hospital in Karachi, Pakistan were reviewed. Patients older than 18 years with decompensation of CLD (i.e. jaundice, ascites, encephalopathy, and/or upper gastrointestinal (GI) bleed) as the primary reason for admission were included. Those admitted for reasons other than decompensation of CLD were excluded. Each patient was followed for 6 wk after index admission to assess mortality and morbidity. The study was conducted after approval from the institutional ethical review committee.
(1) Demographics: age, gender, weight, duration of documented CLD, smoking, and current alcohol use; (2) Clinical presentation: blood pressure, heart rate, temperature, respiratory rate, Glasgow coma scale; (3) CLD complications/decompensation: presence of jaundice, ascites, encephalopathy, esophageal varices, GI bleeding, hepatorenal syndrome, spontaneous bacterial peritonitis, and hepatopulmonary syndrome; (4) NCDs: Diabetes mellitus, HTN, chronic obstructive lung disease, ischemic heart disease, congestive heart failure, peripheral vascular disease, chronic kidney disease (CKD), cerebrovascular disease (prior strokes), acquired immunodeficiency syndrome, cancer, dementia, connective tissue diseases, and peptic ulcer disease; (5) Laboratory markers: complete blood count, electrolytes, liver function tests, serum albumin, creatinine, prothrombin time, C-reactive protein; (6) Scoring Systems: Child-Pugh Score, MELD score, MELD-Na score, and Charlson comorbidity index. While calculating Charlson comorbidity index, patients with Child A disease were given a score of 1 (mild), while those with Child B and C disease were given a score of 3 (moderate to severe) as per standard score; and (7) Primary outcome: To assess mortality within 6 wk of admission and morbidity defined as either prolonged hospital stay > 5 d (120 h) or readmission within 7 d of the index admission.
Sepsis: Sepsis was defined as the presence of any source of infection along with at least two of the following[23]: (1) Temperature > 38 °C (100.4 °F) or < 36 °C (96.8 °F); (2) Heart rate > 90 bpm; (3) Respiratory rate > 20 or PaCO2 < 32 mmHg; and (4) Total leukocyte count (TLC) > 12000/mm³, < 4000/mm³, or > 10% bands.
AKI: AKI was defined as per kidney disease: Improving Global Outcomes Acute Kidney Injury Work Group, and revised consensus recommendations of the International Club of Ascites[24], i.e. increase in serum creatinine ≥ 0.3 mg/dL within 48 h; or a percentage increase serum creatinine ≥ 50% from the baseline which is known, or presumed, to have occurred within the prior 7 d.
The sample size was calculated using Open Epi for proportion, using mortality rate due to infection, and AKI between 36%-38%[16]. Taking into account the 95% confidence interval (CI), 80% power, and an odds ratio (OR) of 1.5, the final sample size was approximately 384 CLD patients.
Data were analyzed using Statistical Package for Social Sciences version 20. The frequency for all variables was calculated. Data were expressed as a mean and standard deviation for normally distributed continuous variables. The significance of association was calculated using the Student t-test. Categorical variables were recorded in their absolute value and analyzed using the chi-squared test. Univariate analysis was used to identify parameters associated with mortality and morbidity. Multiple logistic regressions were done in order to identify independent predictors of poor outcome in these patients. These factors were incorporated in the existing MELD score. Receiver operating characteristic curves of MELD, MELD-Na, and the new score were made. Significance tests and CIs were assessed through the nonparametric bootstrap. The area under the curve was compared between the three scores. Fisher exact test was used to determine the association of NCDs with predictors of mortality. All P values were two-sided and a P value of < 0.05 was considered statistically significant.
Records of 399 patients admitted primarily due to decompensation of liver disease were reviewed. Mean age was 54.3 ± 11.7 years and 64.6% (n = 258) of patients were male. Hepatitis C was found to be the leading cause of CLD. The length of hospital stay of more than five days was 18%, while readmission rate within 1 wk was 7%. Six-week mortality was observed in 13% (n = 52) of patients. Table 1 describes the demographic details of patients.
| Variables | n (%) or | |
| mean ± SD | ||
| Age (yr) | 54.56 ± 11.74 | |
| Gender | Male | 258 (64.6) |
| Female | 136 (34.4) | |
| Etiology (viral) | Hepatitis C | 260 (65.1) |
| Hepatitis B | 31 (7.7) | |
| Hepatitis B + Hepatitis D | 12 (3) | |
| Hepatitis B + Hepatitis C | 6 (1.5) | |
| Non-B, Non-C | 61 (15.2) | |
| (Unknown etiology) | ||
| Alcohol | 20 (5) | |
| Autoimmune hepatitis | 8 (2) | |
| Hemochromatosis | 1 (0.25) | |
| Duration of chronic liver disease (yr) | 4.25 ± 3.71 | |
| NCDs | Diabetes | 148 (37) |
| Hypertension | 96 (24) | |
| Chronic kidney disease | 30 (7.5) | |
| Ischemic heart disease | 23 (5.7) | |
| Chronic obstructive pulmonary disease | 13 (3.2) | |
| Infections on admission | Sepsis | 70 (17.5) |
| Lower respiratory tract infection | 24 (6) | |
| Urinary tract infection | 30 (7.5) | |
| Non-ST Elevation myocardial infarction | 7 (1.75) | |
| Stroke | 3 (0.75) | |
| Decompensation on admission | Ascites | 300 (75.1) |
| Presence of esophageal/gastric varices | 235 (58.8) | |
| Portosystemic encephalopathy | 170 (42.6) | |
| Acute kidney injury | 165 (41.3) | |
| Upper GI bleed | 118 (29.5) | |
| Hepatocellular carcinoma | 98 (24.5) | |
| Hepatorenal syndrome | 86 (21.5) | |
| Spontaneous bacterial peritonitis | 76 (19) | |
| Hepato-hydrothorax | 12 (3) | |
| Investigations | Hemoglobin (g/dL) | 9.81 ± 2.17 |
| Total leukocyte count (× 109/L) | 9.87 ± 6.17 | |
| Platelets (× 109/L) | 121.29 ± 108.12 | |
| Prothrombin time (s) | 17.37 ± 7.87 | |
| International normalizing ratio | 1.63 ± 0.65 | |
| Creatinine (mg/dL) | 1.65 ± 1.37 | |
| Sodium (mmol/L) | 132.3 ± 7.62 | |
| Potassium (mmol/L) | 4.17 ± 0.88 | |
| pH | 7.37 ± 0.12 | |
| Bicarbonate (mmol/L) | 20.13 ± 4.79 | |
| Total bilirubin (mg/dL) | 5.47 ± 7.57 | |
| Alanine transaminase (IU/L) | 78.7 ± 128.21 | |
| Gama glutamyl transferase (IU/L) | 119.26 ± 159.87 | |
| Alkaline phosphatase (IU/L) | 178.35 ± 133.8 | |
| Albumin (g/dL) | 2.59 ± 0.58 | |
| Child class | A | 39 (9.8) |
| B | 142 (35.6) | |
| C | 218 (54.6) | |
| Prognostic scores | MELD score | 18.0 ± 8.55 |
| MELD-Na | 21.73 ± 8.31 | |
| Charlson index | 4.21 ± 1.63 | |
| Charlson age adjusted score | 5.33 ± 2.20 | |
| Outcomes | Mortality | 52 (13) |
| Prolong stay | 72 (18) | |
| Readmission | 28 (7) | |
NCDs were present in 47.4% (n = 189) of patients. AKI, sepsis, and non-ST elevation myocardial infarction (NSTEMI) were present in 41% (n = 165), 17.5% (n = 70), and 1.75% (n = 7) of patients, respectively.
Upon univariate analysis, hypotension, tachycardia, tachypnea, hypoxia, NSTEMI, sepsis, renal insufficiency, encephalopathy, pneumonia, anemia, leukocytosis coagulopathy, high CTP and MELD scores, and frequent admissions in the last 1 to 3 mo were found to be associated with 6 wk mortality.
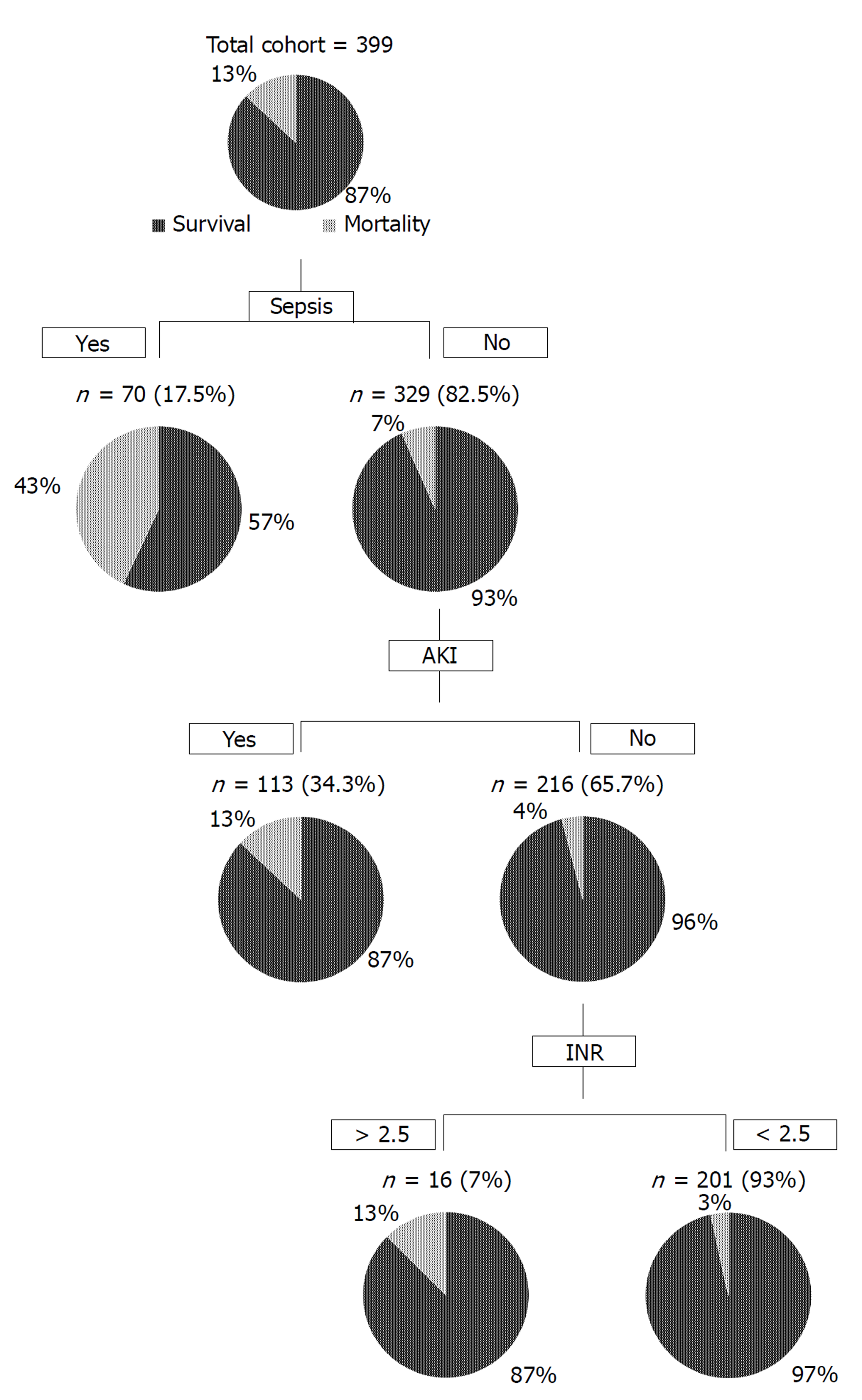
Upon multivariate analysis, sepsis (OR = 6.50; 95%CI: 3.007-14.06; P < 0.001), AKI (OR = 2.69; 95%CI: 1.17-6.20; P = 0.02), and INR (OR = 1.75; 95%CI: 1.14-2.69; P < 0.001) were significant independent predictors of mortality (Table 2). Figure 1 shows a flow diagram of 6 wk mortality for patients with advanced cirrhosis and concomitant sepsis based on the logistic regression model. Sepsis, AKI, and INR were used in a hierarchical pattern based on the OR.
| OR (95%CI) | P value | ||
| NSTEMI | No | 1 | |
| Yes | 16.03 (2.01-127.46) | 0.009 | |
| Sepsis | No | 1 | |
| Yes | 6.50 (3.01-14.06) | < 0.001 | |
| AKI | No | 1 | |
| Yes | 2.69 (1.17-6.20) | 0.02 | |
| INR | 1.75 (1.14-2.69) | < 0.001 | |
Upon multivariate analysis, CKD, low albumin, and high MELD-Na scores were found to be independent factors in predicting morbidity (Table 3).
| OR (95%CI) | P value | ||
| CKD | No | 1 | |
| Yes | 3.18 (1.30-7.82) | 0.01 | |
| MELD-Na | 1.05 (1.01-1.08) | 0.005 | |
| Albumin | 0.55 (0.32-0.92) | 0.02 | |
Upon multivariate analysis, CKD, high TLC, and high Child class were found to affect both mortality and morbidity (Table 4).
| OR (95%CI) | P value | ||
| CKD | No | 1 | |
| Yes | 3.12 (1.21-8.06) | 0.01 | |
| TLC | 1.08 (1.03-1.12) | < 0.001 | |
| Child Class | 3.57 (2.20-5.79) | < 0.001 | |
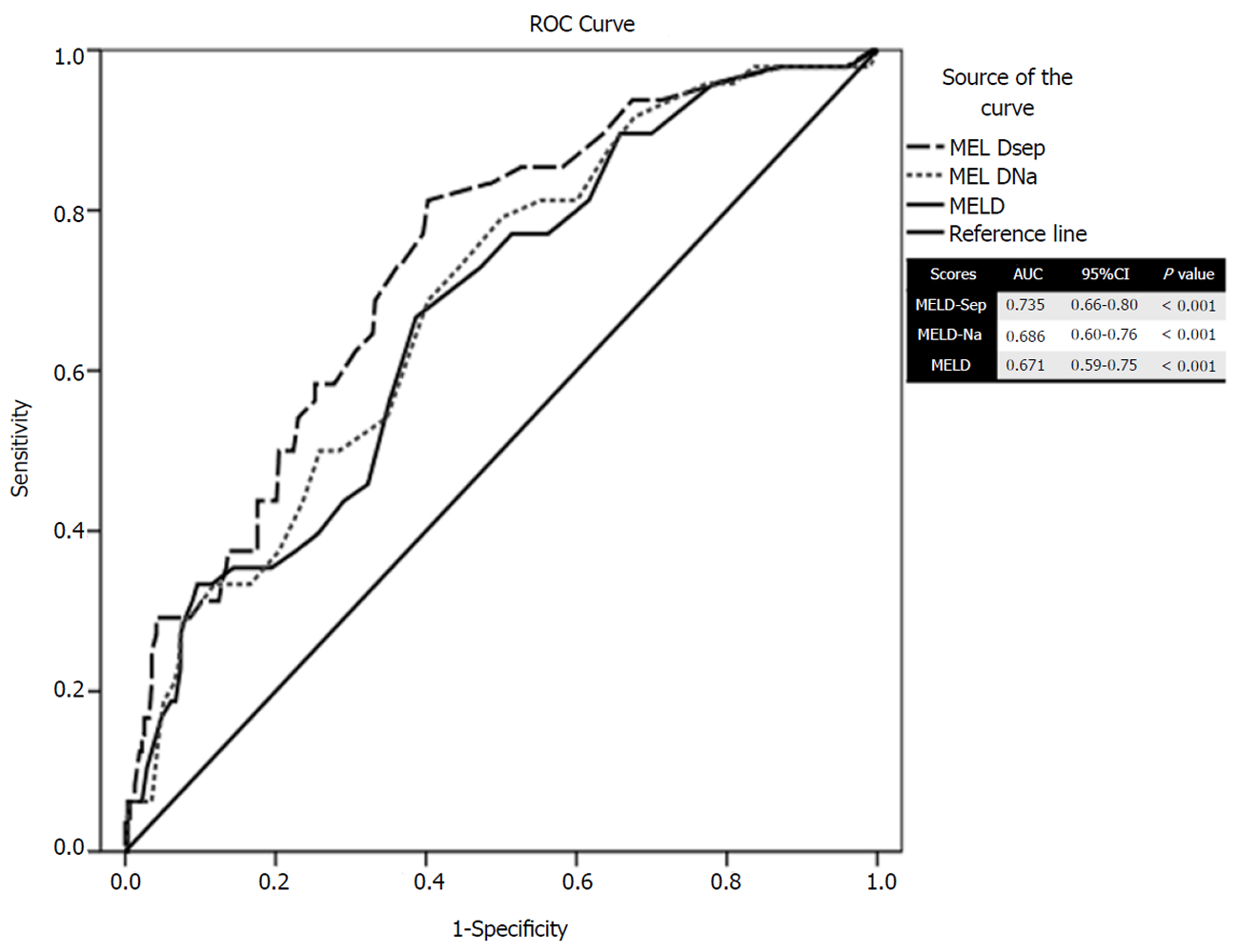
Once the value of sepsis was determined as a significant independent predictor of mortality based on multivariate analysis, we tried to formulate a new score by adding a factor of 6.5 into existing MELD scores. This factor was derived from the odds ratio of sepsis for mortality upon multivariate analysis. The new score labeled as MELD-sep was found to predict mortality better than MELD and MELD-Na as depicted by the area under receiver operating characteristic of 0.735 for MELD-sep in contrast with 0.686 for MELD-Na and 0.671 for MELD score (Figure 2).
NCDs were found to be associated with an increased readmission rate (28.6% without NCDs vs 71.4% with NCDs; P = 0.03). However, there was no effect on length of stay (length of stay > 5 d in 51.4% without NCDs vs 48.6% with NCDs; P = 0.45). Upon multivariate analysis, among all NCDs, only CKD was directly related with increased morbidity (OR = 3.18; 95%CI: 1.30-7.82; P = 0.01). Moreover, the presence of NCDs was not found to be an independent predictor of mortality in our series (mortality rate of 12.4% without NCDs vs 13.9% with NCDs; P = 0.65). Similarly, the presence of multiple comorbid conditions did not appear to impact the mortality directly (mortality rate of 12.2% without NCDs vs 14.1% with 2 or fewer NCDs vs 15.6% with 3 or more NCDS; P = 0.97). However, the presence of NCDs was directly related with NSTEMI, which was a major predictor of mortality in our study (Table 5). Similarly, Charlson comorbidity index was used to calculate the burden of NCDs in our patients, and this index did not appear to predict the outcome in cirrhotic patients.
| NCDs | P value | ||
| Yes | No | ||
| 210 (52.6) | 189 (47.3) | ||
| NSTEMI | 7 (3.3) | 0 | 0.01 |
| Sepsis | 31 (14.8) | 39 (20.6) | 0.14 |
| AKI | 92 (44.7) | 76 (40.2) | 0.41 |
Decompensated liver disease is a state of organ failure related to multi-organ consequences such as encephalopathy, renal insufficiency, volume overload, GI bleeding, infections, and frailty[25-28]. In this study of hospitalized decompensated cirrhotic patients, we evaluated the impact of sepsis and NCDs on patient outcome.
Traditionally, CTP and MELD scores have been used to predict the mortality in cirrhotic patients. However, the group of patients used to create and validate the MELD score was devoid of acute reversible complications such as sepsis[29]. Infections significantly increase the mortality in end-stage liver disease with some studies reporting a 30% death rate in 1 mo[16]. Prevention and treatment of sepsis were shown to reduce mortality in patients with cirrhosis and AKI[17]. AKI is a common and overwhelming complication in patients with end-stage liver disease. Belcher et al[15] also showed AKI to be associated with high mortality and complications in hospitalized patients with cirrhosis. Our study found that AKI, myocardial infarction, sepsis, and coagulopathy on admission were associated with high mortality in cirrhotic patients.
Interestingly, the MELD and Charlson comorbidity index scores were not associated with high mortality in our analysis. However, high Child class appeared to affect both mortality and morbidity. Based on our observations, we propose that the addition of sepsis as a factor in the MELD score gives a better prediction of mortality as compared to conventional MELD and MELD-Na scores in CLD patients admitted with acute decompensation. We also found that CKD, low albumin, and high MELD-Na scores were able to predict morbidity.
Chirapongsathorn et al[30] and Shu et al[31] related longer hospital stays with a high rate of 30-d readmission while Masadeh et al[32] found the opposite relationship. In our analysis, prolonged hospital stay was associated with subsequent early readmission upon univariate analysis but did not stand out as an independent factor upon multivariate analysis.
The prognostic value of chronic NCDs in cirrhotic patients has not been extensively studied. Recently, diabetes has been associated with high mortality[19,33,34], higher readmission rate[35], and a major factor in determining liver-related outcomes in cirrhotic patients[36]. We were unable to relate diabetes directly to mortality and morbidity in our series. However, among NCDs, CKD was found to directly predict morbidity in our series. Moreover, the presence of NCDs was associated with NSTEMI, which was one of the major predictors of mortality in our cohort. This observation indicates NCDs as an important factor that could influence the outcome of CLD patients independent of well-known prognostic variables incorporated in Child-Pugh and MELD scores for advanced liver disease patients.
Despite previous studies supporting the use of the Charlson comorbidity index for prediction of poor outcome in CLD patients[21,22], our study did not find a direct relationship between Charlson comorbidity index and morbidity upon multivariate analysis. This could be due to the difference between patient characteristics. We selectively enrolled patients who were admitted due to decompensated liver disease while previous studies included patients who were labeled as cirrhotic in their database regardless of compensation status[21,22]. Moreover, the follow-up period was longer in the Danish cohort[22].
The present study takes into account readmissions at our center. The possibility of readmissions at other centers was not accounted for. Other limitations include the cross-sectional design, shorter follow-up, and single center focus. Further large-scale multicenter studies with a longer follow-up would be helpful in strengthening the impact of NCDs and sepsis on the determination of outcome in hospitalized cirrhotic patients.
In conclusion, the addition of sepsis improves the predictability of the MELD score as a prognostic marker for mortality in patients with decompensated CLD. Presence of CKD increases morbidity of patients with CLD.
Patients with decompensated chronic liver disease (CLD) are at high risk of complications. Various scores have been used to classify the severity of liver disease and to predict mortality. Recently, diabetes was found to impact mortality in cirrhotic patients. However, the impact of other comorbidities on mortality and morbidity has not been studied. Moreover, the impact of sepsis on available predictability scores has not been determined.
Given the limitations with the use of Child-Pugh and Model for End-Stage Liver Disease (MELD) scores, we wanted to come up with a new score to predict mortality and morbidity.
The objective for this study included determination of sepsis, non-communicable diseases (NCDs), and acute kidney injury (AKI) in patients admitted with decompensated liver disease, along with their impact of NCDs on mortality and morbidity parameters. We also wanted to evaluate whether the addition of any other variable makes MELD a better tool as a prognostic marker.
We performed a retrospective analysis of medical records of patients with CLD admitted at the Aga Khan University Hospital. All adult patients with decompensation of CLD (i.e., jaundice, ascites, encephalopathy, and/or upper gastrointestinal (GI) bleed) as the primary reason for admission were included. Multivariate analysis was performed to assess predictors of 6 wk mortality, prolonged hospital stay (> 5 d), and early readmission (within 7 d).
Six-week mortality rate was 13%. Prolonged hospital stay and readmission rates were 18% and 7%, respectively. NCDs were present in 47.4% of patients. AKI, sepsis, and NSTEMI were present in 41%, 17.5%, and 1.75% patients, respectively. Factors associated with mortality included AKI, NSTEMI, sepsis, and coagulopathy. The factors found responsible for morbidity included chronic kidney disease (CKD), low albumin, and high MELD-Na score. By adding sepsis to the conventional MELD score, the predictability of mortality increased significantly. CKD was found to impact morbidity independently.
This study highlighted multiple factors associated with early mortality, readmission, and prolonged hospital stay. This study also determined the significance of the addition of sepsis in the MELD score to improve its predictability as a prognostic marker for mortality in patients with decompensated CLD. Presence of CKD increased morbidity of patients with CLD.
We need to amend factors linked to mortality, readmission, and prolonged stay not only to control mortality and morbidity, but also to minimize the cost burden by patients.
Manuscript source: Invited manuscript
P-Reviewer: Fedeli U, Reggiani GM S-Editor: Ma RY L-Editor: Filipodia E-Editor:
Specialty type: Gastroenterology and hepatology
Country of origin: Pakistan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Fedeli U, Reggiani GM S- Editor: Ma RY L- Editor: Filipodia E- Editor: Tan WW
| 1. | Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9500] [Cited by in RCA: 9569] [Article Influence: 736.1] [Reference Citation Analysis (0)] |
| 2. | Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. Natl Vital Stat Rep. 2013;61:1-117. [PubMed] |
| 3. | Blachier M, Leleu H, Peck-Radosavljevic M, Valla DC, Roudot-Thoraval F. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol. 2013;58:593-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 879] [Cited by in RCA: 908] [Article Influence: 75.7] [Reference Citation Analysis (0)] |
| 4. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [PubMed] |
| 5. | Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2066] [Article Influence: 82.6] [Reference Citation Analysis (0)] |
| 6. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3676] [Article Influence: 153.2] [Reference Citation Analysis (0)] |
| 7. | Freeman RB Jr. Is waiting time a measure of access to liver transplantation? Is shorter necessarily better? Hepatology. 2007;46:602-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Kamath PS, Kim WR; Advanced Liver Disease Study Group. The model for end-stage liver disease (MELD). Hepatology. 2007;45:797-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1075] [Cited by in RCA: 1225] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 9. | Wiesner R, Lake JR, Freeman RB, Gish RG. Model for end-stage liver disease (MELD) exception guidelines. Liver Transpl. 2006;12:S85-S87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Freeman RB Jr, Gish RG, Harper A, Davis GL, Vierling J, Lieblein L, Klintmalm G, Blazek J, Hunter R, Punch J. Model for end-stage liver disease (MELD) exception guidelines: results and recommendations from the MELD Exception Study Group and Conference (MESSAGE) for the approval of patients who need liver transplantation with diseases not considered by the standard MELD formula. Liver Transpl. 2006;12:S128-S136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 127] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 11. | Heuman DM, Abou-Assi SG, Habib A, Williams LM, Stravitz RT, Sanyal AJ, Fisher RA, Mihas AA. Persistent ascites and low serum sodium identify patients with cirrhosis and low MELD scores who are at high risk for early death. Hepatology. 2004;40:802-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 114] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Luca A, Angermayr B, Bertolini G, Koenig F, Vizzini G, Ploner M, Peck-Radosavljevic M, Gridelli B, Bosch J. An integrated MELD model including serum sodium and age improves the prediction of early mortality in patients with cirrhosis. Liver Transpl. 2007;13:1174-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 187] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 13. | Sharma P, Schaubel DE, Sima CS, Merion RM, Lok AS. Re-weighting the model for end-stage liver disease score components. Gastroenterology. 2008;135:1575-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 14. | Leise MD, Kim WR, Kremers WK, Larson JJ, Benson JT, Therneau TM. A revised model for end-stage liver disease optimizes prediction of mortality among patients awaiting liver transplantation. Gastroenterology. 2011;140:1952-1960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 15. | Belcher JM, Garcia-Tsao G, Sanyal AJ, Bhogal H, Lim JK, Ansari N, Coca SG, Parikh CR; TRIBE-AKI Consortium. Association of AKI with mortality and complications in hospitalized patients with cirrhosis. Hepatology. 2013;57:753-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 286] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 16. | Arvaniti V, D'Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, Burroughs AK. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246-1256, 1256.e1-1256.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 720] [Cited by in RCA: 835] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 17. | Thabut D, Massard J, Gangloff A, Carbonell N, Francoz C, Nguyen-Khac E, Duhamel C, Lebrec D, Poynard T, Moreau R. Model for end-stage liver disease score and systemic inflammatory response are major prognostic factors in patients with cirrhosis and acute functional renal failure. Hepatology. 2007;46:1872-1882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 193] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 18. | Terzic A, Waldman S. Chronic diseases: the emerging pandemic. Clin Transl Sci. 2011;4:225-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 19. | Goh GB, Pan A, Chow WC, Yuan JM, Koh WP. Association between diabetes mellitus and cirrhosis mortality: the Singapore Chinese Health Study. Liver Int. 2017;37:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. [PubMed] |
| 21. | Myers RP, Quan H, Hubbard JN, Shaheen AA, Kaplan GG. Predicting in-hospital mortality in patients with cirrhosis: results differ across risk adjustment methods. Hepatology. 2009;49:568-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Jepsen P, Vilstrup H, Andersen PK, Lash TL, Sørensen HT. Comorbidity and survival of Danish cirrhosis patients: a nationwide population-based cohort study. Hepatology. 2008;48:214-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 23. | Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3146] [Cited by in RCA: 3170] [Article Influence: 264.2] [Reference Citation Analysis (0)] |
| 24. | Angeli P, Ginès P, Wong F, Bernardi M, Boyer TD, Gerbes A, Moreau R, Jalan R, Sarin SK, Piano S. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. J Hepatol. 2015;62:968-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 527] [Article Influence: 52.7] [Reference Citation Analysis (1)] |
| 25. | Tapper EB, Finkelstein D, Mittleman MA, Piatkowski G, Chang M, Lai M. A Quality Improvement Initiative Reduces 30-Day Rate of Readmission for Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2016;14:753-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 127] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 26. | Tapper EB, Jiang ZG, Patwardhan VR. Refining the ammonia hypothesis: a physiology-driven approach to the treatment of hepatic encephalopathy. Mayo Clin Proc. 2015;90:646-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 27. | Volk ML, Tocco RS, Bazick J, Rakoski MO, Lok AS. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107:247-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 363] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 28. | Tapper EB, Finkelstein D, Mittleman MA, Piatkowski G, Lai M. Standard assessments of frailty are validated predictors of mortality in hospitalized patients with cirrhosis. Hepatology. 2015;62:584-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 157] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 29. | Kim HJ, Lee HW. Important predictor of mortality in patients with end-stage liver disease. Clin Mol Hepatol. 2013;19:105-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 30. | Chirapongsathorn S, Talwalkar JA, Kamath PS. Readmission in Cirrhosis: a Growing Problem. Curr Treat Options Gastroenterol. 2016;14:236-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Shu CC, Lin YF, Hsu NC, Ko WJ. Risk factors for 30-day readmission in general medical patients admitted from the emergency department: a single centre study. Intern Med J. 2012;42:677-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Masadeh MM, Zaied A, Hussain F, Spratt H, Soloway R. Adherence to American Association for the Study of Liver Diseases (AASLD) Guidelines and Predictors of Readmission in Cirrhotic Patients: A Single Center Experience. Oalib Journal. 2015;2:1-8. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 33. | Bianchi G, Marchesini G, Zoli M, Bugianesi E, Fabbri A, Pisi E. Prognostic significance of diabetes in patients with cirrhosis. Hepatology. 1994;20:119-125. [PubMed] |
| 34. | D'Amico G, Pasta L, Morabito A, D'Amico M, Caltagirone M, Malizia G, Tinè F, Giannuoli G, Traina M, Vizzini G. Competing risks and prognostic stages of cirrhosis: a 25-year inception cohort study of 494 patients. Aliment Pharmacol Ther. 2014;39:1180-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 373] [Article Influence: 33.9] [Reference Citation Analysis (1)] |
| 35. | Berman K, Tandra S, Forssell K, Vuppalanchi R, Burton JR Jr, Nguyen J, Mullis D, Kwo P, Chalasani N. Incidence and predictors of 30-day readmission among patients hospitalized for advanced liver disease. Clin Gastroenterol Hepatol. 2011;9:254-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 36. | Elkrief L, Chouinard P, Bendersky N, Hajage D, Larroque B, Babany G, Kutala B, Francoz C, Boyer N, Moreau R. Diabetes mellitus is an independent prognostic factor for major liver-related outcomes in patients with cirrhosis and chronic hepatitis C. Hepatology. 2014;60:823-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 133] [Article Influence: 12.1] [Reference Citation Analysis (1)] |