Published online Oct 27, 2018. doi: 10.4254/wjh.v10.i10.708
Peer-review started: July 2, 2018
First decision: July 19, 2018
Revised: August 2, 2018
Accepted: August 6, 2018
Article in press: August 7, 2018
Published online: October 27, 2018
Processing time: 119 Days and 20.8 Hours
Adiponectin is known to play primary roles in the regulation of systemic glucose homeostasis and lipid metabolism. Interestingly, emerging evidence indicates beneficial effects of adiponectin on liver fibrosis; however, the exact mechanisms of this action remain unclear. Herein, we aimed to summarize the recent findings regarding the role of adiponectin in liver fibrogenesis and update the current comprehensive knowledge regarding usefulness of adiponectin-based treatments in liver fibrosis. Adiponectin has been demonstrated to have an anti-fibrotic action in the liver by blocking the activation of hepatic stellate cell-mediated adenosine monophosphate-activated protein kinase and peroxisome proliferator-activated receptor-alpha pathways, which in turn diminish the expression of pro-fibrotic genes. In addition, hyperadiponectinemia was noted in patients with various chronic liver diseases (CLDs)-related liver fibrosis. An increase in circulating adiponectin levels was also found to be associated with the development of liver fibrosis, indicating a role of adiponectin as a non-invasive biomarker for predicting the progression of liver fibrosis. It is therefore reasonable to speculate that adiponectin may be developed as a new therapeutic candidate for the treatment of liver fibrosis. Nonetheless, future observations are still necessary to fully elucidate the extent of the effects of adiponectin on liver fibrotic outcomes, in order to modify adiponectin as an anti-fibrotic therapy that would speed up fibrosis reversal in patients with CLD.
Core tip: Adiponectin plays a protective role against the development of liver fibrosis via inhibition of hepatic stellate cell activation, induced by specific signal transduction pathways. Among patients with chronic liver diseases (CLDs), hyperadiponectinemia is associated with the degree of liver fibrosis. The potential link between adiponectin and the limited progression of liver fibrosis has accelerated attraction in seeking adiponectin as a target for diagnostic detection tools and novel treatment methods. Nonetheless, additional current therapeutic and clinical trials of adiponectin in liver fibrosis are needed. In this context, we reviewed additional potential therapeutic applications of adiponectin in patients with various CLDs in the context of liver fibrosis.
- Citation: Udomsinprasert W, Honsawek S, Poovorawan Y. Adiponectin as a novel biomarker for liver fibrosis. World J Hepatol 2018; 10(10): 708-718
- URL: https://www.wjgnet.com/1948-5182/full/v10/i10/708.htm
- DOI: https://dx.doi.org/10.4254/wjh.v10.i10.708
Liver fibrosis is a leading cause of morbidity and mortality associated with end-stage liver complications in patients with chronic liver disease (CLD). It is presently recognized a reversible wound-healing reaction to chronic hepatic damage. The morphological characteristics of liver fibrosis include the excessive accumulation of extracellular matrix (ECM) components, mainly fibrillar collagens[1]. The complexity of liver fibrosis has hindered attempts to understand its pathology, which remains unclear. However, there is a wide spectrum of stimuli known to influence liver fibrogenesis, such as drugs, alcohol abuse, toxins, metabolic disorders, viral infections, and cholestasis[2]. If the underlying cause of hepatic fibrosis cannot be ameliorated, the majority of CLD patients with severe hepatic fibrosis will develop cirrhosis, hepatic failure, and hepatocellular carcinoma (HCC) and ultimately require hepatic transplantation. This has driven researchers toward the search and development of effective anti-fibrotic approaches focused on constraining the growth of fibrogenic cells and/or prohibiting the synthesis of ECM molecules, which would be helpful in improving the clinical outcomes of patients with CLD. Hepatic stellate cells (HSCs) are classically recognized as primary fibrogenic cells in the liver. Given that HSCs are the primary source of activated myofibroblasts and portal fibroblasts, HSCs unsurprisingly take part in enhancing the synthesis of ECM components and modulating matrix degradation in the injured liver. Generally, quiescent HSCs are dormant and their activity is to deposit retinoids. In response to liver injury, HSCs undergo an activation process, and become myofibroblast-like cells that secrete various cytokines/growth factors and produce ECM proteins[3]. Consequently, elucidating the molecular mechanisms of liver fibrosis and their relevance to HSCs is of paramount importance for the discovery of new therapeutic targets.
Adiponectin, a 28 kDa protein adipocytokine, is mainly produced and secreted into the circulation by white adipose tissue. The primary function of adiponectin is the regulation of carbohydrate and lipid metabolism. However, the full extent of its biological action remains to be elucidated, with a variety of effects on different cell and tissue types, including its immune modulatory, anti-inflammatory[4], and anti-fibrotic properties[5,6]. Regarding its protective effects, an experimental study demonstrated the extensive development of liver fibrosis in adiponectin-knockout mice[6]. Acting via transmembrane receptors, adiponectin regulates HSC proliferation, as well as migration, and induces their apoptosis through the activation of adenosine monophosphate-activated protein kinase (AMPK)[7]. Moreover, adiponectin can attenuate HSC activation and suppress the expression of pro-fibrogenic genes, including collagen I, transforming growth factor-beta 1 (TGF-β1), and alpha-smooth muscle actin (α-SMA)[8], leading to the inhibition of liver fibrogenesis. With such potent effects on HSCs against liver fibrosis, adiponectin may be developed as a novel therapeutic agent in liver fibrosis. As adiponectin can be detected in the circulation and exerts its effects on various cells, it may have prognostic and diagnostic value for several human diseases. Interestingly, hyperadiponectinemia has been documented as highly prevalent in patients with CLD and liver fibrosis[9], thereby establishing the possible influence of adiponectin levels in the development and progression of liver fibrosis. Nevertheless, the underlying mechanisms of association between hyperadiponectinemia and liver fibrosis have yet to be completely elucidated. Therefore, the purpose of this minireview is to summarize an update on experimental and clinical studies that have been focused on the promising beneficial impacts of adiponectin on the treatment of liver fibrosis associated with many aspects of CLDs.
The articles published between 2000-2018 were searched manually from the PubMed and Scopus using the following keywords or combination of keywords: “Adiponectin”, “Adiponectin levels”, “Liver disease”, and “Liver fibrosis”. Initially, titles and abstracts related to the keywords were screened, and further full articles were evaluated for inclusion. Human clinical studies of any design providing circulating adiponectin levels associated with the severity of liver fibrosis in patients with various CLDs were eligible for this review. Articles not written in English-language, letters to the editor, case reports/series, and editorials were excluded from this review. No restrictions on gender, ethnic background, number of study subjects, or publishing year were applied.
Human adiponectin encoded by the Adipo Q gene spanning 17 kb on chromosome locus 3q27 is a multimeric protein hormone and exerts diverse biological functions. The encoded protein comprises an N-terminal signal sequence being a collagenous domain and a C-terminal globular domain maintaining biological properties after cleavage[10]. Pre-secretion, post-translational mechanisms (e.g., hydroxylation and glycosylation) occurring in the collagenous domain of adiponectin at the four lysines have been shown to improve the activity of sub-physiological levels of insulin, which leads to the inhibition of gluconeogenesis in liver cells[11]. The globular domains of adiponectin form three major complexes including trimers, hexamers, and high-molecular-weight (HMW) multimers, classically existing in the circulation[12]. It seems clear that accurate adiponectin folding and assembly are an important step in regulating its complex distribution in the circulation. Although the different oligomeric complexes distributed in the circulation have distinct downstream biological effects on specific target tissues, HMW adiponectin, the dominant form in the circulation, is considered a marker for disease-associated adipocyte dysfunctions[13]. Among adipocytokines, circulating levels of adiponectin have been observed at high levels in healthy individuals, with approximately 0.01% of the total circulating protein ranging from 5 to 30 μg/mL[14]. On the other hand, hypoadiponectinemia has been previously associated with metabolic alterations, including insulin resistance, dyslipidemia, and atherosclerosis[15-17]. It is noteworthy that a physiological level of circulating adiponectin is important for defense against metabolic disorders and may be related to other chronic diseases including chronic obstructive pulmonary disease[18], chronic kidney disease[19], and knee osteoarthritis[20]. Notwithstanding, the precise mechanisms regulating adiponectin levels in the human body remain poorly understood. It has been suggested that there are multiple factors with important roles in regulating adiponectin levels in the human body, including genetics, mechanisms affecting its clearance, and post-translational modifications associated with controlling adiponectin gene expression[21-23]. Moreover, the regulation of adiponectin receptors is thought to be important for facilitating essential physiological functions of adiponectin.
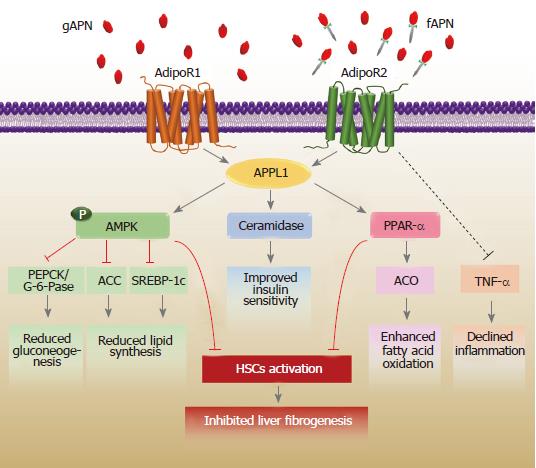
The adiponectin receptors, through which adiponectin acts, include seven-transmembrane domains, consisting of two predominant isoforms: adiponectin receptor type 1 (adipo R1) and adiponectin receptor type 2 (adipo R2). These 2 major receptors have been identified in numerous tissues. In human tissues, both of them are observed in the brain and peripheral tissues. However, adipo R1 is ubiquitously detected, most abundantly in the skeletal muscle. The major expression of adipo R2 is in the liver[24]. The adiponectin receptors bind globular and full-length adiponectin with different affinities. The adipoR1 has a greater affinity for globular adiponectin, whereas adipo R2 has an intermediate affinity for both isoforms. Even though the adiponectin receptors comprise seven-transmembrane domains, their structure and functions are distinct from those of G protein-coupled receptors. Physiologically, the engagement of adiponectin with adipo R1 stimulates the phosphorylation of AMPK, which results in a concomitant suppression of energy-consuming biosynthetic pathways, including lipid synthesis and gluconeogenesis. A second common pathway activated by the adiponectin-adipo R2 axis is the peroxisome proliferator-activated receptor-alpha (PPAR-α) signaling pathway, which regulates fatty acid beta-oxidation[25]. Collectively, the aforementioned pathways boost catabolic processes to renew cellular energy under conditions of energy stress. From a classical perspective, adiponectin takes part in controlling glucose and lipid metabolism, through which it exerts a multitude of beneficial effects such as controlling insulin sensitivity. It is conceivable that adiponectin interacting with its distinct receptors influences the stimulation of an appropriate signaling pathway that becomes different in liver pathology.
It is commonly recognized that adiponectin regulates the metabolism of both glucose and lipid in the liver, and has been implicated in inhibiting gluconeogenesis, as well as activating fatty acid oxidation and glycolytic pathways. These metabolic impacts of adiponectin are activated by the stimulation of adipo R1 and adipo R2. Following the binding of adiponectin to its receptors, the downstream signaling effects of these receptors are associated with the activation of AMPK and the PPAR-α cascade. As a crucial downstream effector of Adipo R1, AMPK is essentially an energy-sensing gauge that is stimulated by AMP and inhibited by ATP. Activation of AMPK inhibits the transcriptional activity of glucose-6-phosphatase (G-6-Pase) and phosphoenolpyruvate carboxykinase (PEPCK), which in turn decreases gluconeogenesis. Through the phosphorylation of acetyl-CoA carboxylase (ACC) converted into an inactive form and malonyl-CoA catalysis, AMPK also induces fatty acid degradation. Malonyl-CoA, the product of the carboxylase reaction, plays an essential role in inhibiting carnitine palmitoyl transferase-1 (CPT-1), which prevents the transport of long-chain fatty acyl-CoA into the mitochondria, resulting in reduced fatty acid biosynthesis. In addition to its effects mediated by the inactivation of PEPCK, G-6-Pase, and ACC signaling, AMPK phosphorylation can limit the activity of sterol regulatory element binding protein-1c (SREBP-1c), which is a transcription factor regulating lipid combustion in the liver[26,27]. This action can cause a reduction in hepatic triglyceride content. It is comprehensible that adiponectin-AMPK signaling can promote fatty acid catabolism and inhibit gluconeogenesis and triglyceride production in the hepatocytes[28]. In parallel with its stimulation of AMPK, adiponectin-adipo R2 axis activates the PPAR-α signaling cascade that increases fatty acid combustion and energy consumption. This mechanism leads to reduced triglyceride accumulation in the liver and thus, increased insulin sensitivity[29].
Although the triggering of adipo R1 and adipo R2 reportedly controls glucose and lipid metabolism by the stimulation of AMPK and PPAR-α signaling cascades, there are additional signaling molecules related to a wide range of beneficial systemic effects of adiponectin in the liver. The pleiotropic actions of adiponectin have been reportedly associated with the activation of its cognate receptor-mediated stimulation of ceramidase activity[30]. Ceramidase is known to take a primary part in the control of ceramide and sphingosine metabolism, which results in the breakdown of ceramide to produce sphingosine for phosphorylation to sphingosine-1-phosphate. Ceramide and sphingosine comprise an important class of bioactive lipids associated with changes in insulin sensitivity, inflammation, and survival[31]. In 2017, Holland et al[32] also demonstrated the significant involvement of adiponectin in declining intracellular ceramide levels, which was accompanied by increased ceramidase activity. This peculiar effect of adiponectin-induced ceramidase signaling was supported in transgenic mice exhibiting an overexpression of adiponectin receptors. Overexpression of adiponectin receptors ameliorated ceramidase activity and induced metabolic improvements in glucose and lipid homeostasis, in addition to insulin sensitivity[32]. These observations provide further evidence regarding the beneficial effects of adiponectin, especially its insulin-sensitizing action, in which adiponectin is activated by its own receptor-mediated stimulation of ceramidase activity.
Besides its primary roles in regulating insulin sensitivity and fatty acid metabolism, adiponectin has been shown to possess anti-inflammatory effects. In particular, adiponectin expands its hepatoprotective effects by decreasing inflammation through blocking the activation of nuclear factor-kappa B (NF-κB)[33] and suppressing the release of tumor necrosis factor-alpha (TNF-α), which is an inflammatory cytokine[4]. The TNF-α that is predominantly secreted by HSCs and Kupffer cells in the liver has a critical effect on hepatic damage, given its capability to promote inflammation and apoptosis in liver cells under the appropriate circumstances of oxidative stress[34]. Adiponectin also limits the production of interleukin-1β, which is a pro-inflammatory cytokine that is responsible for liver injury and initiates the secretion of interleukin-10[35]. The anti-inflammatory actions of adiponectin have also been implicated in macrophage dysfunction[36], through which it suppresses the growth and movement of vascular smooth muscle cells[37] and modulates lymphopoiesis[38]. The pleiotropic biological actions of adiponectin in the liver are illustrated in Figure 1.
One of the critical mechanisms by which adiponectin exerts an anti-fibrogenic effect on the liver is characterized by the induction of the activated phenotype of HSCs. It has been well established that activated HSCs constitutively express both types of adiponectin receptors[39,40], suggesting a potential physiologic action for adiponectin receptor-mediated liver fibrosis in HSCs. As described above, the alteration of HSCs into myofibroblasts is the keystone event of the pathogenesis of liver fibrosis during liver injury. Adiponectin was shown to repress the growth and movement of mouse HSCs stimulated by platelet-derived growth factor (PDGF)[7], which is considered a marker for activated HSCs. An experimental study further demonstrated that adiponectin diminished the effect of TGF-β1-induced expression of connective tissue growth factor (CTGF, also known as fibrogenic gene) on HSCs via suppression of the nuclear translocation of mothers against decapentaplegic homolog 2 (SMAD2)[8]. This is important evidence that supports the hypothesis that adiponectin may have anti-fibrogenic effects that are independent of metabolic actions. In this context, overexpression of adipoR2 in mice was found to have a protective role against the progression of liver fibrosis via the enhancement of PPAR-α signaling with reduced expression of TGF-β1[41]. Moreover, in cultured HSCs, the enhancement of adiponectin expression remarkably worsened HSC proliferation, as well as the expression of α-SMA, and prevented liver fibrosis through the modulation of caspase-mediated HSC apoptosis[8]. Adiponectin expression is considerably down-regulated in activated HSCs, but it is abundantly present in the quiescent phenotype, suggesting a permissive role of adiponectin that favors the quiescent state of HSCs in the physiology of liver fibrosis. In this regard, adiponectin diminishes the development of liver fibrosis by modulating the activity of inhibitor of cytokine signaling-3 (SOCS-3) mediated by the long-form of the leptin receptor (Ob-Rb) and inducing the expression and activation of protein tyrosine phosphatase 1B (PTP1B). As negative regulators of Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling, SOCS-3 and PTP1B inhibit JAK-2/STAT-3, which in turn regulate the formation of extracellular components, including tissue inhibitor of metalloproteinases-1 (TIMP-1) and matrix metalloproteinase-1 (MMP-1)[42].
Indeed, adiponectin protects liver injury and reverses the activation of HSCs in animal models. These effects support the possibility of its role against liver damage and fibrogenesis in humans. In pursuit of developing the clinical advantage of adiponectin as a potential predictor for the progression of liver fibrosis, a number of studies have assessed circulating adiponectin levels in a wide range of CLDs.
Generally, liver fibrosis is a reversible physiologic and pathologic event in response to chronic liver injury that leads to liver cirrhosis and eventually end-stage liver disease. The reversal and prevention of these conditions have come to be critical endpoints in clinical trials with novel anti-fibrotic therapies. A liver biopsy has long remained a definitive diagnostic approach for the staging of fibrosis; however, highly efficient and specific non-invasive tools to assess liver fibrosis will be necessary for monitoring the development and progression of disease. Taking into account the risks for complications with respect to sampling error from a needle liver biopsy, there has been enormous interest in the search and development of noninvasive biological markers. Physical characteristics of adiponectin, e.g., high stability in the circulation, little diurnal variation, and great abundance in the human body have made it a promising biomarker in medical contexts for clinical investigation, prognosis, and therapy of liver fibrosis in patients with CLD.
Given that adiponectin has been shown to have predominantly hepatoprotective and anti-fibrogenic effects in conditions of liver injury, whether circulating adiponectin levels are associated with an increased risk of development of liver fibrosis in CLD remains to be determined. Several studies have been focused on investigating the possible associations between adiponectin concentration and the stage of liver fibrosis in various CLDs, including, liver cirrhosis, biliary atresia (BA), hepatitis C viral infection (HCV), hepatitis B viral infection (HBV), non-alcoholic fatty liver disease (NAFLD), and non-alcoholic steatohepatitis (NASH), as summarized in Table 1. First, in a cross-sectional study of 232 fasting patients with CLD, serum adiponectin levels were significantly elevated in patients with cirrhosis, as compared to those patients with other liver diseases. Serum adiponectin concentrations were also positively associated with surrogate biomarkers of liver fibrogenesis, including transient elastography, fasting serum bile acids, and hyaluronate in patients with CLD[9]. This study supports a recent report in which adiponectin levels were significantly elevated in patients with cirrhosis compared to controls and also associated with the severity of hepatic dysfunction in those patients[43]. The possible relevance of adiponectin in CLD has been attested by our previous studies, which evaluated circulating adiponectin concentrations in patients with cholestatic BA. A case-control study of 106 patients with BA and 40 healthy controls by Udomsinprasert et al[44] demonstrated that patients with BA exhibited considerably greater levels of serum adiponectin than healthy controls. Notably, serum adiponectin levels were positively correlated with the degree of liver fibrosis in patients with BA[44]. When assessing the relationship between circulating adiponectin levels and clinical parameters in patients with BA - particularly liver stiffness scores, serum adiponectin concentrations were also observed to be remarkably higher in patients with significant liver fibrosis than those with insignificant fibrosis, and a direct correlation of serum adiponectin and the severity of liver fibrogenesis was reported[45].
| Reference | Yr | Diagnosis | Study design | Subjects | Significant results |
| Hyperadiponectinemia | |||||
| Balmer et al[9] | 2010 | CLD | Cross-sectional study | 232 fasting patients with CLD, 64 with NAFLD, 71 patients with viral hepatitis, 18 patients with autoimmune disease, 3 patients with alcohol-induced liver disease, 31 patients with elevated liver enzyme of unknown origin, and 45 patients with cirrhosis | Adiponectin levels were substantially increased in cases of cirrhosis Adiponectin levels were positively correlated with surrogate markers of hepatic fibrosis, including transient elastography, fasting serum bile acids, and hyaluronate |
| da Silva et al[43] | 2018 | Cirrhosis | Case-control study | 122 patients with cirrhosis and 30 healthy controls | Patients with CLD had higher adiponectin levels than controls Adiponectin levels were also associated with the severity of liver dysfunction and worse prognosis in those patients |
| Udomsinprasert etal[44] | 2012 | BA | Case-control study | 106 patients with BA and 40 healthy controls | Serum adiponectin levels were significantly higher in BA patients than in healthy controls Adiponectin levels were associated with the severity of fibrosis in BA patients. |
| Honsawek et al[45] | 2011 | BA | Case-control study | 60 patients with BA and 20 healthy controls | BA patients with significant liver fibrosis exhibited remarkably greater serum adiponectin than insignificant fibrosis Serum adiponectin was positively correlated with the degree of fibrosis. |
| Carvalho et al[46] | 2018 | HCV | Case-control study | 33 patients with untreated HCV infection and 30 healthy controls | Patients with HCV infection had higher adiponectin levels, especially those with women |
| Korah et al[47] | 2013 | HCV | Case-control study | 45 untreated men with chronic HCV genotype 4, and 15 healthy men | Serum adiponectin levels were significantly elevated in hepatic fibrosis, but decreased in steatosis |
| Sumie et al[48] | 2011 | HCV | Case-control study | 97 patients with HCC and chronic HCV infection, and 97 patients (controls) with underlying disease | Serum total and HMW adiponectin levels were predictors of liver fibrosis in HCC patients, in response to chronic HCV infection |
| Corbetta et al[49] | 2011 | HCV | Case-control study | 54 patients with chronic HCV hepatitis and healthy controls | Serum adiponectin levels were higher in patients with chronic HCV hepatitis Adiponectin levels were significantly related to the severity of fibrosis in patients with chronic HCV hepatitis |
| Derbala et al[50] | 2009 | HCV | Case-control study | 92 patients with chronic HCV genotype 4 and 66 healthy controls | Adiponectin levels were associated with hepatic fibrosis and inflammation |
| Hsu et al[51] | 2015 | HBV | Case-control study | 187 patients with chronic HBV infection and 187 without chronic HBV infection | Serum adiponectin levels were remarkably correlated with advanced liver fibrosis in elder male HBeAg-negative patients |
| Hui et al[52] | 2007 | HBV | Cross-sectional study | 100 patients with HBV | Patients with fibrosis reduction had a marked decline in serum adiponectin levels after antiviral therapy Adiponectin levels were significantly correlated with fibrosis stage |
| Hypoadiponectinemia | |||||
| Lucero et al[54] | 2017 | NAFLD | Cross-sectional study | 36 patients with NAFLD associated with metabolic syndrome and 24 metabolic syndrome patients without NAFLD | Adiponectin levels were significantly lower in NAFLD patients with metabolic syndrome than those patients without metabolic syndrome Adiponectin levels were associated with metabolic parameters and the degree of liver fibrosis |
| Nazal et al[55] | 2010 | NAFLD | Case-control study | 70 patients with NAFLD and 69 normal controls | NAFLD patients had significantly lower serum adiponectin levels than controls Adiponectin levels were independently associated with liver fibrosis |
| Savvidou et al[56] | 2009 | NAFLD | Cross-sectional study | 42 patients with NAFLD | Adiponectin levels were negatively associated with higher stages of fibrosis Adiponectin levels were independent predictors of advanced fibrosis |
| Yoneda et al[57] | 2007 | NAFLD | Cross-sectional study | 248 patients with NAFLD and type 2 diabetes | A reduction in levels of serum adiponectin was independently associated with the severity of hepatic fibrosis in NAFLD patients with type 2 diabetes |
| Musso et al[58] | 2005 | NASH | Case-control study | 20 patients with biopsy-proven NASH and 45 healthy controls | Serum adiponectin levels were significantly reduced in the NASH group, as compared to control groups Adiponectin levels correlated with the severity of hepatic steatosis and fibrosis |
In addition to its significant involvement in the clinical outcome of BA with respect to the degree of liver fibrosis, a number of studies have reported the relationships between hyperadiponectinemia and the clinical parameters of liver fibrosis in cohorts with hepatitis viral infection. A more recent study by Carvalho et al[46] found that patients with HCV infection had significantly greater adiponectin levels than healthy controls. In 2013, Korah et al[47] examined serum adiponectin levels in patients with chronic HCV genotype 4 associated with steatosis and fibrosis. They also found that 45 men with chronic HCV genotype 4 and advanced fibrosis had significantly increased adiponectin levels, whereas these levels were remarkably reduced in patients with steatosis. Sumie et al[48] added another piece of supporting data, revealing that serum adiponectin levels were predictors of liver fibrosis in patients with HCC, in response to chronic HCV infection. Likewise, when Corbetta et al[49] analyzed circulating adiponectin levels in 54 patients with chronic HCV, they reported increased adiponectin levels. The investigators also reported that serum adiponectin levels were further correlated with the severity of liver fibrosis in those patients. The aforementioned findings suggest that monitoring adiponectin levels may be employed to improve care for liver fibrosis in patients with HCV infection. In support of this hypothesis, a study of Derbala et al[50] also examined circulating adiponectin levels in patients with chronic HCV. They observed that adiponectin concentration was directly associated with hepatic fibrosis and inflammation in the chronic HCV subjects. In addition, strong evidence for a possible correlation between adiponectin levels and progressive liver fibrosis in patients with chronic HBV was recently described by Hsu et al[51]. The authors showed that serum adiponectin levels were independently associated with the development of liver fibrosis in patients with HBV. This information supports the observations of Hui and coworkers, which showed that serum adiponectin levels were significantly reduced in patients with reduced fibrosis after antiviral therapy. In particular, adiponectin levels were also positively associated with the stage of fibrosis[52]. All of these findings support the notion that adiponectin has non-classical metabolic actions, including a potential capacity to suppress fibrosis and therefore, may link its role to the development of hepatic fibrogenesis.
Even though former clinical studies have demonstrated a direct relationship of adiponectin and liver fibrogenesis in patients with various CLDs, the exact mechanism responsible for an increase in circulating adiponectin in liver fibrosis remains uncertain. The possible explanation for these findings might be attributed to a reduction in its clearance. In CLD patients with liver fibrosis, declined adiponectin clearance could result from reduced uptake of adiponectin by liver sinusoidal endothelial cells (LSECs), which may lead to elevated adiponectin levels in the circulation. It is widely known that dysfunction of LSECs is one of pathologic events in liver fibrogenesis. In the healthy liver, LSECs generally promote HSCs quiescence. During the process of liver fibrosis, LSECs undergo phenotypic changes with the loss of several receptors and LSECs fenestration, leading to the capillarization of liver sinusoids and the abnormality of various substances uptake[3]. It has been shown that adiponectin levels and adipoR2 expression are decreased in the LSECs response to liver injury[53]. These phenomena may help explain why hyperadiponectinemia has been observed in CLDs patients with liver fibrosis.
In apparent contrast to the aforementioned studies, circulating adiponectin levels have been shown to be reduced in patients with steatosis and steatohepatitis, such as those with NAFLD and NASH. Most recently, Lucero et al[54] investigated systemic adiponectin levels in 36 patients with NAFLD related to metabolic syndrome and 24 metabolic syndrome patients without NAFLD. They reported that adiponectin levels were significantly elevated in NAFLD patients with metabolic syndrome when compared to those patients without metabolic syndrome. Besides, circulating adiponectin levels were correlated with metabolic parameters and the degree of liver fibrosis in NAFLD patients. Furthermore, a case-control study of 70 patients with NAFLD and 69 healthy controls conducted by Nazal et al[55], explored a possible correlation between adiponectin levels and NAFLD pathology. The authors reported that plasma adiponectin levels were markedly lower in patients with NAFLD than in controls, and they were inversely related to the presence of liver fibrosis. The findings of Savvidou et al[56] provide support to a negative association between adiponectin concentrations and higher stages of fibrosis in patients with NAFLD, suggesting that adiponectin levels can be used as predictors of advanced fibrosis. Supporting this finding, a large cohort study of 248 patients with NAFLD and type 2 diabetes found that reduced adiponectin levels were independently associated with the degree of liver fibrosis[57]. Finally, in a case-control study of 60 patients with NAFLD and 60 healthy controls, patients with NAFLD had markedly reduced plasma adiponectin levels, when compared to controls. Notably, a decline in adiponectin levels was found to be closely associated with the degree of liver fibrosis. Similarly, when Musso et al[58] determined adiponectin levels in 20 patients with biopsy-proven NASH and 45 healthy controls, they observed that low adiponectin levels were associated with the severity of hepatic steatosis and fibrosis in patients with NASH. Based on these findings, hypoadiponectinemia has been proposed as a contributory factor to the development of metabolic dysfunction in NAFLD and NASH. The reasons for these conflicting findings remain unexplained. These are likely due to differences in populations, disease advancement, or measurements applied, or to incomplete control of confounding variables.
The contrasting results regarding hypoadiponectinemia in patients with NAFLD and those with NASH compared to those with other CLDs could be partly attributed to the differences in pathophysiology of the diseases. Indeed, hypoadiponectinemia has been suggested to play a pathogenic role in the pancreatic-cell dysfunction observed in both NAFLD and NASH[59], and accumulated visceral fat can cause a decline in levels of circulating adiponectin[60]. Regardless of the role of environmental and genetic factors, adiponectin appears to be strongly associated with the hepatic phenotype, which is a major cause of morbidity in NAFLD. It has also been discovered that the adiponectin promoter polymorphism rs266729 was associated with the susceptibility of NAFLD, and the subjects with the GG genotype of rs266729 exhibited substantially lesser adiponectin values than those subjects with the GC or CC genotypes[61]. It is tempting to speculate that genetic variation of adiponecin and lifestyles choices causing visceral fat deposition/obesity may lead to reduced circulating adiponetin levels in patients with NAFLD and NASH. From these reports regarding its significant association with liver fibrosis, it is apparent that adiponectin levels in the circulation may be a prognosticator for the development of liver fibrosis in various CLDs.
Given that accumulating data correlate the hepatoprotective functions of adiponectin with restricting HSC proliferation and myofibroblast restoration, which are both pivotal mechanisms of liver fibrogenesis, increases in adiponectin levels and its agonists could be an alternative treatment option for the protection and therapy of liver fibrosis. Currently, there are two prime approaches to increase adiponectin concentrations in the body.
Firstly, recombinant adiponectin, or drugs that bypass adiponectin by directly stimulating AMPK, have been extensively utilized in preclinical models. For example, trials utilizing ADP355, which is an adiponectin-like small synthetic peptide agonist, show promise in inhibiting tumorigenesis in a mouse xenograft breast cancer model[62]. Regarding its protective effects on liver fibrosis, an adiponectin-mimetic peptide analog (ADP355) that docks into the adiponectin receptors binding pocket, possesses potency in modulating multiple anti-fibrogenic mechanisms, in particular, AMPK phosphorylation, and has been shown to eliminate hepatic fibrosis in mice with carbon tetrachloride (CCl4)-induced liver fibrosis. These mechanisms are mediated by the diminishing expression of pro-fibrogenic genes, including α-SMA, desmin, CTGF, TGF-β1, and TIMP-1, but enhanced expression of matrix metalloproteinase-13 (MMP-13) as a marker for restructuring of the collagen matrix[63]. In the light of these considerations, ADP355 may represent an alternative anti-fibrogenic agent in the treatment of liver fibrosis. However, due to the lack of clinical data concerning liver toxicity, further studies assessing the toxic and off-target effects of this agent will be necessary to determine the practicability and clinical efficiency of adiponectin for practical applications
In contrast, one additional method would be to characterize the classes of substances that could bring about an increase in the secretion or expression of adiponectin. Recently, a few studies have suggested that activation of PPAR-γ can enhance the expression and circulating concentrations of adiponectin via regulation of gene transcription[64]. The PPAR-γ cascade is therefore interesting, as it may be associated with direct targets that can modify adiponectin for potential clinical applications. Among the drugs presently in clinical use that are PPAR-γ agonists, the thiazolidinediones (TZDs) exert beneficial anti-proliferative and anti-inflammatory effects via PPAR-γ activation[65], and have been found to induce adipose tissue to release adiponectin into the circulation[66]. As TZDs have been broadly applied to improve insulin sensitivity in patients with diabetes, we cannot discern the specific conditions under which adiponectin has defensive effects against liver fibrosis. Accordingly, the rigorous identification of new drugs that target adiponectin in the treatment of liver fibrosis is presently even more important. However, further research efforts will be needed to validate the safety and efficacy of adiponectin before a standardized treatment regimen can be established.
The close relationships between the pathology of liver fibrogenesis and clinical appearance of the disease affirm the significance of extended basic and translational studies into the pathogenesis of liver fibrosis. To date, the development of anti-fibrotic therapies in fibrosis and/or cirrhosis has been largely unsuccessful. Considerable research conducted over the past several years has demonstrated the multifaceted and potentially contradicting effects of adiponectin as a novel regulator of liver fibrogenesis. The possible relevance of adiponectin has emerged from studies in cell cultures and animal models, in addition to clinical investigations. Indeed, experimental studies document the multiple effects of adiponectin on limiting HSC proliferation and suppressing the expression of pro-fibrogenic genes through specific signal transduction pathways. The possible effect of adiponectin against liver fibrosis is supported by clinical studies that link hyperadiponectinemia to the severity of liver fibrosis in many liver diseases, including BA, HCV, HBV, and liver cirrhosis; whereas reduced adiponectin levels have been reported to be a key factor in the development of metabolic disorders contributing to NAFLD and NASH. Based on these observations, adiponectin could be a plausible noninvasive biochemical marker identifying the severity of liver fibrosis in patients with CLDs. However, additional research is warranted to better comprehend the precise aspect of adiponectin in the pathogenesis of liver fibrosis, which will help to develop adiponectin as circulating indicator for distinct CLD patients who are at risk of developing liver fibrosis.
The authors would like to thank Assistant Professor Jiraphun Jittikoon for kindly reviewing and proofreading the manuscript.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Thailand
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Citores MJ, Pallav K, Reichert MCC S- Editor: Ji FF L- Editor: A E- Editor: Yin SY
| 1. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3381] [Cited by in RCA: 4123] [Article Influence: 206.2] [Reference Citation Analysis (3)] |
| 2. | Rowe IA. Lessons from Epidemiology: The Burden of Liver Disease. Dig Dis. 2017;35:304-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 3. | DeLeve LD. Liver sinusoidal endothelial cells in hepatic fibrosis. Hepatology. 2015;1740-1746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 345] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 4. | Masaki T, Chiba S, Tatsukawa H, Yasuda T, Noguchi H, Seike M, Yoshimatsu H. Adiponectin protects LPS-induced liver injury through modulation of TNF-alpha in KK-Ay obese mice. Hepatology. 2004;40:177-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 312] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 5. | Handy JA, Saxena NK, Fu P, Lin S, Mells JE, Gupta NA, Anania FA. Adiponectin activation of AMPK disrupts leptin-mediated hepatic fibrosis via suppressors of cytokine signaling (SOCS-3). J Cell Biochem. 2010;110:1195-1207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Kamada Y, Tamura S, Kiso S, Matsumoto H, Saji Y, Yoshida Y, Fukui K, Maeda N, Nishizawa H, Nagaretani H. Enhanced carbon tetrachloride-induced liver fibrosis in mice lacking adiponectin. Gastroenterology. 2003;125:1796-1807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 354] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 7. | Adachi M, Brenner DA. High molecular weight adiponectin inhibits proliferation of hepatic stellate cells via activation of adenosine monophosphate-activated protein kinase. Hepatology. 2008;47:677-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 8. | Ding X, Saxena NK, Lin S, Xu A, Srinivasan S, Anania FA. The roles of leptin and adiponectin: a novel paradigm in adipocytokine regulation of liver fibrosis and stellate cell biology. Am J Pathol. 2005;166:1655-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 177] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 9. | Balmer ML, Joneli J, Schoepfer A, Stickel F, Thormann W, Dufour JF. Significance of serum adiponectin levels in patients with chronic liver disease. Clin Sci (Lond). 2010;119:431-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Zhao L, Fu Z, Liu Z. Adiponectin and insulin cross talk: the microvascular connection. Trends Cardiovasc Med. 2014;24:319-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Wang Y, Lam KS, Yau MH, Xu A. Post-translational modifications of adiponectin: mechanisms and functional implications. Biochem J. 2008;409:623-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 293] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 12. | Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, Hara K, Hada Y, Vasseur F, Froguel P. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem. 2003;278:40352-40363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 762] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 13. | Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, Engel J, Brownlee M, Scherer PE. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications fpr metabolic regulation and bioactivity. J Biol Chem. 2003;278:9073-9085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 798] [Cited by in RCA: 820] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 14. | Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746-26749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2259] [Cited by in RCA: 2303] [Article Influence: 76.8] [Reference Citation Analysis (0)] |
| 15. | Matsuda M, Shimomura I, Sata M, Arita Y, Nishida M, Maeda N, Kumada M, Okamoto Y, Nagaretani H, Nishizawa H. Role of adiponectin in preventing vascular stenosis. The missing link of adipo-vascular axis. J Biol Chem. 2002;277:37487-37491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 597] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 16. | Okamoto Y, Kihara S, Funahashi T, Matsuzawa Y, Libby P. Adiponectin: a key adipocytokine in metabolic syndrome. Clin Sci (Lond). 2006;110:267-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 312] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 17. | Chen MC, Lee CJ, Yang CF, Chen YC, Wang JH, Hsu BG. Low serum adiponectin level is associated with metabolic syndrome and is an independent marker of peripheral arterial stiffness in hypertensive patients. Diabetol Metab Syndr. 2017;9:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Bianco A, Mazzarella G, Turchiarelli V, Nigro E, Corbi G, Scudiero O, Sofia M, Daniele A. Adiponectin: an attractive marker for metabolic disorders in Chronic Obstructive Pulmonary Disease (COPD). Nutrients. 2013;5:4115-4125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Lim CC, Teo BW, Tai ES, Lim SC, Chan CM, Sethi S, Wong TY, Sabanayagam C. Elevated serum leptin, adiponectin and leptin to adiponectin ratio is associated with chronic kidney disease in Asian adults. PLoS One. 2015;10:e0122009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Honsawek S, Chayanupatkul M. Correlation of plasma and synovial fluid adiponectin with knee osteoarthritis severity. Arch Med Res. 2010;41:593-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Kedenko L, Lamina C, Kiesslich T, Kapur K, Bergmann S, Waterworth D, Heid IM, Wichmann HE, Kedenko I, Kronenberg F. Genetic polymorphisms of the main transcription factors for adiponectin gene promoter in regulation of adiponectin levels: association analysis in three European cohorts. PLoS One. 2012;7:e52497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Jin D, Sun J, Huang J, Yu X, Yu A, He Y, Li Q, Yang Z. Peroxisome proliferator-activated receptor γ enhances adiponectin secretion via up-regulating DsbA-L expression. Mol Cell Endocrinol. 2015;411:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Shehzad A, Iqbal W, Shehzad O, Lee YS. Adiponectin: regulation of its production and its role in human diseases. Hormones (Athens). 2012;11:8-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 185] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 24. | Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2258] [Cited by in RCA: 2315] [Article Influence: 105.2] [Reference Citation Analysis (0)] |
| 25. | Yamauchi T, Kadowaki T. Adiponectin receptor as a key player in healthy longevity and obesity-related diseases. Cell Metab. 2013;17:185-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 321] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 26. | Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, Park O, Luo Z, Lefai E, Shyy JY. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011;13:376-388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1426] [Cited by in RCA: 1365] [Article Influence: 97.5] [Reference Citation Analysis (0)] |
| 27. | Shklyaev S, Aslanidi G, Tennant M, Prima V, Kohlbrenner E, Kroutov V, Campbell-Thompson M, Crawford J, Shek EW, Scarpace PJ. Sustained peripheral expression of transgene adiponectin offsets the development of diet-induced obesity in rats. Proc Natl Acad Sci USA. 2003;100:14217-14222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 178] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 28. | Misra P. AMP activated protein kinase: a next generation target for total metabolic control. Expert Opin Ther Targets. 2008;12:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 29. | Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3510] [Cited by in RCA: 3504] [Article Influence: 146.0] [Reference Citation Analysis (0)] |
| 30. | Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, Davis KE, Bikman BT, Halberg N, Rutkowski JM. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med. 2011;17:55-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 753] [Cited by in RCA: 723] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 31. | Yang G, Badeanlou L, Bielawski J, Roberts AJ, Hannun YA, Samad F. Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome. Am J Physiol Endocrinol Metab. 2009;297:E211-E224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 243] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 32. | Holland WL, Xia JY, Johnson JA, Sun K, Pearson MJ, Sharma AX, Quittner-Strom E, Tippetts TS, Gordillo R, Scherer PE. Inducible overexpression of adiponectin receptors highlight the roles of adiponectin-induced ceramidase signaling in lipid and glucose homeostasis. Mol Metab. 2017;6:267-275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 133] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 33. | Lira FS, Rosa JC, Pimentel GD, Seelaender M, Damaso AR, Oyama LM, do Nascimento CO. Both adiponectin and interleukin-10 inhibit LPS-induced activation of the NF-κB pathway in 3T3-L1 adipocytes. Cytokine. 2012;57:98-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 34. | Liu C, Tao Q, Sun M, Wu JZ, Yang W, Jian P, Peng J, Hu Y, Liu C, Liu P. Kupffer cells are associated with apoptosis, inflammation and fibrotic effects in hepatic fibrosis in rats. Lab Invest. 2010;90:1805-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 112] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 35. | Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun. 2004;323:630-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 602] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 36. | Tsatsanis C, Zacharioudaki V, Androulidaki A, Dermitzaki E, Charalampopoulos I, Minas V, Gravanis A, Margioris AN. Adiponectin induces TNF-alpha and IL-6 in macrophages and promotes tolerance to itself and other pro-inflammatory stimuli. Biochem Biophys Res Commun. 2005;335:1254-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 163] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 37. | Arita Y, Kihara S, Ouchi N, Maeda K, Kuriyama H, Okamoto Y, Kumada M, Hotta K, Nishida M, Takahashi M. Adipocyte-derived plasma protein adiponectin acts as a platelet-derived growth factor-BB-binding protein and regulates growth factor-induced common postreceptor signal in vascular smooth muscle cell. Circulation. 2002;105:2893-2898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 488] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 38. | Yokota T, Meka CS, Kouro T, Medina KL, Igarashi H, Takahashi M, Oritani K, Funahashi T, Tomiyama Y, Matsuzawa Y. Adiponectin, a fat cell product, influences the earliest lymphocyte precursors in bone marrow cultures by activation of the cyclooxygenase-prostaglandin pathway in stromal cells. J Immunol. 2003;171:5091-5099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 97] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 39. | Bertolani C, Marra F. Role of adipocytokines in hepatic fibrosis. Curr Pharm Des. 2010;16:1929-1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Caligiuri A, Bertolani C, Guerra CT, Aleffi S, Galastri S, Trappoliere M, Vizzutti F, Gelmini S, Laffi G, Pinzani M. Adenosine monophosphate-activated protein kinase modulates the activated phenotype of hepatic stellate cells. Hepatology. 2008;47:668-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 41. | Tomita K, Oike Y, Teratani T, Taguchi T, Noguchi M, Suzuki T, Mizutani A, Yokoyama H, Irie R, Sumimoto H. Hepatic AdipoR2 signaling plays a protective role against progression of nonalcoholic steatohepatitis in mice. Hepatology. 2008;48:458-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 42. | Handy JA, Fu PP, Kumar P, Mells JE, Sharma S, Saxena NK, Anania FA. Adiponectin inhibits leptin signalling via multiple mechanisms to exert protective effects against hepatic fibrosis. Biochem J. 2011;440:385-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 43. | da Silva TE, Costa-Silva M, Correa CG, Denardin G, Alencar MLA, Coelho MSPH, Muraro-Wildner L, Luiza-Bazzo M, González-Chica DA, Dantas-Correa EB. Clinical Significance of Serum Adiponectin and Resistin Levels in Liver Cirrhosis. Ann Hepatol. 2018;17:286-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 44. | Udomsinprasert W, Honsawek S, Anomasiri W, Chongsrisawat V, Vejchapipat P, Poovorawan Y. Elevated adiponectin is associated with poor outcome in children with biliary atresia. Asian Biomedicine. 2012;6:369-376. |
| 45. | Honsawek S, Chayanupatkul M, Chongsrisawat V, Theamboonlers A, Praianantathavorn K, Udomsinprasert W, Vejchapipat P, Poovorawan Y. Serum adiponectin and transient elastography as non-invasive markers for postoperative biliary atresia. BMC Gastroenterol. 2011;11:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 46. | Carvalho RF, Atta AM, de Oliveira IS, Santos TPS, Santos JPA, Schinoni MI, de Sousa-Atta MLB. Adiponectin levels and insulin resistance among patients with chronic hepatitis C. Acta Trop. 2018;178:258-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 47. | Korah TE, El-Sayed S, Elshafie MK, Hammoda GE, Safan MA. Significance of serum leptin and adiponectin levels in Egyptian patients with chronic hepatitis C virus associated hepatic steatosis and fibrosis. World J Hepatol. 2013;5:74-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 48. | Sumie S, Kawaguchi T, Kuromatsu R, Takata A, Nakano M, Satani M, Yamada S, Niizeki T, Torimura T, Sata M. Total and high molecular weight adiponectin and hepatocellular carcinoma with HCV infection. PLoS One. 2011;6:e26840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 49. | Corbetta S, Redaelli A, Pozzi M, Bovo G, Ratti L, Redaelli E, Pellegrini C, Beck-Peccoz P, Spada A. Fibrosis is associated with adiponectin resistance in chronic hepatitis C virus infection. Eur J Clin Invest. 2011;41:898-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 50. | Derbala M, Rizk N, Al-Kaabi S, Amer A, Shebl F, Al Marri A, Aigha I, Alyaesi D, Mohamed H, Aman H. Adiponectin changes in HCV-Genotype 4: relation to liver histology and response to treatment. J Viral Hepat. 2009;16:689-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 51. | Hsu CS, Liu WL, Chao YC, Lin HH, Tseng TC, Wang CC, Chen DS, Kao JH. Adipocytokines and liver fibrosis stages in patients with chronic hepatitis B virus infection. Hepatol Int. 2015;9:231-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 52. | Hui CK, Zhang HY, Lee NP, Chan W, Yueng YH, Leung KW, Lu L, Leung N, Lo CM, Fan ST. Serum adiponectin is increased in advancing liver fibrosis and declines with reduction in fibrosis in chronic hepatitis B. J Hepatol. 2007;47:191-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 53. | Sun LJ, Yu JW, Shi YG, Zhang XY, Shu MN, Chen MY. Hepatitis C virus core protein induces dysfunction of liver sinusoidal endothelial cell by down-regulation of silent information regulator 1. J Med Virol. 2018;90:926-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 54. | Lucero D, Miksztowicz V, Gualano G, Longo C, Landeira G, Álvarez E, Zago V, Brites F, Berg G, Fassio E. Nonalcoholic fatty liver disease associated with metabolic syndrome: Influence of liver fibrosis stages on characteristics of very low-density lipoproteins. Clin Chim Acta. 2017;473:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 55. | Nazal L, Riquelme A, Solís N, Pizarro M, Escalona A, Burotto M, Méndez JI, Saint-Jean C, Concha MJ, Giovanni S. Hypoadiponectinemia and its association with liver fibrosis in morbidly obese patients. Obes Surg. 2010;20:1400-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 56. | Savvidou S, Hytiroglou P, Orfanou-Koumerkeridou H, Panderis A, Frantzoulis P, Goulis J. Low serum adiponectin levels are predictive of advanced hepatic fibrosis in patients with NAFLD. J Clin Gastroenterol. 2009;43:765-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 57. | Yoneda M, Iwasaki T, Fujita K, Kirikoshi H, Inamori M, Nozaki Y, Maeyama S, Wada K, Saito S, Terauchi Y. Hypoadiponectinemia plays a crucial role in the development of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus independent of visceral adipose tissue. Alcohol Clin Exp Res. 2007;31:S15-S21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 58. | Musso G, Gambino R, Biroli G, Carello M, Fagà E, Pacini G, De Michieli F, Cassader M, Durazzo M, Rizzetto M. Hypoadiponectinemia predicts the severity of hepatic fibrosis and pancreatic Beta-cell dysfunction in nondiabetic nonobese patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2005;100:2438-2446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 148] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 59. | Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1564] [Cited by in RCA: 1532] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 60. | Burgert TS, Taksali SE, Dziura J, Goodman TR, Yeckel CW, Papademetris X, Constable RT, Weiss R, Tamborlane WV, Savoye M. Alanine aminotransferase levels and fatty liver in childhood obesity: associations with insulin resistance, adiponectin, and visceral fat. J Clin Endocrinol Metab. 2006;91:4287-4294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 271] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 61. | Hsieh CJ, Wang PW, Hu TH. Association of adiponectin gene polymorphism with nonalcoholic fatty liver disease in Taiwanese patients with type 2 diabetes. PLoS One. 2015;10:e0127521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 62. | Otvos L Jr, Haspinger E, La Russa F, Maspero F, Graziano P, Kovalszky I, Lovas S, Nama K, Hoffmann R, Knappe D, Cassone M, Wade J, Surmacz E. Design and development of a peptide-based adiponectin receptor agonist for cancer treatment. BMC Biotechnol. 2011;11:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 144] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 63. | Kumar P, Smith T, Rahman K, Thorn NE, Anania FA. Adiponectin agonist ADP355 attenuates CCl4-induced liver fibrosis in mice. PLoS One. 2014;9:e110405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 64. | Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, Nagaretani H, Matsuda M, Komuro R, Ouchi N. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001;50:2094-2099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1280] [Cited by in RCA: 1252] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 65. | Yki-Järvinen H. Thiazolidinediones. N Engl J Med. 2004;351:1106-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1612] [Cited by in RCA: 1541] [Article Influence: 73.4] [Reference Citation Analysis (0)] |
| 66. | Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, Wagner JA, Wu M, Knopps A, Xiang AH. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004;279:12152-12162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 852] [Cited by in RCA: 859] [Article Influence: 39.0] [Reference Citation Analysis (0)] |