Published online Oct 27, 2018. doi: 10.4254/wjh.v10.i10.772
Peer-review started: April 6, 2018
First decision: May 16, 2018
Revised: June 14, 2018
Accepted: June 27, 2018
Article in press: June 28, 2018
Published online: October 27, 2018
Processing time: 205 Days and 15.8 Hours
Presented here is the clinical course of a 63-year-old patient with a central, large and unresectable hepatocellular carcinoma (HCC) with liver metastases and tumor invasion of the portal and hepatic veins. After the tumor had been diagnosed, the patient was immediately treated with proton beam therapy (PBT), at a total dose of 60 Gy (relative biological effectiveness) in 20 fractions administered within 4 wk. To manage the respiratory movements, at the Rinecker Proton Therapy Center, apneic oxygenation was given daily, under general anesthesia. The patient tolerated both the PBT and general anesthesia very well, and did now show any signs of acute or late toxicity. The treatment was followed by constant reductions in the tumor marker alpha-fetoprotein and the cholestatic parameters gamma-glutamyltransferase and alkaline phosphatase. The patient commenced an adjuvant treatment with sorafenib, given at 6-wk intervals, after the PBT. Follow-up with regular magnetic resonance imaging has continued for 40 mo so far, demonstrating remarkable shrinkage of the HCC (maximal diameter dropping from approximately 13 cm to 2 cm). To date, the patient remains free of tumor recurrence. PBT served as a safe and effective treatment method for an unresectable HCC with vascular invasion.
Core tip: Hepatocellular carcinoma (HCC) is one of the most common cancers in Asia. Patients with unresectable tumor disease require more options for in-principle curative therapies. We report here a patient with a large unresectable HCC due to vascular invasion and satellite metastases, who showed remarkable tumor shrinkage after completing proton beam therapy 4 years ago and who is still free of tumor recurrence to date.
- Citation: Lin YL. Proton beam therapy in apneic oxygenation treatment of an unresectable hepatocellular carcinoma: A case report and review of literature. World J Hepatol 2018; 10(10): 772-779
- URL: https://www.wjgnet.com/1948-5182/full/v10/i10/772.htm
- DOI: https://dx.doi.org/10.4254/wjh.v10.i10.772
Hepatocellular carcinoma (HCC) is one of the most common tumor diseases worldwide, with particularly high incidence in the Asia-Pacific region, and is often associated with liver cirrhosis owing to alcohol abuse and chronic viral hepatitis[1]. Surgical resection and liver transplantation are the first-line curative treatments for large HCC, while small HCC (preferably < 2 cm in diameter) in an accessible location, away from critical structures, can be cured by local ablative techniques. For patients with large unresectable tumors, such as those with advanced tumor extension and vascular invasion, other locoregional treatment modalities are usually considered as palliative options; these include arterial catheter-based treatments, ablative techniques and radiation therapy.
In past years, many convincing results were published on the effectiveness and tolerability of charged-particle therapy with dose-escalating proton and heavy ion beam as a curative-intent treatment of unresectable HCC[2-4]. Based on the physical property of Bragg peak, proton beams deposit the dose maximum at a predefined depth in the tumor. Behind the maximal deposit, the dose drops rapidly, having no exit dose. This is a significant advantage in comparison to photon radiotherapy and enables the dose escalation to improve the local control. Because the surrounding uninvolved liver parenchyma can be spared from the unnecessary radiation dose, even larger tumor volume can be treated with proton beam therapy (PBT) without risk of fatal radiation-induced liver disease (RILD)[5,6].
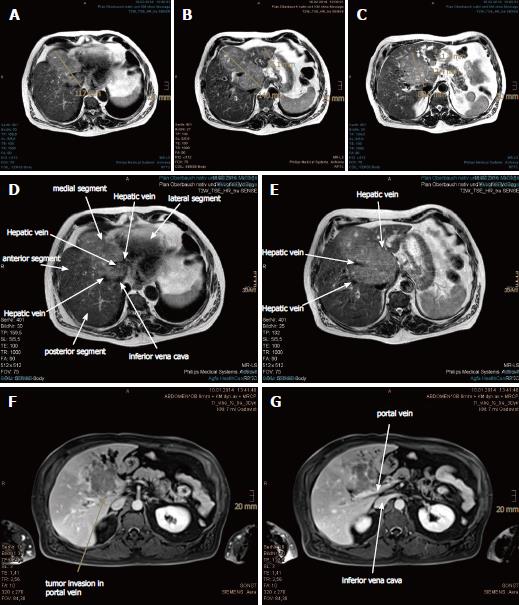
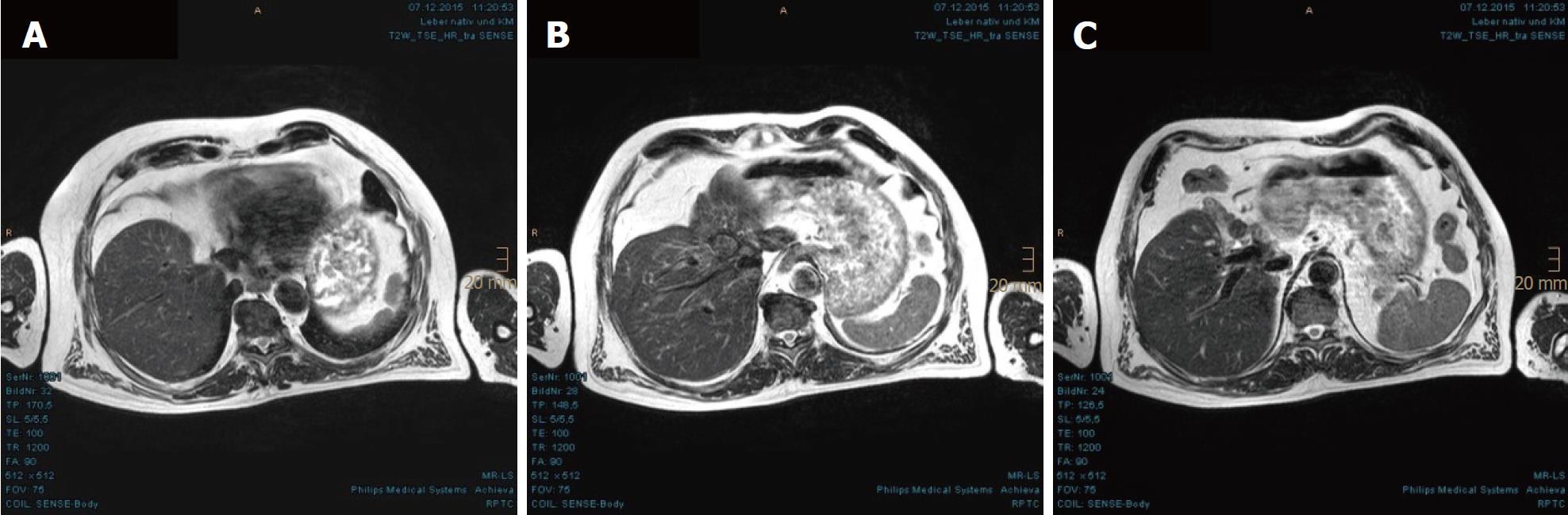
In November 2013, a routine abdominal sonography of a 63-year-old German male revealed a huge tumor in the liver. Subsequent magnetic resonance imaging (MRI) and computed tomography (CT) scan of the abdomen revealed the main tumor mass to be of about 13.4 cm × 7.1 cm in size, encompassing segments I, IV, V and VIII, as well as satellite metastases in segments II and III. The tumor involved all three hepatic veins and the portal vein, compressing the inferior vena cava (Figure 1). Liver biopsy, taken in January 2014, confirmed a diagnosis of HCC, Edmondson-Steiner-grade II, with partially cirrhotic parenchymal modification. The additional esophagogastroduodenoscopy and colonoscopy demonstrated chronic gastritis with helicobacter pylori infection and sigmoid diverticulosis, but no evidence of other gastrointestinal malignancy. The patient reported not feeling any discomfort but having lost 12 kg of weight, which he had attributed to a dietary prescription to address his decompensated diabetes mellitus. Blood test (hepatic function panel) showed remarkably increased levels of gamma-glutamyltransferase (GGT) and alkaline phosphatase (AP), and moderately elevated levels of total bilirubin, glutamic-oxalocetic transaminase (GOT), glutamic-pyruvic transaminase (GPT) and cholesterol.
Because the initial consulting hospital classified this case of HCC as Child-Pugh score A, BCLC stage B, palliative treatments with transarterial chemoembolization (TACE) and sorafenib were recommended in the tumor board review. However, extended vascular invasion and tumor size > 10 cm are contraindications for TACE. The patient was informed about best supportive care and approximate survival time of 6 mo. He contacted our institute, the Rinecker Proton Therapy Center (RPTC), and was treated with PBT from February to March 2014. A total dose of 60.00 Gy (relative biological effectiveness, RBE) administered in 20 fractions within 4 wk was applied to both the main liver tumor and the satellite metastases, with a safety margin of 3 mm.
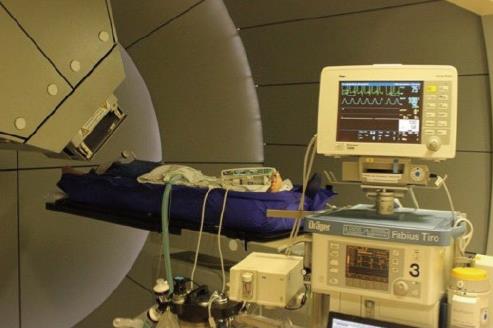
For precise targeting by PBT, special techniques to control the respiratory movements are required. At RPTC, we use apneic oxygenation (AO) with total intravenous anesthesia and oral intubation, which prolongs the safe apnea time during the irradiation (Figure 2). After preoxygenation, the artificial ventilation is stopped during the apneic phase. The patient stays connected, with delivery of 1 L/min oxygen and constant airway pressure. Due to the disproportion between the rate of oxygen removal from the alveoli compared to the rate of carbon dioxide delivery to them, the barometric pressure in the alveoli sinks, pushing the oxygen to move from the upper airway to the alveoli. That means, the consumed oxygen is replaced but the carbon dioxide is not removed, and the arterial partial pressure of carbon dioxide (PaCO2) rises in the time following, in a range of 2-4 mmHg/min[7,8]. According to our standard operating procedure, the oxygen saturation during AO should not fall below 97%, and PaCO2 is not allowed to exceed 61 mmHg.
The patient tolerated the PBT under general anesthesia daily, without any considerable toxicity (Common Terminology Criteria for Adverse Events, grade 0). At the first follow-up (after 6 wk of treatment), the patient showed remarkable reduction in the tumor marker alpha-fetoprotein (AFP) (from 109 μg/L to 34 μg/L; normal range: < 15 ng/mL) and decrease in GGT (from 64 μkat/L to 25 μkat/L; normal range: < 1.00 μkat/L). From this time forward, the patient also commenced with targeted therapy, i.e., tyrosine kinase inhibitor (sorafenib) and continued in his normal occupational activities (working as a driver). At 5 mo after the PBT, routine blood test showed leukocytosis (12.7 Gpt/L; normal range: 4.0-10.9 Gpt/L) with elevated C-reactive protein (101 mg/L; normal range: < 5.0 mg/L). Respiratory or urinary infection was excluded. The patient denied experience of fever but complained of night sweats, and was treated with antibiotics by his oncologist. Because of diarrhea, the sorafenib was stopped for a few months and restarted with the dosage halved (to 400 mg daily). At the end of 2014, both the leukocyte count and C-reactive protein level finally decreased and the GGT continued to fall (to 3.3 μkat/L). At 20 mo after the PBT, the AFP was within normal range (2.8 μg/L), remaining stable through the last measurement in June 2017 (2.5 μg/L). The thoracic CT scan at 19 mo after the PBT also did not reveal any suspicious metastasis.
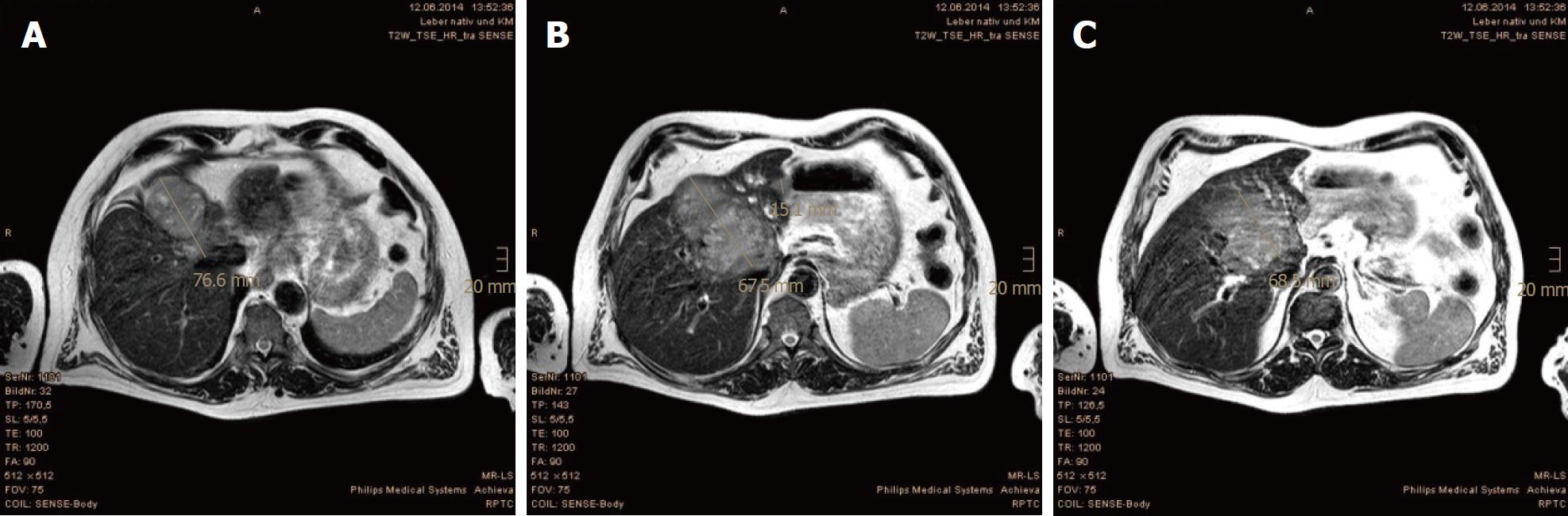
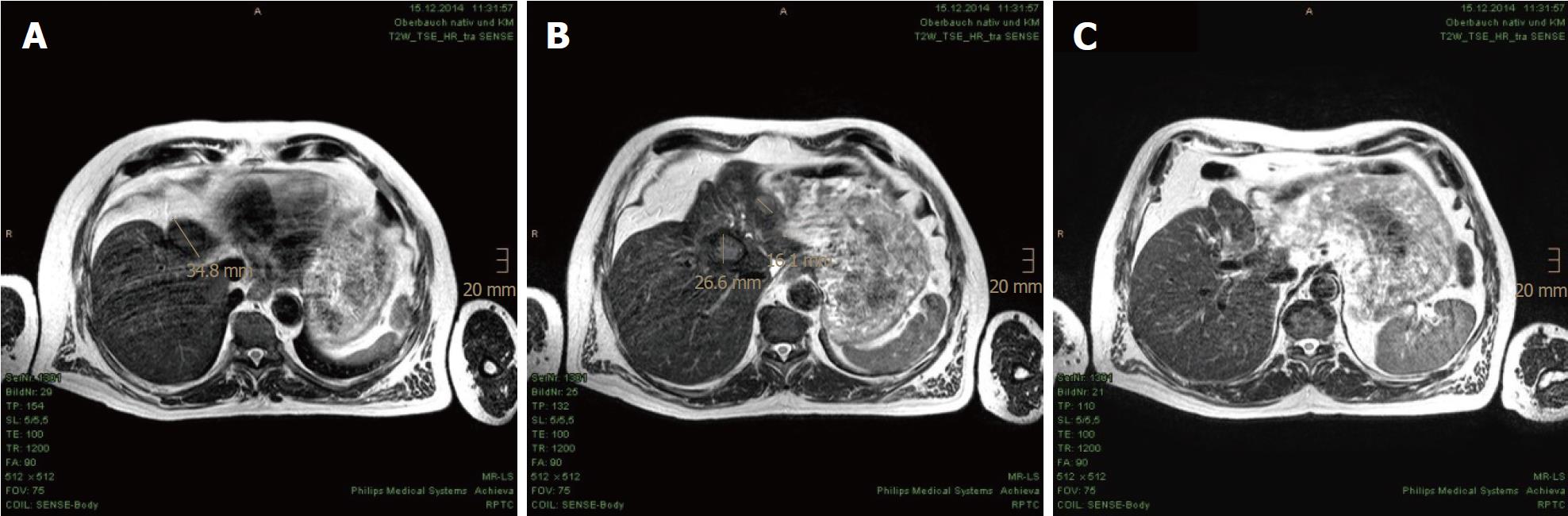
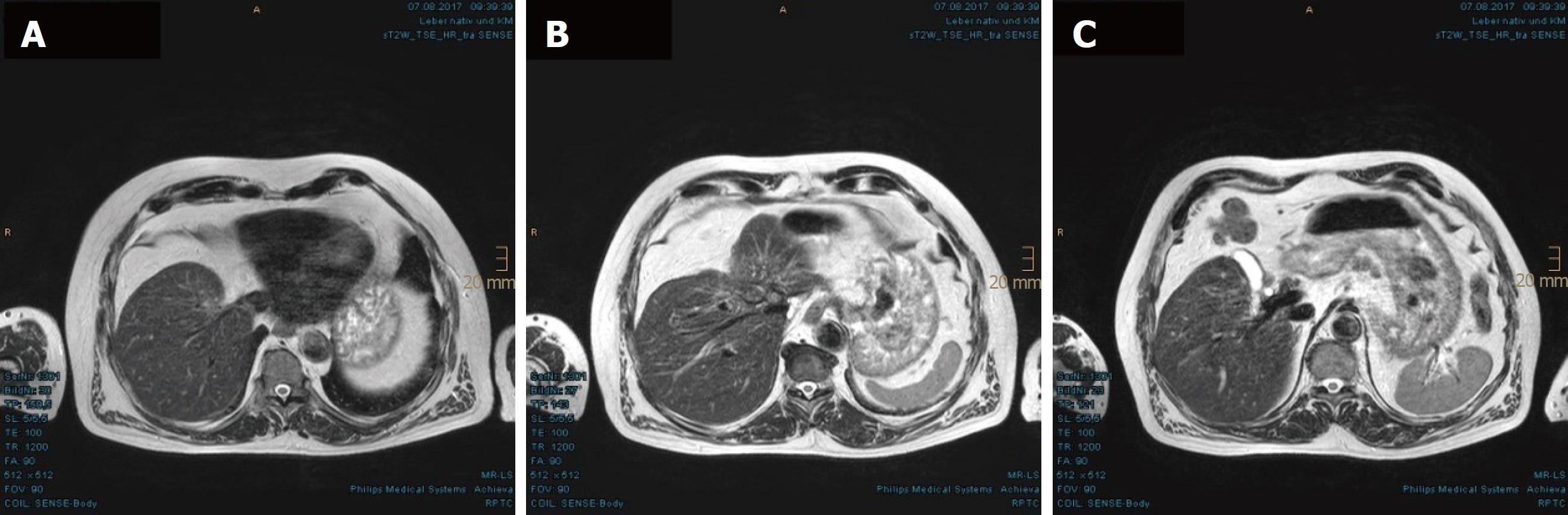
In the first MRI control, taken 3 mo after the PBT, significant size reductions of the main tumor in the hepatic hilum and the satellite metastases in the left hepatic lobe were observed. The segmental cholestasis had also regressed, consistent with the falling GGT and AP (Figure 3). In the subsequent check-ups, continuous tumor shrinkage with indentation of the liver contour was observed, as if the patient had undergone a liver resection (Figure 4 and Figure 5). The latest MRI scan, performed in August 2017, revealed a residual nodule of approximately 2 cm × 2 cm without pathological enhancement in the hilar region (Figure 6). No signs of ascites, or new distant or lymph node metastases have been found in the 40-mo period following the PBT. To date, the patient is still receiving semi-dosed sorafenib and participating in regular follow-up visits with his oncologist as well as in an annual MRI scan performed at the RPTC.
Patients with unresectable HCC are usually not deemed to be curable because surgical resection and liver transplantation are considered as the only curative options. According to guidelines, TACE and sorafenib are the standard of palliative care for unresectable HCC. Other local treatment methods, such as radiofrequency ablation, cryoablation and radioembolization, are often combined with the TACE and sorafenib regimen to improve the total response[9]. Yet, the therapeutic efficacy of combined treatments, such as that of radiofrequency ablation after TACE, is limited by the tumor size and morphology[10].
Katsanos et al[11] analyzed 55 randomized controlled trials on mono- or combined treatments with TACE for unresectable HCC and concluded that TACE in combination with external radiotherapy or local liver ablation may distinctly improve tumor response and the survival rates of patients. Choi et al[12] reviewed the different radiotherapy techniques and summarized the results of radiotherapy, partially combined with liver transplantation, TACE and concomitant chemotherapy, for HCC in each tumor stage. Radiotherapy can amend the total therapeutic assortments and outcomes of HCC by improving local control, enabling downstaging and treating unresectable HCC with vascular invasion or multiple intrahepatic metastases. The latest National Comprehensive Cancer Network (NCCN) guidelines[13] consider external-beam radiation therapy as a category 2B option for patients with unresectable HCC or those with contraindication for operation due to comorbidity. Besides, stereotactic body radiation therapy can be recommended as alternative to ablation and embolization techniques, particularly after their failure or in case of their contraindication. Because prospective randomized controlled trials evaluating the outcome of various techniques of external-beam radiation therapy vs ablation and arterially directed therapies are still pending or ongoing, clear guidelines of treatment recommendations for large unresectable HCC, specifically with vascular invasion and intrahepatic metastasis, are still missing. Mostly, only when further interventional treatments are no longer possible, will radiation therapy be considered.
The aim of modern radiotherapy techniques is to achieve delivery of an effective high-dose in the tumor, while sparing the surrounding structures as much as possible. If the liver function is restricted by coexistent liver disease, such as cirrhosis and/or viral hepatitis, the more essential the role of sparing the surrounding noncancerous liver tissues becomes. Crane et al[14] pointed out the challenges of radiotherapy for large HCC due to proximity of stomach and intestines, underlying liver disease, radiosensitivity of liver parenchyma and respiratory and interfractional motions. Because partial resection of the liver (with only 25% remnant) has been demonstrated as well tolerated by noncirrhotic patients[15], it allows radiation therapy of partial liver with higher doses if there is enough functional liver parenchyma left.
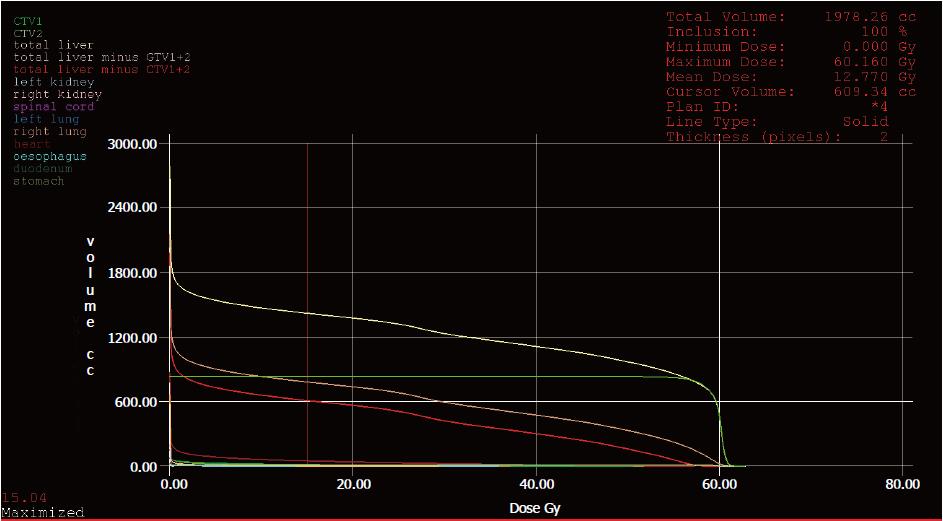
In order to prevent RILD, our institute uses the following dose constraints of liver: at least 700 cc of uninvolved liver parenchyma should receive less than 15 Gy (RBE), and the mean dose of liver should be less than 13 Gy (RBE). This is the Quantitative Analyses of Normal Tissue Effects in the Clinic (commonly known as QUANTEC) recommendation for stereotactic body radiation therapy of liver tumor with three fractions[16]. In the case of our patient, presented herein, the total liver volume prior to PBT was 2789 cc (including 638 cc gross tumor volume), and the mean dose of uninvolved liver parenchyma (i.e., total liver volume with subtraction of clinical target volume) was 12.77 Gy (RBE). The volume of uninvolved liver that received less than 15 Gy (RBE) was approximately 1370 cc (Figure 7), while the remnant liver volume after tumor regression was 1470 cc, as measured by MRI scan at 40 mo after the PBT.
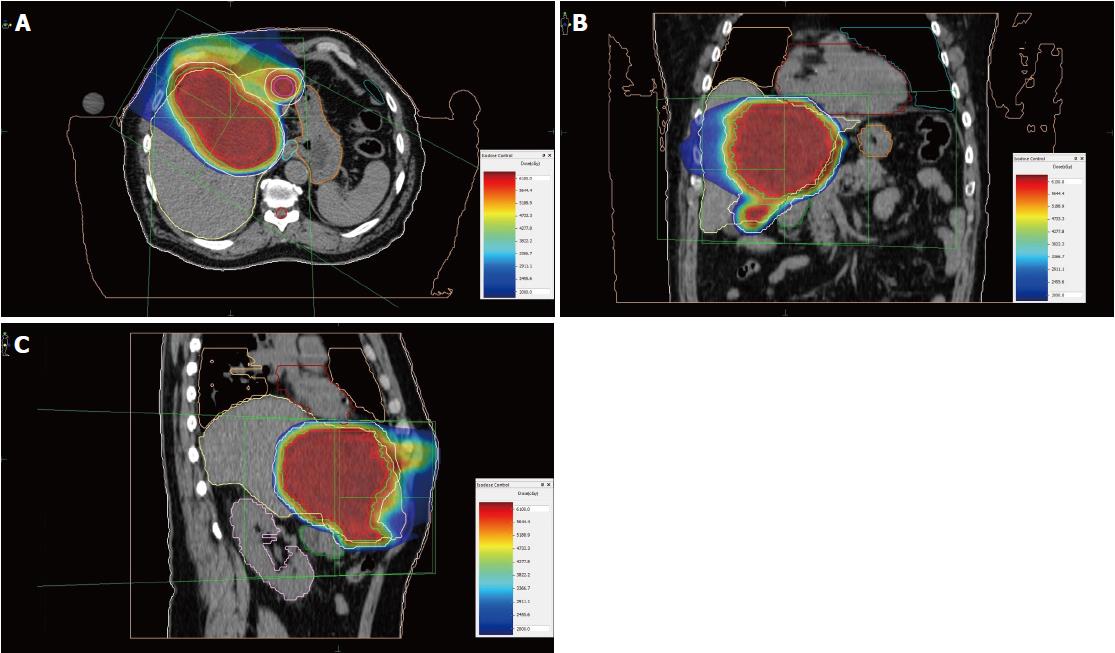
Unlike photon radiotherapy, PBT requires less irradiation fields to encompass the target. Therefore, the dose burden of uninvolved liver parenchyma and other surrounding organs at risk, such as heart, stomach, intestines, spinal cord and kidney, as well as the risk of radiation-induced secondary malignancies is significantly reduced by the dosimetric advantage of proton beams[17,18]. Leung et al[19] compared the cost-utility of stereotactic radiation therapy and PBT, assuming PBT is a cost-effective therapy for inoperable advanced HCC due to the improved quality-adjusted life years. The spot scanning technique used at RPTC also facilitates more conformity to the extent of the target, in comparison to common scattering PBT[20]. In our case, we only used two irradiation fields, from 0 and 300 degrees, to embrace the main tumor in the central hepatic region and the satellite metastases in the left lobe (Figure 8). Irradiation of each field lasted 2.5-3.5 min.
The main challenge of radiotherapy for movable organs, in particular lungs and liver, is to manage the intra- and interfractional motion of the tumor. Measures like implantation of fiducial markers, respiratory gating or tracking, breath-hold method, abdominal compression and 4-dimensional CT simulation are applied in different institutions[21,22]. A unique feature of our center is the use of AO to control respiratory motion and to reduce the safety margin. To assess whether the patient can withstand apneic oxygenation, besides general preanesthesia evaluation, additional tests, such as body plethysmography, echocardiography and arterial blood gas test, can be required in case of relevant cardiopulmonary comorbidity. Since the inauguration of RPTC in 2009, we have treated over 500 patients with the apneic method. Up to the end of 2017, the total number of general anesthesia sessions was more than 6000, while the total time, including introduction and discharge, was 55 min on average. The longest period for AO was over 9 min, and the average apneic duration was approximately 3 min. Statistically speaking, the most treated tumor entities for the use of AO were thoracic malignancies and liver metastases.
Although the patient presented in our case report had several adverse prognostic factors, his case demonstrates that large unresectable HCC with vascular invasion and intrahepatic metastases can be treated excellently with PBT. RPTC has long-standing experience in PBT of movable tumors with AO, which securely solves the problem of organ motion, permits the reduction of safety margin and consequently the side effects, like RILD, and requires the least compliance of patients concerning breath control and abandonment of fiducial markers.
In a routine abdominal sonography of a 63-year-old asymptomatic male, a large central tumor in the liver was detected.
According to the abdominal sonography, the magnetic resonance imaging and the pathologically increased tumor marker alpha-fetoprotein and liver function panel, a hepatic malignancy was urgently suspected.
Liver cirrhosis, cholangiocellular carcinoma, liver angiosarcoma, liver metastases of non-hepatic origin.
The laboratory tests showed significant increase of the tumor marker alpha-fetoprotein and the cholestatic parameters gamma-glutamyltransferase and alkaline phosphatase.
Magnetic resonance imaging and computed tomography of the abdomen revealed the main tumor mass to be of about 13.4 cm × 7.1 cm in size, encompassing segments I, IV, V and VIII, as well as satellite metastases in segments II and III. The tumor involved all three hepatic veins and the portal vein, compressing the inferior vena cava.
A liver biopsy confirmed a diagnosis of hepatocellular carcinoma (HCC), Edmondson-Steiner-grade II, with partially cirrhotic parenchymal modification.
The patient was treated with proton beam therapy (PBT) in apneic oxygenation at the Rinecker Proton Therapy Center, at a total dose of 60 Gy (relative biological effectiveness) in 20 fractions.
We use the apneic oxygenation to manage respiratory motion of the tumor and to reduce the safety margin, which enables the sparing of uninvolved liver parenchyma and other surrounding organs at risk as well as a dose escalation.
The patient is free of tumor recurrence in a period of 4 years after the treatment. PBT is a safe and effective therapy for large unresectable HCC with vascular invasion and intrahepatic metastases.
CARE Checklist (2013) statement: The author has read the CARE Checklist (2013), and the manuscript was prepared and revised according to the CARE Checklist (2013).
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Iliescu L, Lin ZY S- Editor: Ji FF L- Editor: A E- Editor: Song H
| 1. | Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1628] [Cited by in RCA: 1639] [Article Influence: 204.9] [Reference Citation Analysis (0)] |
| 2. | Skinner HD, Hong TS, Krishnan S. Charged-particle therapy for hepatocellular carcinoma. Semin Radiat Oncol. 2011;21:278-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Hong TS, Wo JY, Yeap BY, Ben-Josef E, McDonnell EI, Blaszkowsky LS, Kwak EL, Allen JN, Clark JW, Goyal L. Multi-Institutional Phase II Study of High-Dose Hypofractionated Proton Beam Therapy in Patients With Localized, Unresectable Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. J Clin Oncol. 2016;34:460-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 332] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 4. | Fukuda K, Okumura T, Abei M, Fukumitsu N, Ishige K, Mizumoto M, Hasegawa N, Numajiri H, Ohnishi K, Ishikawa H. Long-term outcomes of proton beam therapy in patients with previously untreated hepatocellular carcinoma. Cancer Sci. 2017;108:497-503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Pan CC, Kavanagh BD, Dawson LA, Li XA, Das SK, Miften M, Ten Haken RK. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys. 2010;76:S94-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 517] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 6. | Thomas TO, Hasan S, Small W Jr, Herman JM, Lock M, Kim EY, Mayr NA, Teh BS, Lo SS. The tolerance of gastrointestinal organs to stereotactic body radiation therapy: what do we know so far? J Gastrointest Oncol. 2014;5:236-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 7. | Frumin MJ, Epstein RM, Cohen G. Apneic oxygenation in man. Anesthesiology. 1959;20:789-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 249] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 8. | Kolettas A, Grosomanidis V, Kolettas V, Zarogoulidis P, Tsakiridis K, Katsikogiannis N, Tsiouda T, Kiougioumtzi I, Machairiotis N, Drylis G. Influence of apnoeic oxygenation in respiratory and circulatory system under general anaesthesia. J Thorac Dis. 2014;6 Suppl 1:S116-S145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 9. | Murata S, Mine T, Sugihara F, Yasui D, Yamaguchi H, Ueda T, Onozawa S, Kumita S. Interventional treatment for unresectable hepatocellular carcinoma. World J Gastroenterol. 2014;20:13453-13465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 10. | Yamakado K, Nakatsuka A, Ohmori S, Shiraki K, Nakano T, Ikoma J, Adachi Y, Takeda K. Radiofrequency ablation combined with chemoembolization in hepatocellular carcinoma: treatment response based on tumor size and morphology. J Vasc Interv Radiol. 2002;13:1225-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 151] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Katsanos K, Kitrou P, Spiliopoulos S, Maroulis I, Petsas T, Karnabatidis D. Comparative effectiveness of different transarterial embolization therapies alone or in combination with local ablative or adjuvant systemic treatments for unresectable hepatocellular carcinoma: A network meta-analysis of randomized controlled trials. PLoS One. 2017;12:e0184597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 12. | Choi SH, Seong J. Strategic Application of Radiotherapy for Hepatocellular Carcinoma. Clin Mol Hepatol. 2018; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 13. | Benson AB 3rd, D‘Angelica MI, Abbott DE, Abrams TA, Alberts SR, Saenz DA, Are C, Brown DB, Chang DT, Covey AM, Hawkins W, Iyer R, Jacob R, Karachristos A, Kelley RK, Kim R, Palta M, Park JO, Sahai V, Schefter T, Schmidt C, Sicklick JK, Singh G, Sohal D, Stein S, Tian GG, Vauthey JN, Venook AP, Zhu AX, Hoffmann KG, Darlow S. NCCN Guidelines Insights: Hepatobiliary Cancers, Version 1.2017. J Natl Compr Canc Netw. 2017;15:563-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 254] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 14. | Crane CH, Koay EJ. Solutions that enable ablative radiotherapy for large liver tumors: Fractionated dose painting, simultaneous integrated protection, motion management, and computed tomography image guidance. Cancer. 2016;122:1974-1986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 15. | Guglielmi A, Ruzzenente A, Conci S, Valdegamberi A, Iacono C. How much remnant is enough in liver resection? Dig Surg. 2012;29:6-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 247] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 16. | Marks LB, Yorke ED, Jackson A, Ten Haken RK, Constine LS, Eisbruch A, Bentzen SM, Nam J, Deasy JO. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76:S10-S19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1352] [Cited by in RCA: 1172] [Article Influence: 78.1] [Reference Citation Analysis (1)] |
| 17. | Lischalk JW, Repka MC, Unger K. Radiation therapy for hepatobiliary malignancies. J Gastrointest Oncol. 2017;8:279-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Mondlane G, Gubanski M, Lind PA, Ureba A, Siegbahn A. Comparative study of the calculated risk of radiation-induced cancer after photon- and proton-beam based radiosurgery of liver metastases. Phys Med. 2017;42:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Leung HWC, Chan ALF. Cost-utility of stereotactic radiation therapy versus proton beam therapy for inoperable advanced hepatocellular carcinoma. Oncotarget. 2017;8:75568-75576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | McGowan SE, Burnet NG, Lomax AJ. Treatment planning optimisation in proton therapy. Br J Radiol. 2013;86:20120288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 21. | Hong TS, DeLaney TF, Mamon HJ, Willett CG, Yeap BY, Niemierko A, Wolfgang JA, Lu HM, Adams J, Weyman EA. A prospective feasibility study of respiratory-gated proton beam therapy for liver tumors. Pract Radiat Oncol. 2014;4:316-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Lock MI, Klein J, Chung HT, Herman JM, Kim EY, Small W, Mayr NA, Lo SS. Strategies to tackle the challenges of external beam radiotherapy for liver tumors. World J Hepatol. 2017;9:645-656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |