Published online Dec 27, 2018. doi: 10.4254/wjh.v10.i12.898
Peer-review started: September 13, 2018
First decision: October 5, 2018
Revised: November 8, 2018
Accepted: November 15, 2018
Article in press: November 16, 2018
Published online: December 27, 2018
Processing time: 105 Days and 4.3 Hours
The introduction of a direct-acting antiviral (DAA) for patients with hepatitis C virus (HCV) infection, could lead to higher sustained virologic response (SVR) rates with fewer adverse events, and it could shorten the treatment duration relative to the interferon era. Although most recent clinical studies have demonstrated that the occurrence rates of hepatocellular carcinoma (HCC) are decreased by SVR with both interferon-based and interferon-free-regimens, there are several reports about the unexpected observation of high rates of early tumor occurrence and recurrence in patients with HCV-related HCC undergoing interferon-free therapy despite SVR. Several mechanisms of HCC occurrence and rapid immunological changes, including cytokines and chemokines during and after DAA treatment, have also been reported. We focused on the possibilities that HCC occurs or recurs during and after DAA treatment, based on the reported clinical and basic studies. Further studies and observations will be needed to determine the short-term and long-term effects on hepatocarcinogenesis caused by the eradication of HCV with DAAs. New serum biomarkers and a follow-up system for HCV-patients with SVR should be established.
Core tip: The incidence of hepatocellular carcinoma (HCC) in hepatitis C virus (HCV) patients with sustained virologic response (SVR) after direct-acting antiviral (DAA) treatment is a serious health issue. We focused on the role of DAA treatment in hepatocarcinogenesis. DAAs may also lead to rapid changes in immune status through interactions between the host and HCV. Changes in the immune system may play a role in the progression of HCC. Further observations are needed to determine the effects on hepatocarcinogenesis caused by the eradication of HCV with DAAs.
- Citation: Sasaki R, Kanda T, Kato N, Yokosuka O, Moriyama M. Hepatitis C virus-associated hepatocellular carcinoma after sustained virologic response. World J Hepatol 2018; 10(12): 898-906
- URL: https://www.wjgnet.com/1948-5182/full/v10/i12/898.htm
- DOI: https://dx.doi.org/10.4254/wjh.v10.i12.898
Hepatocellular carcinoma (HCC) is the sixth most common cancer type[1] and the third most likely cause of cancer related deaths[2]. HCC is associated with chronic liver disease and cirrhosis in > 90% of cases[3]. In the West and Japan, hepatitis C virus (HCV) infection is one of the leading causes of chronic hepatitis, cirrhosis, and HCC. HCV affects approximately 130-210 million people worldwide, or 2%-3% of the world’s population[4]. Approximately 20%-30% of chronically HCV infected patients show liver cirrhosis[5], and 1%-4% of cirrhotic patients develop HCC per year[6]. HCC is characterized by a 5-year survival rate of 10%-12%[7].
Recently, several regimens of direct-acting antiviral (DAA) combinations have been developed for the treatment of chronic HCV infection[8]. The introduction of DAA agents has improved sustained virologic response (SVR) rates to approximately 90% and shortened treatment duration[9]. DAAs also help to overcome interferon non-responsiveness[10]. SVR is associated with improved overall survival in HCV infected patients. Recurrence-free survival in HCV infected patients who have undergone resection or locoregional therapy for HCC is also improved by SVR[11].
According to studies from the interferon era, the survival benefit in HCC patients infected with HCV has been postulated to occur through anti-inflammatory, antiangiogenic, and antiviral properties, and interferon-based antiviral therapies were associated with improved outcomes in HCC patients who were infected with HCV during long-term observation[12,13]. However, treatment with DAA therapy can promptly eradicate serum HCV ribose nucleic acid (RNA), and liver failure, including HCC, may occur after the achievement of SVR[14]. In 2016, two articles suggested an unexpectedly higher rate of early occurrence and recurrence of HCC in HCV-infected patients who were treated with DAAs[15,16]. Both had relatively shorter-term follow-up periods after the end of treatment (EOT). However, several articles presenting the opposite data or the data from longer-term follow-up periods have been published. A conclusion has not been reached in this matter and several studies are still ongoing. Considering these circumstances, this report focuses on hepatocarcinogenesis after DAA treatment, which will be discussed based on clinical points of view.
Interferon-free regimens with DAA combination can be used to treat HCV-infected individuals who cannot be treated with interferon-based regimens, such as older patients, patients with comorbidities, patients with cirrhosis, or patients with a history of HCC[17]. HCC recurrence or HCC occurrence, respectively, has been defined as the appearance of HCC in a patient with or without history of HCC [18].
In general, HCV infected patients with advanced liver fibrosis tend to develop HCC, compared to those with mild or moderate liver fibrosis[19]. Patients whose HCC has been curatively treated, also have a much higher risk of recurrence of HCC[20].
In 2016, Conti et al[15] reported that DAA therapy induced SVR in 91% of patients. During a 24-wk follow-up, HCC occurrence and recurrence, respectively, were detected in 9 of 285 patients (3.16%) and in 17 of 59 patients (28.8%); a total of 26 patients developped HCC. They also demonstrated that neither HCV genotype nor therapeutic DAA regimen correlated to HCC occurrence or HCC recurrence[15]. Similarly, Reig et al[16] reported an unexpectedly high rate and pattern of tumor recurrence coinciding with HCV clearance, suggesting the possible disruption of immune tumor surveillance. In their study[16], 8 (13.8%), 45 (77.6%), 2 (3.4%), 3 (5.2%) patients were HCV genotypes 1a, 1b, 3 and 4, respectively.
Guarino et al[18] extensively reviewed the association between DAA and HCC in patients with chronic HCV infection. They reported that, among 11 and 18 studies, the HCC occurrence and recurrence rates ranged from 0 to 7.4% and from 0 to 54.4%, respectively, although their observation periods were relatively shorter.
Li et al[21] reported that the short-term incidence of HCC is not increased after the eradication of HCV with DAA and mentioned that the previous reports about higher rates of HCC associated with DAAs may be related to the fact that those patients had a higher risk of developing HCC. Notably, this study also suggests that some patients have a higher risk of developing HCC after achieving SVR with DAA. It is important to elucidate the mechanism of the development of HCC after achieving SVR with DAA and to investigate the patients’ characteristics. Thus, the rates of HCC occurrence or recurrence varied from a clinical point of views.
HCV-infected patients have a decreased risk of HCC after achieving SVR by interferon treatment[11,22]. Previous studies reported that biomarkers including aspartate aminotransferase (AST), old age, liver cirrhosis and higher posttreatment alpha-fetoprotein (AFP) can predict HCC in patients after interferon therapy[23]. Toyoda et al[24] suggested that an elevated indicator of liver fibrosis, the FIB-4 index at SVR24, is also a predictor of HCC development in SVR patients. The FIB-4 index was a prediction of 5-year survival in HCV infected patients in the interferon-era[25].
Nguyen et al[26] suggested that AFP decreased significantly from pretreatment (median 7.2 ng/mL) to EOT (4.2 ng/mL) and at 12 wk after treatment (4.2 ng/mL) with DAAs. Liver inflammation increased AFP values in the absence of HCC. Of interest, they suggested that the pattern for normalization of AFP with entecavir showed a shorter period and gradual reduction compared to patients treated with pegylated-interferon.
Similarly, Nagaoki et al[27] showed that serum AFP levels decreased to similar levels at SVR24 both in the pegylated-interferon plus ribavirin and the DAAs treatment groups, and similar rates of HCC development existed in these two HCV genotype 1 infected patients groups (the cumulative HCC development rates after 1-, 3- and 5-years were 1.5%, 10% and 19% and 1.5%, 10% and 12%, respectively). These data suggested the possible reduced potential for HCC development by DAA treatment is as same as that of interferon-based treatment.
Moreover, Tag-Adeen et al[28] showed significant improvement in the FIB-4 index after achieving SVR by DAA in HCV genotype 4 infected patients. However, they also showed that achieving SVR did not guarantee improvement in cirrhosis (61% of cirrhotic patients showed liver stiffness > 12.5 kPa), and cirrhotic patients still had a risk for HCC development despite achieving SVR by DAA. Thus, from the clinical point of view, several liver fibrosis markers may be helpful for the early detection of HCC occurrence and recurrence.
Previous reports suggested that DAA changes the cytokine/chemokine levels compared to the pretreatment levels, and it may be related to hepatocarcinogenesis. Sung et al[29] investigated the level of type I interferon, interferon-β in HCV genotype 1b infected patients. Type I interferons bind to a common cell surface receptor, resulting in the activation of the Jak-STAT signal transduction system[30]. Interferon-β may be important not only to prevent patients with acute hepatitis C from developing chronic infection[31] but also to reduce the risk of HCC[32]. After DAA treatment, the expression levels of interferon-β, interferon-induced protein 44 (IFI44) and C-X-C motif chemokine ligand 10 (CXCL10) significantly decreased and rapidly normalized at EOT in the peripheral blood mononuclear cells (PBMCs)[29]. IFI44 and CXCL10 correlated with the pretreatment expression level of interferon-β.
Carlton-Smith et al[33] exhibited similar interferon-stimulated gene results in PBMC at treatment week four and EOT and showed a reduction in CXCL10, CXCL11 and macrophage inflammatory protein (MIP)-1β levels. Hengst et al[34] suggested that the expression level of 22 cytokines/chemokines including type I interferon and CXCL10 decreased significantly from baseline to 12 wk after treatment by DAA treatment in patients with HCV genotypes 1, 2, 3 and 4. CXCL10 level was increased in interferon-based therapies by the responsiveness to interferon. In contrast, the interferon system, including CXCL10, is not increased during DAA treatment. A similar effects were also observed in patients infected with HCV genotype 2 or 3 who were treated with DAA[35]. The interferon-stimulated intrahepatic and peripheral gene expression declines with HCV eradication[36].
DAA treatment increased the serum vascular endothelial growth factor (VEGF) level[37,38], and it remained stably elevated at the 3-mo follow-up[38]. These were observed in patients with HCV genotypes 1a, 1b, 2, 3 and 4. VEGF was significantly related to the serum angiopoietin-2 level, and angiopoietin-2 expression in HCC or in cirrhotic tissue before DAAs was related to the risk of HCC recurrence or occurrence. They[38] showed that the extremely high expression of angiopoietin-2 in recurrence of HCC and de novo HCC had its counterpart in the increased levels of circulating VEGF during DAA therapy. Therefore, they suggested that the interaction between the local overexpression of angiopoietin-2 by the slow blood flow of portal hypertension arising through the progression of chronic liver damage and circulating VEGF is a risk factor for developing HCC that is linked with the advanced stage of cirrhosis in patients treated with DAAs[38].
Tumor necrosis factor (TNF)-α, known as an important inflammatory mediator that induces immune responses, was originally found to induce tumor lysis. TNF induces the cellular apoptosis of hepatoma cell lines and HCV core and NS5A proteins block TNF-induced cellular apoptosis[39,40]. TNF-α related apoptosis-inducing ligand (TRAIL) also induces the apoptosis of human hepatic stellate cells and HCV blocks TRAIL-induced cellular apoptosis[41]. Spaan et al[42] reported that there is down-regulation in TRAIL-mediated killing by NK cells during DAA therapy in patients infected with HCV genotype 1b. Further studies will be needed. Thus, rapid changes in several cytokines and chemokines are observed during and after DAA treatment and may have several effects on HCC occurrence and recurrence.
Several reports showed that a rapid decreased or normalized immuno-surveillance causes early HCC recurrence or occurrence after DAA therapy. The activating receptor natural killer group 2, member D (NKG2D) and its ligands play a crucial role in the immune response to HCC. Reduced NKG2D ligand expression in HCC correlates with early recurrence[43]. NKG2D predicts the early emergence of HCC after interferon-free DAAs[44].
Major histocompatibility complex class I-related chain A (MICA), which is one of the human ligands of NKG2D, has been known to be a key molecule in viral HCC immune surveillance, as the interaction with NKG2D triggers NK cell-mediated cytotoxicity toward the stressed cells[45,46]. Moreover, HCC sheds membrane-bound MICA as soluble MICA and down-regulates the expression of NKG2D on the NK cell surface because escape immune surveillance[47]. Chu et al[44] reported that 12% of DAA-treated HCV genotype 1 infected patients developed HCC recurrence or occurrence within 24 wk after EOT. They suggested that a rapid decrease in NKG2D levels at EOT correlated with early HCC emergence in DAA-treated patients. Of interest, this phenomenon was not found in patients treated with the interferon-based regimen. These data may suggest a risk of early HCC after interferon-free DAA treatment, different from interferon-based therapy. Golden-Mason et al[48] reported that the frequency of clusters of differentiation (CD) of 56bright immature NKs decreased 2 wk after DAA therapy started and was maintained at SVR12 in HCV genotype 1 infected patients. Moreover, the downregulation of receptors of cytotoxic signaling including TRAIL, NKp30 and NKp46, was observed 12 wk after DAA therapy started and was maintained at SVR12[48]. They suggest that rapid viral clearance induced by DAA therapy normalizes NK cell function and reduces cytotoxic activity.
The other mechanism was explained by the numbers of peripheral FOXP3+CD25+CD4+ regulatory T cells[49]. In their report[49], peripheral CD4+ T cells numbers persisted in DAA treatment groups even approximately 51 wk after EOT in HCV genotype 1 infected patients. In HCV genotype 1a/1b patients, DAA therapy reduced the T-cell compartment in the peripheral blood and re-differentiation of the T lymphocyte memory compartment and resulted in a reduction in the expression of the coinhibitory molecule T cell immunoglobulin and immunoreceptor tyrosine-based inhibition motif domains (TIGITs) in bulk T lymphocytes[50]. They reported that HCV eradication after DAA therapy involves immune reconstitution[50]. These immune reconstitutions may support the successful treatment of oral lichen planus after DAA therapy[51]. Thus, rapid immunological changes, including in NKG2D systems are observed during and after DAA treatment, and they may have several effects on HCC occurrence and recurrence.
In the interferon era, genome-wide association studies (GWAS) identified that several genetic variants in close proximity to interleukin 28B (IL28B; also known as interferon-lambda 3) variants were strongly associated with the response to pegylated-interferon-α plus ribavirin therapy for chronic HCV infected patients[52-54]. Moreover, IL28B variations are independent predictors of the progression of hepatic fibrosis[55,56] and seems to be involved in the hepatocarcinogenesis[57,58]. However, with the induction of interferon-free regimens, the importance of IL28B genetic variants may be diminishing in HCV genotype 1 infected patients[59]. There have been some reports that showed the relation between single nucleotide polymorphisms (SNPs) and hepatocarcinogenesis.
Lange et al[60] revealed a SNP in HLA complex P5 (HCP5) rs2244546, which is a upstream of MICA, as a strong predictor of HCV-related HCC. The differentially methylated cytosine-phosphate-guanine (dmCpG) loci were also reported[61]. The dmCpG loci were highly enriched for enhancers, promoters, or CpG islands and the surrounding regions and were hypermethylated in HCC infected with HCV. The dmCpG loci were associated with cellular growth and proliferation although this report has several limitations[61].
Of interest, Matsuura et al[62] investigated GWAS data on hepatocarcinogenesis specifically in HCV-infected patients after the eradication of HCV by interferon-based therapy. There was no difference of development of HCC in HCV genotype 1 or 2 infected patients after eradication of HCV. They found a strong association between the SNP rs17047200, located within the intron of the tolloid like 1 gene (TLL1) on chromosome 4, and the development of HCC, and it played a role in hepatic fibrogenesis. It is uncertain whether interferon-free therapy can inhibit TLL1 after the eradication of HCV. Future studies are needed to evaluate this point.
HCV-mediated enhancement of microRNA miR-373 impairs the JAK/STAT signaling pathway[63]. MicroRNAs associated with HCV-related immunopathogenesis which were found to be enriched in exosomes of HCV viremic patients (in particular, miR-122-5p, miR-222-3p, miR-146a, miR-150-5p, miR-30c, miR-378a-3p and miR-20a-5p), were markedly reduced by DAA therapy. Enrichment of immunomodulatory microRNAs in exosomes of HCV patients was correlated with their inhibitory activity on innate immune cell functions[64]. DAAs against HCV may have an impact on extracellular vesicles including microRNAs, leading to immunomodulation. Thus, several host genetic factors and microRNAs are change during and after DAA treatment, which may have several effects on HCC occurrence and recurrence.
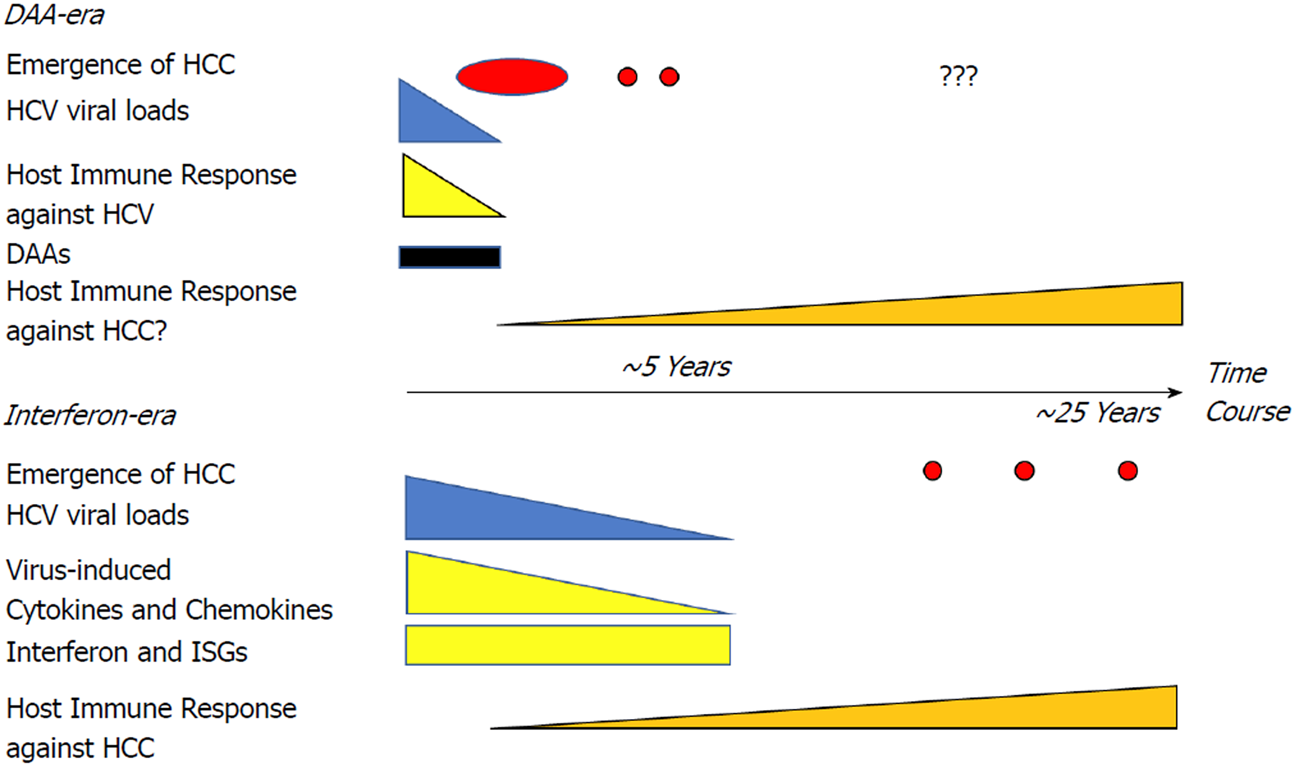
DAA therapy is more efficacious for HCV eradication with fewer side effects. The use of DAAs does not increase the occurrence or recurrence of HCC according to clinical trials. However, the mechanism that altered the immunological balance because of a rapid decrease of HCV viral load in the short-term after DAA therapy may contribute to early tumor development (Figure 1). Sasaki et al[65] demonstrated the changes of complement cascades and neutralizing antibodies after SVR by DAA. Complement-dependent cytotoxic effects[66] and neutralizing antibodies[67] are also important for HCC cells survival. Thus, a longer follow-up period and basic research are required to establish whether there is a risk or advantage of HCC recurrence or occurrence with interferon-free therapy. Moreover, new serum biomarkers that may be altered by DAA therapy should be investigated in the follow-up of HCV-patients with SVR after DAA and interferon-based regimens.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Carneiro BM, Pandey VN, Arriagada GL S- Editor: Dou Y L- Editor: A E- Editor: Tan WW
| 1. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3588] [Article Influence: 276.0] [Reference Citation Analysis (4)] |
| 2. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11831] [Article Influence: 845.1] [Reference Citation Analysis (4)] |
| 3. | Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63:844-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 929] [Cited by in RCA: 1104] [Article Influence: 100.4] [Reference Citation Analysis (1)] |
| 4. | Smith DJ, Combellick J, Jordan AE, Hagan H. Hepatitis C virus (HCV) disease progression in people who inject drugs (PWID): A systematic review and meta-analysis. Int J Drug Policy. 2015;26:911-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 5. | Hoofnagle JH, Seeff LB. Peginterferon and ribavirin for chronic hepatitis C. N Engl J Med. 2006;355:2444-2451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 353] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 6. | Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35-S50. [PubMed] |
| 7. | Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, Brú C, Rodés J, Bruix J. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 904] [Article Influence: 34.8] [Reference Citation Analysis (1)] |
| 8. | Geddawy A, Ibrahim YF, Elbahie NM, Ibrahim MA. Direct Acting Anti-hepatitis C Virus Drugs: Clinical Pharmacology and Future Direction. J Transl Int Med. 2017;5:8-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 130] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 9. | Pawlotsky JM. New hepatitis C therapies: the toolbox, strategies, and challenges. Gastroenterology. 2014;146:1176-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 412] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 10. | Flohr H, Breull W. Effect of etafenone on total and regional myocardial blood flow. Arzneimittelforschung. 1975;25:1400-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Manthravadi S, Paleti S, Pandya P. Impact of sustained viral response postcurative therapy of hepatitis C-related hepatocellular carcinoma: a systematic review and meta-analysis. Int J Cancer. 2017;140:1042-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Miyake Y, Takaki A, Iwasaki Y, Yamamoto K. Meta-analysis: interferon-alpha prevents the recurrence after curative treatment of hepatitis C virus-related hepatocellular carcinoma. J Viral Hepat. 2010;17:287-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Inagaki Y, Sugimoto K, Shiraki K, Tameda M, Kusagawa S, Nojiri K, Ogura S, Yamamoto N, Takei Y, Ito M. The long-term effects of splenectomy and subsequent interferon therapy in patients with HCV-related liver cirrhosis. Mol Med Rep. 2014;9:487-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Werner JM, Adenugba A, Protzer U. Immune Reconstitution After HCV Clearance With Direct Antiviral Agents: Potential Consequences for Patients With HCC? Transplantation. 2017;101:904-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Conti F, Buonfiglioli F, Scuteri A, Crespi C, Bolondi L, Caraceni P, Foschi FG, Lenzi M, Mazzella G, Verucchi G. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol. 2016;65:727-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 698] [Article Influence: 77.6] [Reference Citation Analysis (0)] |
| 16. | Reig M, Mariño Z, Perelló C, Iñarrairaegui M, Ribeiro A, Lens S, Díaz A, Vilana R, Darnell A, Varela M. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol. 2016;65:719-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 803] [Article Influence: 89.2] [Reference Citation Analysis (0)] |
| 17. | Kanda T, Yasui S, Nakamura M, Suzuki E, Arai M, Ooka Y, Ogasawara S, Chiba T, Saito T, Haga Y. Real-World Experiences with the Combination Treatment of Ledipasvir plus Sofosbuvir for 12 Weeks in HCV Genotype 1-Infected Japanese Patients: Achievement of a Sustained Virological Response in Previous Users of Peginterferon plus Ribavirin with HCV NS3/4A Inhibitors. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Guarino M, Sessa A, Cossiga V, Morando F, Caporaso N, Morisco F; Special Interest Group on “Hepatocellular carcinoma and new anti-HCV therapies” of the Italian Association for the Study of the Liver. Direct-acting antivirals and hepatocellular carcinoma in chronic hepatitis C: A few lights and many shadows. World J Gastroenterol. 2018;24:2582-2595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 19. | Takano S, Yokosuka O, Imazeki F, Tagawa M, Omata M. Incidence of hepatocellular carcinoma in chronic hepatitis B and C: a prospective study of 251 patients. Hepatology. 1995;21:650-655. [PubMed] |
| 20. | Shiratori Y, Shiina S, Teratani T, Imamura M, Obi S, Sato S, Koike Y, Yoshida H, Omata M. Interferon therapy after tumor ablation improves prognosis in patients with hepatocellular carcinoma associated with hepatitis C virus. Ann Intern Med. 2003;138:299-306. [PubMed] |
| 21. | Li DK, Ren Y, Fierer DS, Rutledge S, Shaikh OS, Lo Re V 3rd, Simon T, Abou-Samra AB, Chung RT, Butt AA. The short-term incidence of hepatocellular carcinoma is not increased after hepatitis C treatment with direct-acting antivirals: An ERCHIVES study. Hepatology. 2018;67:2244-2253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 125] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 22. | Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158:329-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 651] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 23. | Wu CK, Chang KC, Hung CH, Tseng PL, Lu SN, Chen CH, Wang JH, Lee CM, Tsai MC, Lin MT. Dynamic α-fetoprotein, platelets and AST-to-platelet ratio index predict hepatocellular carcinoma in chronic hepatitis C patients with sustained virological response after antiviral therapy. J Antimicrob Chemother. 2016;71:1943-1947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Toyoda H, Kumada T, Tada T, Kiriyama S, Tanikawa M, Hisanaga Y, Kanamori A, Kitabatake S, Ito T. Risk factors of hepatocellular carcinoma development in non-cirrhotic patients with sustained virologic response for chronic hepatitis C virus infection. J Gastroenterol Hepatol. 2015;30:1183-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 25. | Vergniol J, Foucher J, Terrebonne E, Bernard PH, le Bail B, Merrouche W, Couzigou P, de Ledinghen V. Noninvasive tests for fibrosis and liver stiffness predict 5-year outcomes of patients with chronic hepatitis C. Gastroenterology. 2011;140:1970-1979, 1979.e1-1979.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 308] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 26. | Nguyen K, Jimenez M, Moghadam N, Wu C, Farid A, Grotts J, Elashoff D, Choi G, Durazo FA, El-Kabany MM. Decrease of Alpha-fetoprotein in Patients with Cirrhosis Treated with Direct-acting Antivirals. J Clin Transl Hepatol. 2017;5:43-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Nagaoki Y, Imamura M, Aikata H, Daijo K, Teraoka Y, Honda F, Nakamura Y, Hatooka M, Morio R, Morio K. The risks of hepatocellular carcinoma development after HCV eradication are similar between patients treated with peg-interferon plus ribavirin and direct-acting antiviral therapy. PLoS One. 2017;12:e0182710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Tag-Adeen M, Sabra AM, Akazawa Y, Ohnita K, Nakao K. Impact of hepatitis C virus genotype-4 eradication following direct acting antivirals on liver stiffness measurement. Hepat Med. 2017;9:45-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Sung PS, Lee EB, Park DJ, Lozada A, Jang JW, Bae SH, Choi JY, Yoon SK. Interferon-free treatment for hepatitis C virus infection induces normalization of extrahepatic type I interferon signaling. Clin Mol Hepatol. 2018;24:302-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Deonarain R, Alcamí A, Alexiou M, Dallman MJ, Gewert DR, Porter AC. Impaired antiviral response and alpha/beta interferon induction in mice lacking beta interferon. J Virol. 2000;74:3404-3409. [PubMed] |
| 31. | Sasaki R, Kanda T, Nakamoto S, Haga Y, Nakamura M, Yasui S, Jiang X, Wu S, Arai M, Yokosuka O. Natural interferon-beta treatment for patients with chronic hepatitis C in Japan. World J Hepatol. 2015;7:1125-1132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Yoshida H, Shiratori Y, Moriyama M, Arakawa Y, Ide T, Sata M, Inoue O, Yano M, Tanaka M, Fujiyama S. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT Study Group. Inhibition of Hepatocarcinogenesis by Interferon Therapy. Ann Intern Med. 1999;131:174-181. [PubMed] |
| 33. | Carlton-Smith C, Holmes JA, Naggie S, Lidofsky A, Lauer GM, Kim AY, Chung RT; of the ACTG A5327 study group. IFN-free therapy is associated with restoration of type I IFN response in HIV-1 patients with acute HCV infection who achieve SVR. J Viral Hepat. 2018;25:465-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Hengst J, Falk CS, Schlaphoff V, Deterding K, Manns MP, Cornberg M, Wedemeyer H. Direct-Acting Antiviral-Induced Hepatitis C Virus Clearance Does Not Completely Restore the Altered Cytokine and Chemokine Milieu in Patients With Chronic Hepatitis C. J Infect Dis. 2016;214:1965-1974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 116] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 35. | Carlin AF, Aristizabal P, Song Q, Wang H, Paulson MS, Stamm LM, Schooley RT, Wyles DL. Temporal dynamics of inflammatory cytokines/chemokines during sofosbuvir and ribavirin therapy for genotype 2 and 3 hepatitis C infection. Hepatology. 2015;62:1047-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 36. | Meissner EG, Wu D, Osinusi A, Bon D, Virtaneva K, Sturdevant D, Porcella S, Wang H, Herrmann E, McHutchison J. Endogenous intrahepatic IFNs and association with IFN-free HCV treatment outcome. J Clin Invest. 2014;124:3352-3363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 180] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 37. | Villani R, Facciorusso A, Bellanti F, Tamborra R, Piscazzi A, Landriscina M, Vendemiale G, Serviddio G. DAAs Rapidly Reduce Inflammation but Increase Serum VEGF Level: A Rationale for Tumor Risk during Anti-HCV Treatment. PLoS One. 2016;11:e0167934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 38. | Faillaci F, Marzi L, Critelli R, Milosa F, Schepis F, Turola E, Andreani S, Vandelli G, Bernabucci V, Lei B. Liver Angiopoietin-2 Is a Key Predictor of De Novo or Recurrent Hepatocellular Cancer After Hepatitis C Virus Direct-Acting Antivirals. Hepatology. 2018;68:1010-1024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 39. | Ray RB, Meyer K, Steele R, Shrivastava A, Aggarwal BB, Ray R. Inhibition of tumor necrosis factor (TNF-alpha)-mediated apoptosis by hepatitis C virus core protein. J Biol Chem. 1998;273:2256-2259. [PubMed] |
| 40. | Kanda T, Steele R, Ray R, Ray RB. Inhibition of intrahepatic gamma interferon production by hepatitis C virus nonstructural protein 5A in transgenic mice. J Virol. 2009;83:8463-8469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 41. | Basu A, Saito K, Meyer K, Ray RB, Friedman SL, Chang YH, Ray R. Stellate cell apoptosis by a soluble mediator from immortalized human hepatocytes. Apoptosis. 2006;11:1391-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Spaan M, van Oord G, Kreefft K, Hou J, Hansen BE, Janssen HL, de Knegt RJ, Boonstra A. Immunological Analysis During Interferon-Free Therapy for Chronic Hepatitis C Virus Infection Reveals Modulation of the Natural Killer Cell Compartment. J Infect Dis. 2016;213:216-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 120] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 43. | Kamimura H, Yamagiwa S, Tsuchiya A, Takamura M, Matsuda Y, Ohkoshi S, Inoue M, Wakai T, Shirai Y, Nomoto M. Reduced NKG2D ligand expression in hepatocellular carcinoma correlates with early recurrence. J Hepatol. 2012;56:381-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 44. | Chu PS, Nakamoto N, Taniki N, Ojiro K, Amiya T, Makita Y, Murata H, Yamaguchi A, Shiba S, Miyake R. On-treatment decrease of NKG2D correlates to early emergence of clinically evident hepatocellular carcinoma after interferon-free therapy for chronic hepatitis C. PLoS One. 2017;12:e0179096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 45. | Kumar V, Kato N, Urabe Y, Takahashi A, Muroyama R, Hosono N, Otsuka M, Tateishi R, Omata M, Nakagawa H. Genome-wide association study identifies a susceptibility locus for HCV-induced hepatocellular carcinoma. Nat Genet. 2011;43:455-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 288] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 46. | Goto K, Kato N. MICA SNPs and the NKG2D system in virus-induced HCC. J Gastroenterol. 2015;50:261-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 47. | Li JJ, Pan K, Gu MF, Chen MS, Zhao JJ, Wang H, Liang XT, Sun JC, Xia JC. Prognostic value of soluble MICA levels in the serum of patients with advanced hepatocellular carcinoma. Chin J Cancer. 2013;32:141-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 48. | Golden-Mason L, McMahan RH, Kriss MS, Kilgore AL, Cheng L, Dran RJ, Wieland A, Rosen HR. Early and late changes in natural killer cells in response to ledipasvir/sofosbuvir treatment. Hepatol Commun. 2018;2:364-375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 49. | Langhans B, Nischalke HD, Krämer B, Hausen A, Dold L, van Heteren P, Hüneburg R, Nattermann J, Strassburg CP, Spengler U. Increased peripheral CD4+ regulatory T cells persist after successful direct-acting antiviral treatment of chronic hepatitis C. J Hepatol. 2017;66:888-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 50. | Burchill MA, Golden-Mason L, Wind-Rotolo M, Rosen HR. Memory re-differentiation and reduced lymphocyte activation in chronic HCV-infected patients receiving direct-acting antivirals. J Viral Hepat. 2015;22:983-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 51. | Nagao Y, Nakasone K, Maeshiro T, Nishida N, Kimura K, Kawahigashi Y, Tanaka Y, Sata M. Successful Treatment of Oral Lichen Planus with Direct-Acting Antiviral Agents after Liver Transplantation for Hepatitis C Virus-Associated Hepatocellular Carcinoma. Case Rep Gastroenterol. 2017;11:701-710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 52. | Pár A, Pár G, Tornai I, Szalay F, Várszegi D, Fráter E, Papp M, Lengyel G, Fehér J, Varga M. IL28B and IL10R -1087 polymorphisms are protective for chronic genotype 1 HCV infection and predictors of response to interferon-based therapy in an East-Central European cohort. BMC Res Notes. 2014;7:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 53. | Akkarathamrongsin S, Thong VD, Payungporn S, Poovorawan K, Prapunwattana P, Poovorawan Y, Tangkijvanich P. IFNL3 (IL28B) and IFNL4 polymorphisms are associated with treatment response in Thai patients infected with HCV genotype 1, but not with genotypes 3 and 6. J Med Virol. 2014;86:1482-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 54. | Miyamura T, Kanda T, Nakamoto S, Wu S, Fujiwara K, Imazeki F, Yokosuka O. Hepatic STAT1-nuclear translocation and interleukin 28B polymorphisms predict treatment outcomes in hepatitis C virus genotype 1-infected patients. PLoS One. 2011;6:e28617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 55. | Tamaki N, Kurosaki M, Higuchi M, Takada H, Nakakuki N, Yasui Y, Suzuki S, Tsuchiya K, Nakanishi H, Itakura J. Genetic Polymorphisms of IL28B and PNPLA3 Are Predictive for HCV Related Rapid Fibrosis Progression and Identify Patients Who Require Urgent Antiviral Treatment with New Regimens. PLoS One. 2015;10:e0137351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 56. | Domagalski K, Pawłowska M, Kozielewicz D, Dybowska D, Tretyn A, Halota W. The Impact of IL28B Genotype and Liver Fibrosis on the Hepatic Expression of IP10, IFI27, ISG15, and MX1 and Their Association with Treatment Outcomes in Patients with Chronic Hepatitis C. PLoS One. 2015;10:e0130899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 57. | Eurich D, Boas-Knoop S, Bahra M, Neuhaus R, Somasundaram R, Neuhaus P, Neumann U, Seehofer D. Role of IL28B polymorphism in the development of hepatitis C virus-induced hepatocellular carcinoma, graft fibrosis, and posttransplant antiviral therapy. Transplantation. 2012;93:644-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 58. | Sato M, Kato N, Tateishi R, Muroyama R, Kowatari N, Li W, Goto K, Otsuka M, Shiina S, Yoshida H. IL28B minor allele is associated with a younger age of onset of hepatocellular carcinoma in patients with chronic hepatitis C virus infection. J Gastroenterol. 2014;49:748-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 59. | Thompson AJ, Clark PJ, Singh A, Ge D, Fellay J, Zhu M, Zhu Q, Urban TJ, Patel K, Tillmann HL. Genome-wide association study of interferon-related cytopenia in chronic hepatitis C patients. J Hepatol. 2012;56:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 60. | Lange CM, Bibert S, Dufour JF, Cellerai C, Cerny A, Heim MH, Kaiser L, Malinverni R, Müllhaupt B, Negro F. Comparative genetic analyses point to HCP5 as susceptibility locus for HCV-associated hepatocellular carcinoma. J Hepatol. 2013;59:504-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 61. | Song MA, Kwee SA, Tiirikainen M, Hernandez BY, Okimoto G, Tsai NC, Wong LL, Yu H. Comparison of genome-scale DNA methylation profiles in hepatocellular carcinoma by viral status. Epigenetics. 2016;11:464-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 62. | Matsuura K, Sawai H, Ikeo K, Ogawa S, Iio E, Isogawa M, Shimada N, Komori A, Toyoda H, Kumada T. Genome-Wide Association Study Identifies TLL1 Variant Associated With Development of Hepatocellular Carcinoma After Eradication of Hepatitis C Virus Infection. Gastroenterology. 2017;152:1383-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 63. | Mukherjee A, Di Bisceglie AM, Ray RB. Hepatitis C virus-mediated enhancement of microRNA miR-373 impairs the JAK/STAT signaling pathway. J Virol. 2015;89:3356-3365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 64. | Santangelo L, Bordoni V, Montaldo C, Cimini E, Zingoni A, Battistelli C, D’Offizi G, Capobianchi MR, Santoni A, Tripodi M. Hepatitis C virus direct-acting antivirals therapy impacts on extracellular vesicles microRNAs content and on their immunomodulating properties. Liver Int. 2018;38:1741-1750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 65. | Sasaki R, Meyer K, Moriyama M, Kato N, Yokosuka O, Ray RB, Aurora R, Ray R, Kanda T. Rapid hepatitis C virus clearance by antivirals correlates with immune status of infected patients. J Med Virol. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 66. | Li W, Jian YB. Antitumor necrosis factor-α antibodies as a noveltherapy for hepatocellular carcinoma. Exp Ther Med. 2018;16:529-536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 67. | Zhou G, Sprengers D, Boor PPC, Doukas M, Schutz H, Mancham S, Pedroza-Gonzalez A, Polak WG, de Jonge J, Gaspersz M. Antibodies Against Immune Checkpoint Molecules Restore Functions of Tumor-Infiltrating T Cells in Hepatocellular Carcinomas. Gastroenterology. 2017;153:1107-1119.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 318] [Article Influence: 39.8] [Reference Citation Analysis (0)] |