Published online Nov 27, 2018. doi: 10.4254/wjh.v10.i11.877
Peer-review started: July 17, 2018
First decision: August 20, 2018
Revised: September 13, 2018
Accepted: October 11, 2018
Article in press: October 11, 2018
Published online: November 27, 2018
Processing time: 133 Days and 14.3 Hours
To evaluate the diagnostic value of dobutamine stress echocardiography (DSE) and myocardial perfusion scintigraphy (MPS) in predicting coronary artery disease (CAD) in cirrhotic patients listed for liver transplantation (LT), using invasive coronary angiography (ICA) as gold-standard.
Retrieval of studies was based on Medical Subject Headings and Health Sciences Descriptors, which were combined using Boolean operators. Searches were run on the electronic databases Scopus, Web of Science, EMBASE, MEDLINE (PubMed), BIREME (Biblioteca Regional de Medicina), LILACS (Latin American and Caribbean Health Sciences Literature), Cochrane Library for Systematic Reviews and Opengray.eu. There was no language or date of publication restrictions. The reference lists of the studies retrieved were searched manually.
The search strategy retrieved 322 references for DSE and 90 for MPS. In the final analysis, 10 references for DSE and 10 for MPS were included. Pooled sensitivity was 28% and 61% for DSE and MPS and specificity was 82% and 74%, for diagnosis of CAD using ICA as gold-standard, respectively.
DSE and MPS do not have adequate sensitivity for determination of whether CAD is present, despite having significant specificity.
Core tip: The concept of cardiac involvement in cirrhotic patients has been changing as patients listed for liver transplantation (LT) have become older and sicker. We aimed to evaluate the diagnostic value of dobutamine stress echocardiography (DSE) and myocardial perfusion scintigraphy (MPS) in predicting coronary artery disease (CAD) in cirrhotic patients listed for LT, using invasive coronary angiography as gold-standard. A systematic review and meta-analysis was performed, including 10 references for DSE and 10 for MPS. We concluded that DSE and MPS do not have adequate sensitivity for determination of whether CAD is present, despite having significant specificity.
- Citation: Soldera J, Camazzola F, Rodríguez S, Brandão A. Cardiac stress testing and coronary artery disease in liver transplantation candidates: Meta-analysis. World J Hepatol 2018; 10(11): 877-886
- URL: https://www.wjgnet.com/1948-5182/full/v10/i11/877.htm
- DOI: https://dx.doi.org/10.4254/wjh.v10.i11.877
When liver transplantation (LT) programs were beginning three decades ago, it was believed that the systemic vasodilation that occurs in end-stage liver disease (ESLD) might be able to protect patients from coronary artery disease (CAD)[1]. Nevertheless, studies have shown that CAD is more prevalent in cirrhotic patients than previously suspected. In a cohort with high risk for CAD, 26% of the patients had previously unknown CAD on routine invasive coronary angiography (ICA)[2].
The cardiac profile for LT candidates has been changing, because they are now older and sicker[3]. Data from the United Network for Organ Sharing (UNOS) show that the proportion of LT recipients over the age of 65 years in the United States increased from 9.6% in 2003 to 16.3% in 2013[4]. This has been a cause for major concern regarding perioperative cardiac risk. For example, a publication from 1996 predicted that around 50% of patients with significant CAD would die from cardiac complications in the perioperative period[5]. However, in a more recent study, the presence of obstructive CAD did not significantly impact post-LT survival, when modern treatment of CAD pre-LT is taken into account[6]. Furthermore, patients with ESLD have a specific type of cardiovascular sickness, currently known as cirrhotic cardiomyopathy, whose role in LT survival is yet to be established[7].
These findings suggest a real need for protocols for cardiac evaluation of patients awaiting LT - particularly for cirrhotic patients. The American Association for the Study of Liver Diseases (AASLD) published a guideline in 2005 that recommends myocardial stress testing for every patient referred for LT[8]. Nevertheless, the guideline published in 2012 by the American Heart Association (AHA) and the American College of Cardiology (ACC)[9], suggested that myocardial stress testing should be reserved for patients with three or more CAD risk factors. A score has recently been published for evaluation of perioperative cardiac risk, but it has yet to be validated further[10].
The aim of this systematic review with meta-analysis is to summarize the evidence related to the diagnostic value of two non-invasive cardiac stress testing methods: Dobutamine stress echocardiography (DSE) and myocardial perfusion scintigraphy (MPS), for the diagnosis of CAD in cirrhotic pre-LT patients, using ICA as gold-standard.
This study was carried out in accordance with the recommendations contained in the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA-P) guidelines[11]. Our systematic review was registered with the International Prospective Register of Systematic Reviews (PROSPERO), maintained by York University, on 17 August 2015 and was last updated on 5 April 2018 [registration No. 10.15124/CRD42015025391 (http://www.crd.york.ac.uk/prospero/)].
Studies were retrieved using Medical Subject Headings (MeSH) and Health Sciences Descriptors (DeCS), which were combined with Boolean operators. Searches were run on the electronic databases Scopus, Web of Science, Embase, Medline (PubMed), BIREME (Biblioteca Regional de Medicina), LILACS (Latin American and Caribbean Health Sciences Literature), Cochrane Library for Systematic Reviews and Opengray.eu. There was no language or date of publication restrictions. The reference lists of the retrieved studies were submitted to manual search. The search strategies used for each test and each database are shown in Supplemental material. Databases were last searched between August and September of 2015.
Cohort or case-control studies were eligible for selection, hence it was analyzed the diagnostic accuracy of DSE and/or MPS in adult patients with cirrhosis submitted for pre-LT evaluation. The tests had to be performed as a part of cardiac evaluation before LT. Studies were excluded if they did not meet these inclusion criteria. If there was more than one study published using the same population, the most recent study was selected for the analysis. Studies published only as abstracts were included, as long as the data available made analysis possible. The outcome measured was a diagnosis of CAD using ICA as gold standard.
An initial screening of titles and abstracts was the first stage to select potentially relevant papers. The second step was the analysis of the full-length papers. Two independent reviewers (Jonathan Soldera, Fabio Camazzola) extracted data using a standardized data extraction form after assessing and reaching consensus on eligible studies. The same reviewers separately assessed each study and extracted data about the characteristics of the subjects, the diagnostic accuracy for DCE and MPS and the outcomes measured. A third party (Santiago Rodriguez) was responsible for divergences in data extraction, clearing them when required. Quality of evidence regarding diagnostic accuracy was evaluated according of the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2)[12].
In anticipation of possible heterogeneity between the populations of the studies, a random-effects DerSimonian and Laird model was used. Data regarding the tests’ diagnostic accuracy was collected. The measures of diagnostic accuracy chosen were specificity, sensitivity, likelihood ratio and diagnostic odds ratio. Heterogeneity was assessed using the I2 statistic. MetaDisc 1.4 was used for diagnostic accuracy. The small number of studies included made funnel plot analysis impossible.
The search strategy retrieved 322 references for DSE and 90 for MPS. After analyzing titles and abstracts, 111 references for DSE and 24 for MPS were excluded because they were duplicates and the full texts were retrieved for 60 references on DSE and 26 on MPS. In the final analysis, 10 references were included for DSE and 10 for MPS. Flowcharts illustrating the search strategies are shown in Supplemental Figures 1 and 2, respectively. Studies included were either a case-control study or a prospective or historical cohort study.
Data were collected after the conclusion of a systematic review of the 10 studies included in the diagnostic analysis that used ICA as the gold-standard. The data extracted are summarized in Table 1.
| Ref. | TP | FP | FN | TN | Total number of patients in the study | Proportion of patients who underwent ICA | Definition of patients included | Criteria for ICA indication | Lesion for definition of positive ICA | QUADAS-2 quality analysis criteria |
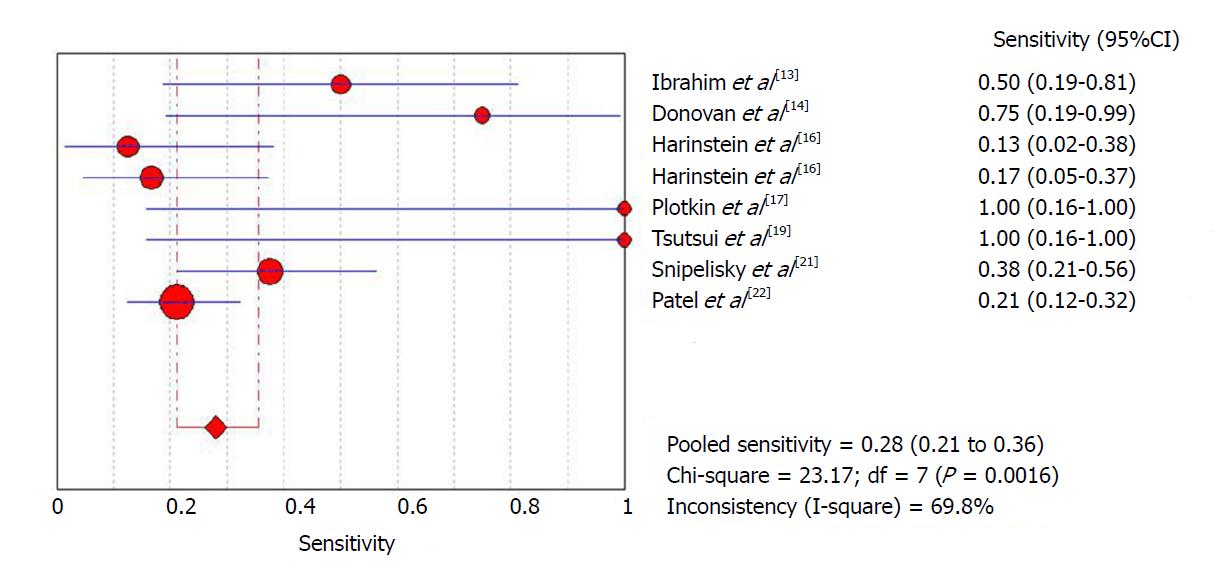
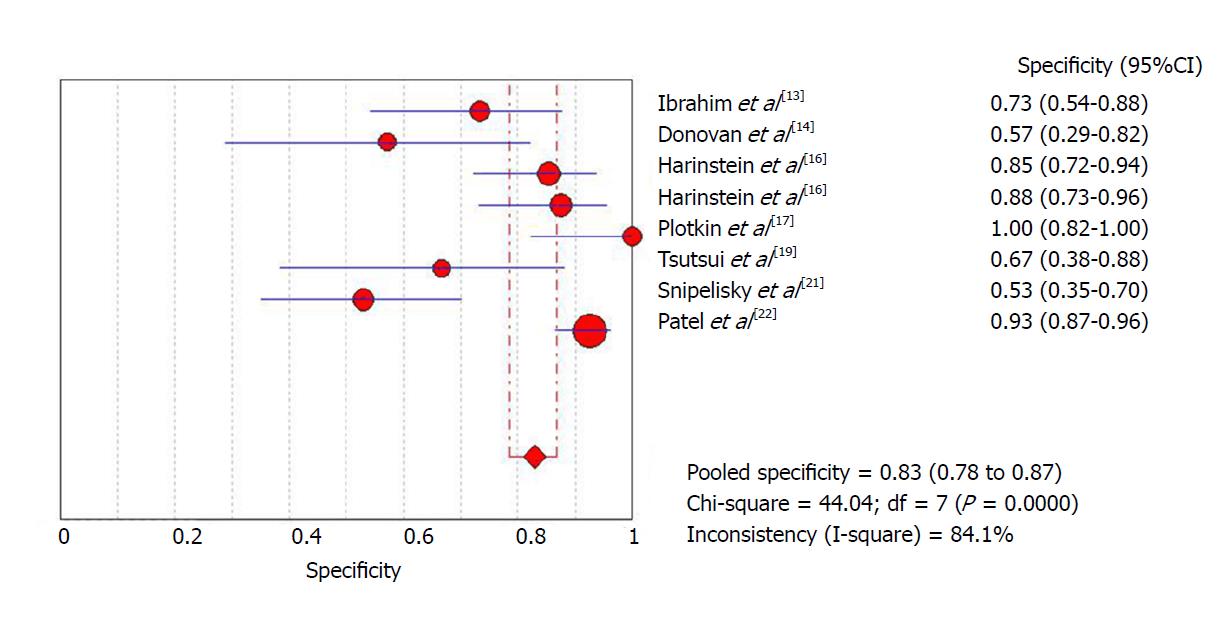
| Ibrahim et al[13] | 5 | 8 | 5 | 22 | 366 | 10.9% | Cirrhotic patients in pre-LT evaluation | High risk patients/positive DSE | NA | RB: P + I - R + F ? AC: P + I ? R + |
| Donovan et al[14] | 3 | 6 | 1 | 8 | 190 | 9.5% | Cirrhotic patients in pre-LT evaluation | High risk patients/positive DSE | > 50% | RB: P + I + R + F + AC: P + I + R + |
| Findlay et al[15] | 1 | 6 | 0 | 0 | 117 | 6% | Cirrhotic patients in pre-LT evaluation | Transplanted patients | > 70% | RB: P + I + R + F + AC: P – I + R + |
| Harinstein et al[16] | 2 | 7 | 14 | 41 | 105 | 61% | Cirrhotic patients in pre-LT evaluation | High risk patients/positive DSE | > 70% | RB: P + I + R + F + AC: P + I + R + |
| Harinstein et al[16] | 4 | 5 | 20 | 35 | 105 | 61% | Cirrhotic patients in pre-LT evaluation | High risk patients/positive DSE | > 50% | RB: P + I + R + F + AC: P + I + R + |
| Plotkin et al[17] | 2 | 0 | 0 | 19 | 40 | 52.5% | Cirrhotic patients in pre-LT evaluation | High risk patients/positive DSE | > 70% | RB: P + I + R + F + AC: P + I + R + |
| Ramrakhiani et al[18] | 4 | 10 | 0 | 0 | 201 | 7% | Cirrhotic patients in pre-LT evaluation | High risk patients/positive DSE | > 70% | RB: P + I – R – F ? AC: P + I – R – |
| Tsutsui et al[19] | 2 | 5 | 0 | 10 | 230 | 7.4% | Cirrhotic patients in pre-LT evaluation | High risk patients/positive DSE | > 50% | RB: P + I + R + F + AC: P + I + R + |
| Umphrey et al[20] | 0 | 0 | 0 | 9 | 157 | 5.7% | Cirrhotic patients in pre-LT evaluation | High risk patients | > 70% | RB: P + I + R + F + AC: P + I + R + |
| Snipelisky et al[21] | 12 | 16 | 20 | 18 | 66 | 100% | Cirrhotic patients in pre-LT evaluation | High risk patients/positive DSE | > 50% | RB: P + I + R + F + AC: P + I + R + |
| Patel et al[22] | 15 | 10 | 56 | 124 | 420 | 48.8% | Cirrhotic patients in pre-LT evaluation | High risk patients/positive DSE | > 70% | RB: P + I + R + F + AC: P + I + R + |
A minority of the patients included in these studies underwent ICA and they were generally higher risk patients with positive DSE findings or multiple risk factors. Data for risk factors specifically for the patients who underwent ICA were not available for most studies, therefore the data on risk factors described refer to the whole study population, as summarized in Supplemental Table 1.
The initial meta-analysis was performed including all studies. Global sensitivity was 28% [95% confidence interval (CI): 21.2%-35.6%] with high heterogeneity (I2 = 69%) (Figure 1), specificity was 82.9% (95%CI: 78.5%-86.8%) with high heterogeneity (I2 = 84.1%) (Figure 2) and the diagnostic odds ratio was 2.09 (95%CI: 0.96-4.58) with moderate heterogeneity (I2 = 47.5%) (Supplemental Figure 3). The positive likelihood ratio was 1.7 (95%CI: 1.06-2.7) with moderate heterogeneity (I2 = 51.4%) (Supplemental Figure 4) and the negative likelihood ratio was 0.92 (95%CI: 0.81-1.04) with little heterogeneity (I2 = 18.8%) (Supplemental Figure 5). An asymmetrical Receiver Operating Characteristic (ROC) curve is provided in Supplemental Figure 6.
A meta-regression was performed using the subsets of patients from each of the study samples who had undergone ICA and no statistically significant association was detected between this variable and the diagnostic odds ratio (P = 0.0586).
In order to attempt to reduce heterogeneity between studies, a sub-analysis was performed of sensitivity and specificity according to the definition of a positive ICA result employed by each study. Studies that used a positive ICA defined as any number of lesions with at least one greater than 70%, had a sensitivity of 21% (95%CI: 13.4%-31.3%) with high heterogeneity (I2 = 71%) and a specificity of 91.5% (95%CI: 86.8%-95%) with high heterogeneity (I2 = 63.5%), while studies that defined positive ICA as any number of lesions, with at least one greater than 50%, had a sensitivity of 36.1% (95%CI: 25.1%-48.3%) with high heterogeneity (I2 = 66.3%) and a specificity of 69.9% (95%CI: 61.4%-77.6%) with high heterogeneity (I2 = 68%).
Data were collected after conclusion of a systematic review of the 10 studies included in the diagnostic analysis that used ICA as the gold-standard. The data extracted are summarized in Table 2.
| Ref. | TP | FP | FN | TN | Total number of patients in the study | Proportion of patients who underwent ICA | Definition of patients included | Criteria for ICA indication | Lesion for definition of positive ICA | QUADAS-2 quality analysis criteria |
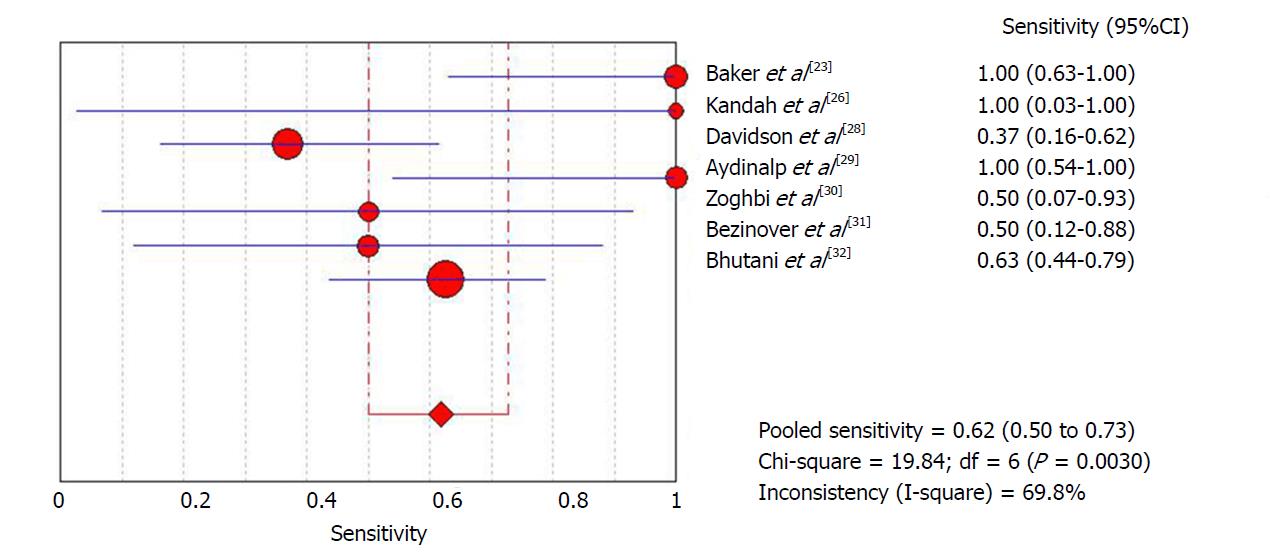
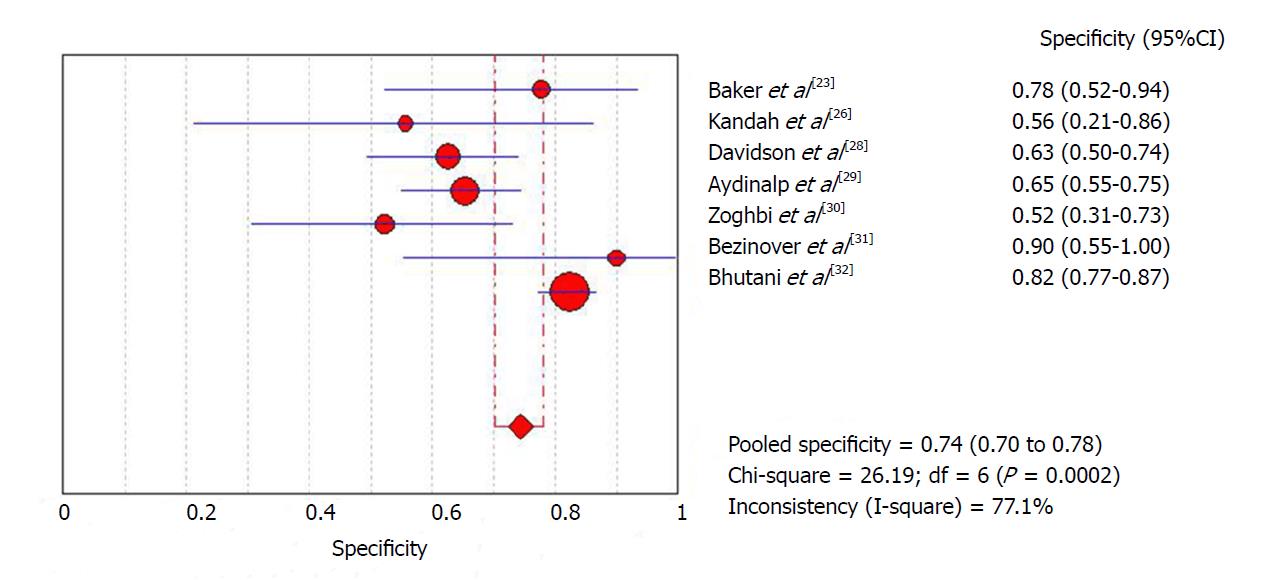
| Baker et al[23] | 8 | 4 | 0 | 14 | 74 | 35.1% | Cirrhotic patients in pre-LT evaluation with cardiac risk factors | High risk patients/positive MPS | > 70% | RB: P – I + R + F + AC: P – I + R + |
| Kryzhanovski et al[24] | 0 | 1 | 0 | 0 | 63 | 1.6% | Cirrhotic patients in pre-LT evaluation with cardiac risk factors | High risk patients/positive MPS | > 70% | RB: P – I + R + F + AC: P – I + R + |
| Senzolo et al[25] | 0 | 2 | 0 | 0 | 24 | 8.3% | Cirrhotic patients in pre-LT evaluation | Positive MPS | > 70% | RB: P – I + R + F + AC: P – I + R + |
| Kandiah et al[26] | 1 | 4 | 0 | 5 | 93 | 10.7% | Cirrhotic patients in pre-LT evaluation | High risk patients/positive MPS | > 70% | RB: P – I + R + F + AC: P – I + R + |
| Oprea-Lager et al[27] | 1 | 1 | 0 | 0 | 156 | 1.2% | Cirrhotic patients in pre-LT evaluation | High risk patients/positive MPS | > 70% | RB: P – I + R + F + AC: P – I + R + |
| Davidson et al[28] | 7 | 24 | 12 | 40 | 83 | 100% | Cirrhotic patients in pre-LT evaluation with cardiac risk factors | High risk patients/positive MPS | > 70% | RB: P + I + R + F + AC: P + I + R + |
| Aydinalp et al[29] | 6 | 34 | 0 | 64 | 389 | 26.7% | Cirrhotic patients in pre-LT evaluation | High risk patients/positive MPS | > 50% | RB: P + I + R + F + AC: P + I + R + |
| Zoghbi et al[30] | 2 | 11 | 2 | 12 | 87 | 31% | Cirrhotic patients in pre-LT evaluation | High risk patients/positive MPS | > 70% | RB: P – I + R + F + AC: P – I + R + |
| Bezinover et al[31] | 3 | 1 | 3 | 9 | 173 | 9.2% | Cirrhotic patients in pre-LT evaluation | High risk patients/positive MPS | NA | RB: P + I + R + F + AC: P + I + R + |
| Bhutani et al[32] | 20 | 46 | 12 | 215 | 414 | 70.7% | Cirrhotic patients in pre-LT evaluation | High risk patients/positive MPS | > 70% | RB: P + I + R + F + AC: P + I + R + |
As with DSE, a minority of the patients included in these studies underwent ICA, and they were generally higher risk patients with a positive MPS result or multiple risk factors. As with DSE, data for risk factors specifically for the patients who underwent ICA were not available for most studies, therefore the data for risk factors described refer to the whole study population, as summarized in Supplemental Table 2.
The diagnostic data were used for meta-analysis. The initial meta-analysis was performed including all studies. Global sensitivity was 61.8% (95%CI: 50%-72.8%) with high heterogeneity (I2 = 69.8%) (Figure 3), specificity was 74.3% (95%CI: 70.2%-78.2%) with high heterogeneity (I2 = 77.1%) (Figure 4) and the diagnostic odds ratio was 4.74 (95%CI: 1.51-14.8) with high heterogeneity (I2 = 61.9%) (Supplemental Figure 7). The positive likelihood ratio was 2.26 (95%CI: 1.47-3.48) with high heterogeneity (I2 = 63.5%) (Supplemental Figure 8) and the negative likelihood ratio was 0.57 (95%CI: 0.32-1.02) with high heterogeneity (I2 = 62.7%) (Supplemental Figure 9). An asymmetrical ROC curve is provided in Supplemental Figure 10.
A meta-regression was performed using the subsets of patients from each of the study samples who had undergone ICA and no statistically significant association was detected between this variable and the diagnostic odds ratio (P = 0.4984).
In order to attempt to reduce heterogeneity between studies, a sub-analysis was performed of sensitivity and specificity according to the definition of a positive ICA result employed by each study. Studies that used a positive ICA defined as any number of lesions with at least one greater than 70% had a sensitivity of 59.4% (95%CI: 46.4%-71.5%) with high heterogeneity (I2 = 70.5%) and specificity of 76.3% (95%CI: 71.6%-80.5%) with high heterogeneity (I2 = 80%). In another sub-analysis, including only the four studies in which ICA was performed for all patients, sensitivity was 57.1% (95%CI: 44%-69.5%) with high heterogeneity (I2 = 71.1%) and specificity was 75.5% (95%CI: 71.4%-79.7%) with high heterogeneity (I2 = 84.2%).
It is essential to understand the role of CAD in cirrhosis and LT patients. There is a need to improve pre-LT diagnostic tools because the age of LT candidates is rising and the proportion of NASH patients has been increasing. This systematic review is the largest current meta-analysis of diagnostic data for DSE and MPS in pre-LT patients. It increases the data available in a previous study of DSE as a diagnostic and prognostic tool for LT candidates, published by Nguyen et al[33], which found that DSE had a high negative predictive value for adverse outcomes post-LT.
Among the general population, a prior meta-analysis of five studies found that both DSE and MPS are accurate for detection of CAD, with sensitivity of 85% and specificity of 87%[34] for DSE and sensitivity of 83% and specificity of 77% for MPS[35]. However, this meta-analysis found much lower sensitivity values for diagnosis of CAD in patients awaiting LT, while specificity rates did not vary so much. This could have happened because results for stress testing might be false due to modifications in hemodynamics caused by ESLD, such as high-output cardiac failure, cirrhotic cardiomyopathy, anemia and the use of beta blockers[36,37].
Nevertheless, the most used method for pre-LT cardiac stress testing is DSE, since cirrhotic patients have a low tolerance of exercise[38]. When compared to ergometric cardiac stress testing, DSE has higher sensitivity (67% vs 88%) and specificity (71% vs 83%)[39-41]. The prognostic value of MPS has also been evaluated previously, with a hazard ratio of 3.17 for all-cause mortality for a group with reversible perfusion defect when compared to a group without perfusion defect[27].
The goal of both tests is to detect significant CAD prior to LT. In a high risk cohort in whom all patients underwent ICA and half had arterial systemic hypertension or diabetes, a 60% prevalence of CAD was found - one third with severe disease. Presence of moderate to severe CAD was associated with the presence of two or more cardiac risk factors[2]. If needed, ICA and stenting, seem to be safe in cirrhotic patients, taking precaution with the doubling of anti-platelet blockade in patients with esophageal varices[42]. The presence of CAD is associated with a poorer prognosis post-LT[43-45], although, Wray et al[6] did not detect a change in prognosis in the cohort they described. One must keep in mind also that pre-LT cardiac evaluation is costly and is not free from risks. In a previous study by Fili et al[46], the study protocol failed to demonstrate improvement in prognosis, but did raise costs.
One meta-analysis has found that DSE is superior to MPS among patients undergoing major vascular surgery - a positive DSE meant higher relative risk for perioperative MACE and all-cause mortality, when compared to MPS[47]. The prognostic role of DSE and MPS in patients undergoing kidney transplantation has been studied by two meta-analyses, which found these tests to be accurate in predicting outcomes, with DSE performing better than MPS in their analysis. Nevertheless, in this context, a normal non-invasive stress test did not necessarily exclude the possibility of adverse cardiac outcomes[48,49].
Analyzing the data collected and presented in this meta-analysis, it can be concluded that DSE and MPS offer limited accuracy for predicting CAD diagnoses. They both have low sensitivity and moderate specificity, which does not make them the ideal tests for pre-LT cardiac risk evaluation, as they also do not predict adverse outcomes with accuracy[50]. This is consistent with the latest ACC/AHA guidelines, which describes non-invasive stress testing as of low sensitivity and specificity for detecting CAD in liver-transplant candidates[9]. Nevertheless, the high specificity found in this meta-analysis show that both DSE and MPS are useful for identifying patients with CAD. Notwithstanding, a negative stress test does not exclude the presence of CAD.
The element most likely to affect the results of this meta-analysis is selection of patients with indications for both LT and ICA. Generally, physicians happen to be more cautious in referring sicker and older patients for LT, which might mean that this group of patients is under-represented in this meta-analysis. Also, ICA is generally ordered only for high-risk patients with a positive DSE or MPS, and a positive ICA can lead to de-listing for LT, or even death before LT, due to advanced heart conditions.
This heterogeneity of indications for DSE and MPS as part of pre-LT evaluation is reflected in the heterogeneity found in this meta-analysis, which is high throughout. Sub-analyses and meta-regressions were attempted in order to minimize heterogeneity, but with no substantial success. A major limitation is that, in most studies, just a few patients were referred for ICA, generally those with higher risk or a positive non-invasive stress test, which might over represent the proportion of CAD in pre-LT patients.
The results of this meta-analysis call into question the AASLD rationale of recommending routine non-invasive stress testing in pre-LT cardiac evaluation, since DSE and MPS both have low sensitivity for detecting CAD and did not predict outcomes adequately. Nevertheless, further prospective studies with standardized and homogenous patient characteristics are necessary in order to arrive at a better understanding of the value of pre-LT cardiac evaluation and a better-grounded decision on whether it is more cost-effective to follow AASLD[8] or ACC/AHA recommendations[9]. Initiatives such as development of the CAR-OLT score might help clarify this problem[10]. This paper’s strengths are its complete search strategy, performed in multiple databases. Nevertheless, results are just for pre-LT candidates; hence only patients referred for LT because of ESLD were reviewed.
The results of this systematic review and meta-analysis can also have been limited due to a post-referral bias, since patients with previously known serious cardiac conditions are generally not referred for LT. Early revascularization, in the general population, might lead to a significant change in the history of CAD and a better survival. This is somewhat unclear for ESLD patients. Because of the small number of studies and their limitations, the quality of evidence in the meta-analysis was low throughout, which might have negatively impacted this review.
In conclusion, this meta-analysis found that among few and limited studies, DSE and MPS are of limited value for predicting positive ICA. Their low sensitivity might make them inadequate for pre-LT cardiac evaluation. Prospective studies with larger samples are needed to better define an adequate test for predicting CAD in pre-LT patients.
The concept of cardiac involvement with coronary artery disease (CAD) in cirrhotic patients has been changing as patients listed for liver transplantation (LT) have become older and sicker. A previous study of dobutamine stress echocardiography (DSE) as a diagnostic and prognostic tool for LT candidates, published by Nguyen et al, which found that DSE had a high negative predictive value for adverse outcomes post-LT. This study tries to elucidate the problem of CAD screening in pre-LT patients.
There is a real need for protocols for cardiac evaluation of patients awaiting LT - particularly for cirrhotic patients. The American Association for the Study of Liver Diseases (AASLD) published a guideline in 2005 that recommends myocardial stress testing for every patient referred for LT. Nevertheless, the guideline published in 2012 by the American Heart Association (AHA and the American College of Cardiology (ACC), suggested that myocardial stress testing should be reserved for patients with three or more CAD risk factors. Better understanding the use of these tools might lead to better choices for pre-LT patients and better prognosis post-LT.
To evaluate the diagnostic value of DSE and myocardial perfusion scintigraphy (MPS) in predicting CAD in cirrhotic patients listed for LT, using invasive coronary angiography (ICA) as gold-standard. This could help clinicians choose the best test for predicting adverse cardiac events post-LT.
A systematic review and meta-analysis was performed. Searches were run on the electronic databases Scopus, Web of Science, EMBASE, MEDLINE (PubMed), BIREME (Biblioteca Regional de Medicina), LILACS (Latin American and Caribbean Health Sciences Literature), Cochrane Library for Systematic Reviews and Opengray.eu. There was no language or date of publication restrictions. The reference lists of the studies retrieved were searched manually.
The search strategy retrieved 322 references for DSE and 90 for MPS. In the final analysis, 10 references for DSE and 10 for MPS were included. Pooled sensitivity was 28% and 61% for DSE and MPS and specificity was 82% and 74%, for diagnosis of CAD using ICA as gold-standard, respectively.
This study found that DSE and MPS do not have adequate sensitivity for determination of whether CAD is present, despite having significant specificity. There is a need for better tools in order to detect CAD in pre-LT patients. It is not feasible to determine whether AASLD or AHA/ACC is correct, hence both tests underperformed. It is proposed a hypothesis that new methods, tests or scores are need in order to clarify this question, which could impact pre-LT decisions in the future.
It is possible to conclude that current evidence regarding pre-LT cardiac stress testing is lacking, and future research are bound to focus into solving this important clinical question. A comprehensive study, cohort or randomized, is necessary in order to gather more information on the utility and feasibility of the use of current and future tests in order to determine the presence of pre-LT CAD.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gencdal G, Milovanovic T S- Editor: Ji FF L- Editor: A E- Editor: Tan WW
| 1. | McCaughan GW, Crawford M, Sandroussi C, Koorey DJ, Bowen DG, Shackel NA, Strasser SI. Assessment of adult patients with chronic liver failure for liver transplantation in 2015: who and when? Intern Med J. 2016;46:404-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Tiukinhoy-Laing SD, Rossi JS, Bayram M, De Luca L, Gafoor S, Blei A, Flamm S, Davidson CJ, Gheorghiade M. Cardiac hemodynamic and coronary angiographic characteristics of patients being evaluated for liver transplantation. Am J Cardiol. 2006;98:178-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 160] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 3. | Xia VW, Taniguchi M, Steadman RH. The changing face of patients presenting for liver transplantation. Curr Opin Organ Transplant. 2008;13:280-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Kim WR, Lake JR, Smith JM, Skeans MA, Schladt DP, Edwards EB, Harper AM, Wainright JL, Snyder JJ, Israni AK. OPTN/SRTR 2013 Annual Data Report: liver. Am J Transplant. 2015;15 Suppl 2:1-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 250] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 5. | Plotkin JS, Scott VL, Pinna A, Dobsch BP, De Wolf AM, Kang Y. Morbidity and mortality in patients with coronary artery disease undergoing orthotopic liver transplantation. Liver Transpl Surg. 1996;2:426-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 198] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 6. | Wray C, Scovotti JC, Tobis J, Niemann CU, Planinsic R, Walia A, Findlay J, Wagener G, Cywinski JB, Markovic D. Liver transplantation outcome in patients with angiographically proven coronary artery disease: a multi-institutional study. Am J Transplant. 2013;13:184-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Ruiz-del-Arbol L, Serradilla R. Cirrhotic cardiomyopathy. World J Gastroenterol. 2015;21:11502-11521. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 96] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 8. | Murray KF, Carithers RL Jr; AASLD. AASLD practice guidelines: Evaluation of the patient for liver transplantation. Hepatology. 2005;41:1407-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 516] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 9. | Lentine KL, Costa SP, Weir MR, Robb JF, Fleisher LA, Kasiske BL, Carithers RL, Ragosta M, Bolton K, Auerbach AD. Cardiac disease evaluation and management among kidney and liver transplantation candidates: a scientific statement from the American Heart Association and the American College of Cardiology Foundation: endorsed by the American Society of Transplant Surgeons, American Society of Transplantation, and National Kidney Foundation. Circulation. 2012;126:617-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 198] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 10. | VanWagner LB, Ning H, Whitsett M, Levitsky J, Uttal S, Wilkins JT, Abecassis MM, Ladner DP, Skaro AI, Lloyd-Jones DM. A point-based prediction model for cardiovascular risk in orthotopic liver transplantation: The CAR-OLT score. Hepatology. 2017;66:1968-1979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 11. | Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7463] [Cited by in RCA: 8254] [Article Influence: 825.4] [Reference Citation Analysis (0)] |
| 12. | Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM; QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6953] [Cited by in RCA: 9589] [Article Influence: 684.9] [Reference Citation Analysis (0)] |
| 13. | Ibrahim A, Schuster A, Alraies MC, Sonny A, Cywinski JB, Jaber WA. Liver transplant candidates: to stress or not to stress? Circulation. 2014;130:A11773. |
| 14. | Donovan CL, Marcovitz PA, Punch JD, Bach DS, Brown KA, Lucey MR, Armstrong WF. Two-dimensional and dobutamine stress echocardiography in the preoperative assessment of patients with end-stage liver disease prior to orthotopic liver transplantation. Transplantation. 1996;61:1180-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 182] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Findlay JY, Keegan MT, Pellikka PP, Rosen CB, Plevak DJ. Preoperative dobutamine stress echocardiography, intraoperative events, and intraoperative myocardial injury in liver transplantation. Transplant Proc. 2005;37:2209-2213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Harinstein ME, Flaherty JD, Ansari AH, Robin J, Davidson CJ, Rossi JS, Flamm SL, Blei AT, Bonow RO, Abecassis M. Predictive value of dobutamine stress echocardiography for coronary artery disease detection in liver transplant candidates. Am J Transplant. 2008;8:1523-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Plotkin JS, Benitez RM, Kuo PC, Njoku MJ, Ridge LA, Lim JW, Howell CD, Laurin JM, Johnson LB. Dobutamine stress echocardiography for preoperative cardiac risk stratification in patients undergoing orthotopic liver transplantation. Liver Transpl Surg. 1998;4:253-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 101] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Ramrakhiani C, Bacon BR, St Vrain J, Befeler AS, Ramrakhiani S, Labovitz AJ. 2-D and Dobutamine Stress echocardiography in the pre- operative evaluation of patients undergoing orthotopic liver transplantation. Gastroenterology. 2001;120:A371-A371. [DOI] [Full Text] |
| 19. | Tsutsui JM, Mukherjee S, Elhendy A, Xie F, Lyden ER, O’Leary E, McGrain AC, Porter TR. Value of dobutamine stress myocardial contrast perfusion echocardiography in patients with advanced liver disease. Liver Transpl. 2006;12:592-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Umphrey LG, Hurst RT, Eleid MF, Lee KS, Reuss CS, Hentz JG, Vargas HE, Appleton CP. Preoperative dobutamine stress echocardiographic findings and subsequent short-term adverse cardiac events after orthotopic liver transplantation. Liver Transpl. 2008;14:886-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Snipelisky D, Levy M, Shapiro B. Utility of dobutamine stress echocardiography as part of the pre-liver transplant evaluation: an evaluation of its efficacy. Clin Cardiol. 2014;37:468-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Patel S, Kiefer TL, Ahmed A, Ali ZA, Tremmel JA, Lee DP, Yeung AC, Fearon WF. Comparison of the frequency of coronary artery disease in alcohol-related versus non-alcohol-related endstage liver disease. Am J Cardiol. 2011;108:1552-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Baker S, Chambers C, McQuillan P, Janicki P, Kadry Z, Bowen D, Bezinover D. Myocardial perfusion imaging is an effective screening test for coronary artery disease in liver transplant candidates. Clin Transplant. 2015;29:319-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Kryzhanovski VA, Beller GA. Usefulness of preoperative noninvasive radionuclide testing for detecting coronary artery disease in candidates for liver transplantation. Am J Cardiol. 1997;79:986-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Senzolo M, Bassanello M, Graziotto A, Zucchetta P, Cillo U, Maraglino G, Loreno M, Bellotto F, Davià G, Burra P. Microvascular autonomic dysfunction may justify false-positive stress myocardial perfusion imaging in patients with liver cirrhosis undergoing liver transplantation. Transplant Proc. 2008;40:1916-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Kandiah K, Steeds R, Thorburn D. The role of Myocardial Perfusion Imaging (MPI) in the assessment of cardiovascular risk in patients referred with end-stage liver failure for liver transplantation. J Hepatol. 2009;50:S178. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Oprea-Lager DE, Sorgdrager BJ, Jukema JW, Scherptong RW, Ringers J, Coenraad MJ, van Hoek B, Stokkel MP. Clinical value of myocardial perfusion scintigraphy as a screening tool in liver transplant candidates. Liver Transpl. 2011;17:261-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Davidson CJ, Gheorghiade M, Flaherty JD, Elliot MD, Reddy SP, Wang NC, Sundaram SA, Flamm SL, Blei AT, Abecassis MI. Predictive value of stress myocardial perfusion imaging in liver transplant candidates. Am J Cardiol. 2002;89:359-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 95] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Aydinalp A, Bal U, Atar I, Ertan C, Aktaş A, Yildirir A, Ozin B, Mudderisoglu H, Haberal M. Value of stress myocardial perfusion scanning in diagnosis of severe coronary artery disease in liver transplantation candidates. Transplant Proc. 2009;41:3757-3760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Zoghbi GJ, Patel AD, Ershadi RE, Heo J, Bynon JS, Iskandrian AE. Usefulness of preoperative stress perfusion imaging in predicting prognosis after liver transplantation. Am J Cardiol. 2003;92:1066-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Bezinover D, Bowman J, Baker S, Kadry Z, Uemura T, McQuillan P, Mets B, Chambers CE. Use of myocardial perfusion imaging for the evaluation of liver transplant candidates. Liver Transpl. 2013;19:S108. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Bhutani S, Tobis J, Gevorgyan R, Sinha A, Suh W, Honda HM, Vorobiof G, Packard RR, Steadman R, Wray C. Accuracy of stress myocardial perfusion imaging to diagnose coronary artery disease in end stage liver disease patients. Am J Cardiol. 2013;111:1057-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | Nguyen P, Plotkin J, Fishbein TM, Laurin JM, Satoskar R, Shetty K, Taylor AJ. Dobutamine stress echocardiography in patients undergoing orthotopic liver transplantation: a pooled analysis of accuracy, perioperative and long term cardiovascular prognosis. Int J Cardiovasc Imaging. 2013;29:1741-1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 34. | Picano E, Molinaro S, Pasanisi E. The diagnostic accuracy of pharmacological stress echocardiography for the assessment of coronary artery disease: a meta-analysis. Cardiovasc Ultrasound. 2008;6:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 35. | de Jong MC, Genders TS, van Geuns RJ, Moelker A, Hunink MG. Diagnostic performance of stress myocardial perfusion imaging for coronary artery disease: a systematic review and meta-analysis. Eur Radiol. 2012;22:1881-1895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 36. | Krag A, Bendtsen F, Burroughs AK, Møller S. The cardiorenal link in advanced cirrhosis. Med Hypotheses. 2012;79:53-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 37. | Singhal A, Mukerji AN, Thomaides A, Karachristos A, Maloo M, Sanchez B, Keresztury M, Santora TA, Jain A. Chronotropic incompetence on dobutamine stress echocardiography in candidates for a liver transplant. Exp Clin Transplant. 2013;11:546-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | Krahwinkel W, Ketteler T, Gödke J, Wolfertz J, Ulbricht LJ, Krakau I, Gülker H. Dobutamine stress echocardiography. Eur Heart J. 1997;18 Suppl D:D9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Pellikka PA, Nagueh SF, Elhendy AA, Kuehl CA, Sawada SG; American Society of Echocardiography. American Society of Echocardiography recommendations for performance, interpretation, and application of stress echocardiography. J Am Soc Echocardiogr. 2007;20:1021-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 529] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 40. | Meneghelo RS, Araújo CGS, Stein R, Mastrocolla LE, Albuquerque PF, Serra SM. III Diretrizes da Sociedade Brasileira de Cardiologia sobre teste ergométrico. Arq Bras Cardiol. 2010;95:1-29. [RCA] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 41. | Geleijnse ML, Krenning BJ, van Dalen BM, Nemes A, Soliman OI, Bosch JG, Galema TW, ten Cate FJ, Boersma E. Factors affecting sensitivity and specificity of diagnostic testing: dobutamine stress echocardiography. J Am Soc Echocardiogr. 2009;22:1199-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 42. | Russo MW, Pierson J, Narang T, Montegudo A, Eskind L, Gulati S. Coronary artery stents and antiplatelet therapy in patients with cirrhosis. J Clin Gastroenterol. 2012;46:339-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 43. | Yong CM, Sharma M, Ochoa V, Abnousi F, Roberts J, Bass NM, Niemann CU, Shiboski S, Prasad M, Tavakol M. Multivessel coronary artery disease predicts mortality, length of stay, and pressor requirements after liver transplantation. Liver Transpl. 2010;16:1242-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 44. | Azarbal B, Poommipanit P, Arbit B, Hage A, Patel J, Kittleson M, Kar S, Kaldas FM, Busuttil RW. Feasibility and safety of percutaneous coronary intervention in patients with end-stage liver disease referred for liver transplantation. Liver Transpl. 2011;17:809-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 45. | Diedrich DA, Findlay JY, Harrison BA, Rosen CB. Influence of coronary artery disease on outcomes after liver transplantation. Transplant Proc. 2008;40:3554-3557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 46. | Filì D, Vizzini G, Biondo D, Pietrosi G, Volpes R, Palazzo U, D’Antoni A, Petridis I, Luca A, Gridelli B. Clinical burden of screening asymptomatic patients for coronary artery disease prior to liver transplantation. Am J Transplant. 2009;9:1151-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 47. | Kertai MD, Boersma E, Bax JJ, Heijenbrok-Kal MH, Hunink MG, L’talien GJ, Roelandt JR, van Urk H, Poldermans D. A meta-analysis comparing the prognostic accuracy of six diagnostic tests for predicting perioperative cardiac risk in patients undergoing major vascular surgery. Heart. 2003;89:1327-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 160] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 48. | Wang LW, Fahim MA, Hayen A, Mitchell RL, Baines L, Lord S, Craig JC, Webster AC. Cardiac testing for coronary artery disease in potential kidney transplant recipients. Cochrane Database Syst Rev. 2011;12:CD008691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 49. | Wang LW, Masson P, Turner RM, Lord SW, Baines LA, Craig JC, Webster AC. Prognostic value of cardiac tests in potential kidney transplant recipients: a systematic review. Transplantation. 2015;99:731-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 50. | Soldera J, Camazzola F, Rodríguez S, Brandão A. Dobutamine stress echocardiography, myocardial perfusion scintigraphy, invasive coronary angiography, and post-liver transplantation events: Systematic review and meta-analysis. Clin Transplant. 2018;32:e13222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |