Published online Oct 27, 2018. doi: 10.4254/wjh.v10.i10.662
Peer-review started: April 10, 2018
First decision: May 17, 2018
Revised: July 26, 2018
Accepted: August 1, 2018
Article in press: August 1, 2018
Published online: October 27, 2018
Processing time: 200 Days and 16 Hours
The complement system is a key component of the body’s immune system. When abnormally activated, this system can induce inflammation and damage to normal tissues and participate in the development and progression of a variety of diseases. In the past, many scholars believed that alcoholic liver disease (ALD) is induced by the stress of ethanol on liver cells, including oxidative stress and dysfunction of mitochondria and protease bodies, causing hepatocyte injury and apoptosis. Recent studies have shown that complement activation is also involved in the genesis and development of ALD. This review focuses on the roles of complement activation in ALD and of therapeutic intervention in complement-activation pathways. We intend to provide new ideas on the diagnosis and treatment of ALD.
Core tip: In this review, we cited evidence that the complement system is involved in the pathogenesis of each stage of alcoholic liver disease (ALD) that include fatty liver, alcoholic hepatitis, and fibrosis/cirrhosis, and we also summarized the complement regulation in ALD. We intend to provide new ideas on the treatment of ALD.
- Citation: Lin CJ, Hu ZG, Yuan GD, Lei B, He SQ. Complements are involved in alcoholic fatty liver disease, hepatitis and fibrosis. World J Hepatol 2018; 10(10): 662-669
- URL: https://www.wjgnet.com/1948-5182/full/v10/i10/662.htm
- DOI: https://dx.doi.org/10.4254/wjh.v10.i10.662
Liver disease caused by long-term excessive ethanol drinking is a major cause of chronic liver disease. As the global incidence of alcoholic liver disease (ALD) increases year by year, it has become a serious threat to human health. Almost all heavy drinkers have fatty liver, 10%-20% of which develop into alcoholic hepatitis, cirrhosis, and even hepatocellular carcinoma[1]. Exploration of the mechanisms of alcohol-induced liver injury and repair is extremely important in developing methods for preventing and treating ALD.
The complement system plays an important beneficial role in the immune system: Complement activation promotes target-cell lysis, with the associated elimination of exogenous pathogens. Yet, the complement system is a “double-edged sword”, as the excessive activation of complement can induce inflammation and lead to autoimmune diseases, such as autoimmune kidney disease, glomerular nephritis, acute lung injury, and others[2-5]. Most plasma complement components are synthesized in liver cells. Thus, the liver becomes the main target of damage by complement activation[6-8]. This connection is likely due to the direct effects of alcohol that activate complement, but not because the liver is a major producer of complement proteins. Several studies have illustrated that complement activation is involved in the development of ALD[8-14] (Table 1).
| Study | Complement component | Alcoholic liver disease | Species |
| Järveläinen et al[10] | C1, Crry, CD59 | Alcohol-induced injury | Rat |
| Bykov et al[11] | C3 | Liver steatosis | Mouse |
| Pritchard et al[8] | C3, C5, DAF | Fatty liver | Mouse |
| He et al[7] | C3 | Liver steatosis | Mouse |
| Cohen et al[13] | C1q | Alcohol-induced injury | Mouse |
| Wlazlo et al[27] | C3 | Liver steatosis | Human |
| Shen et al[6] | C3 | Alcoholic hepatitis | Mouse |
| Maslowska et al[30] | Factor D | Alcoholic hepatitis | Mouse |
Most ingested ethanol is absorbed into the blood circulation, and soon reaches each organ of the body. About 90% of the ingested ethanol is metabolized in the liver[15], and most is metabolized by alcohol dehydrogenase and aldehyde dehydrogenase to form acetic acid, which can be used as substrate in the tricarboxylic acid cycle to produce energy. With excessive drinking, the body can activate another metabolic pathway, i.e., the microsomal ethanol oxidation system (MEOS), which catalyzes ethanol mainly by cytochrome P450 2El in Kupffer cells. The MEOS can over produced reactive oxygen species and reactive nitrogen species, which may exceed the body’s antioxidant capacity. Free radicals produced via the MEOS pathway exert a series of toxic effects: Membrane-lipid peroxidation, intracellular protease degeneration, oxidative modification of DNA, and others, which eventually lead to necrosis or apoptosis of hepatocytes[14,16-18]. A small percentage of ethanol is metabolized by fatty acid ethyl ester synthase to produce fatty acid ethyl ester through the non-oxidative pathway. Fatty acid ethyl ester has cytotoxicity, which can further injure the liver and pancreas[19]. Thus the liver becomes the main organ damaged by ethanol. Chronic ethanol exposure results in decreased protease activity in liver cells, imbalance of the liver’s detoxification function, and overproduction of acetaldehyde, thus inducing hepatic oxidative stress and complement activation; all these activities can injure hepatocytes[20].
The complement system consists of more than 30 kinds of proteins with enzyme-like activities which are inherent components, regulatory proteins, and complement receptors. Complement regulatory proteins include plasma soluble factors, membrane binding proteins, homologous restriction factor, and membrane inhibitors of reactive lysis. Because the complement system is involved in inflammation and immune regulation, it plays an important role in regulation of pathophysiological functions[21].
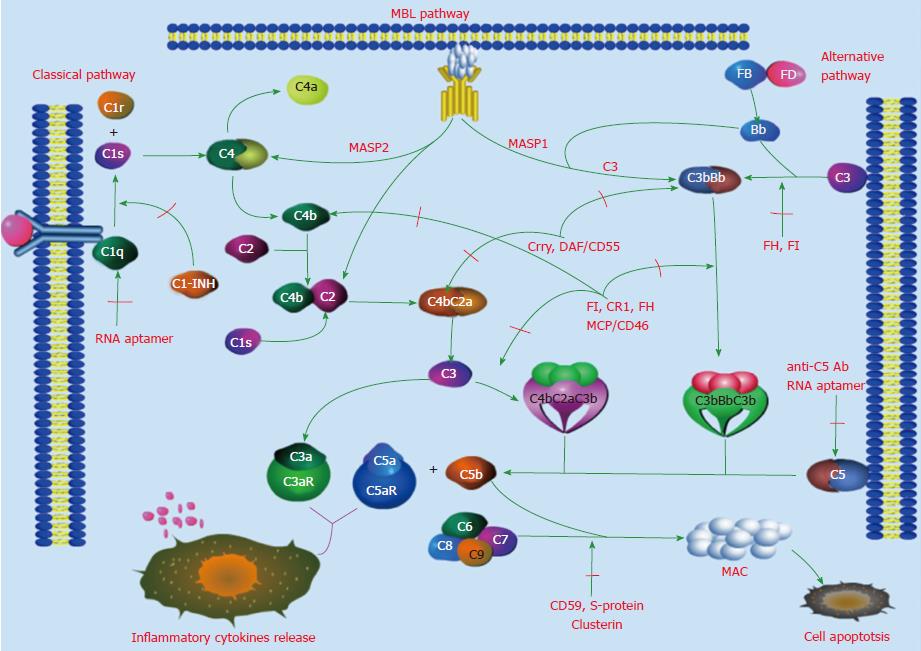
Complement is activated by three pathways: The classical, mannan-binding lectin (MBL), and alternative pathways. The three pathways start with different mechanisms, but they end with a common terminal pathway, as shown in Figure 1. The classical pathway is the main mechanism of immune responses. In it, C1q identifies immune complexes, followed by the activation of C1r and C1s. Activated C1s cleaves C2 and C4 to form C3 convertase (C4bC2a), which cleaves C3 to form C5 convertase (C4bC2aC3b). In contrast to activation of the classical pathway, activation of the lectin pathway does not depend on immune complexes. In this pathway, the cascade of enzymatic reactions proceeds in this sequence: MBL identifies the pathogens to form MBL-associated serine proteases (MASP1, MASP2); MASP1 directly cleaves C3 to form C3 convertase (C3bBb), MASP2 cleaves C4 and C2 in a manner similar to that of C1s, forming C3 convertase (C4bC2a), which continues to cleave C5 to form C5 convertase (C4bC2aC3b). Thus, this pathway can cross-promote the classical and alternative pathways. The alternative pathway is activated with hydrolysis of C3 into C3(H2O), factor B and factor D, the activation of which is also independent of immune complexes, and participates in the defense mechanisms of the early stage of inflammation[13,22-24]. The above three pathways merge into the terminal pathway, in which C5 convertase cleaves C5 to form C5a and C5b, and C5b combines with C6, C7, C8 and C9 to form the membrane attack complex (MAC). Formation of the MAC leads to cell lysis and induces cells to release inflammatory cytokines.
ALD progresses in three distinct stages: Fatty liver, alcoholic hepatitis, and fibrosis/cirrhosis. In this review, we cite evidence that the complement system is involved in the pathogenesis of each of these stages.
The liver is the main site of fat metabolism. Disorders of fat metabolism, caused by various factors, can lead to excessive fat accumulation in the liver cells, i.e., fatty liver. Long-term heavy drinking is the main independent risk factor of fatty liver disease[25], but its pathogenesis is not clearly defined. Liu et al[26] found that gut microbiota played a synergistic role in the liver response, and the complement system was suppressed in fatty liver which was partially due to increased blood lactic acid from enriched Lactobacillus. Abnormal complement activation reportedly enhances the sensitivity of steatotic livers to ischemia and reperfusion injury, which leads to the development of fatty liver[27,28]. Järveläinen et al[10] found that deposition of complement C1, C3, and C8 was increased, and the expression of membrane-binding proteins, complement receptor 1-related protein y (Crry), and CD59 was decreased in the liver cells of a mouse ALD model. These findings proved that alcohol-induced complement activation can result in ALD, at least in an experimental model. In a study in mice chronically exposed to ethanol, Cohen et al[13] found that lipid deposition in liver cells as well as values of liver-related serum enzymes (alanine aminotransferase and aspartate amino transferase) increased significantly; various degrees of liver cell apoptosis were also found. Moreover, with knock out of the C1q gene, hepatic steatosis in the mice was significantly decreased[13]. This study illustrated that complement activation could be associated with ethanol-induced hepatic steatosis.
Bykov et al[11] fed C3+/+ and C3-/- mice a high fat and high alcohol diet, respectively, and found that hepatic steatosis and significant increases in triglyceride values occurred in the C3+/+mice, whereas C3-/-mice were protected from ethanol-induced liver injury; research by Stewart et al[9] yielded similar results.
At the complement activation pathways, C3a converted to C3adesAg, C3adesArg which known as acylation stimulating protein had been shown to have lipogenic activity via its receptor C5L2, and promoted triglyceride storage in adipocytes[29,30] It was also found that C3adesArg was involved in the triglyceride metabolism[31].
Thus, activation of complement C1 and C3 appears to play a significant role in promoting fatty accumulation in the liver. Further definition of the relationship between activated complement C1, C3 and lipid metabolism in the liver may aid in the development of methods for intervention and treatment of alcoholic fatty liver disease. Besides C1 and C3, complement C5 also is involved in lipid metabolism. Bavia et al[32,33] found that the activation of complement C5 by high-dose ethanol exposure can affect the distribution of lipid in liver cells and serum. Less lipid and cholesterol is deposited in hepatocytes of C5- mice than in hepatocytes of C5+mice, and values of IL-17, which are involved in the synthesis and metabolism of lipid and cholesterol, are higher in C5- mice than in C5+mice[34,35]. The above-cited reports indicate that activation of C5 may play a role in the development of alcoholic fatty liver.
ALD has many potential pathogenic factors, such as endotoxin, which may lead to complement activation and deposition in the liver cells. Shen et al[6] found that complement activation was involved in humans with ALD. Cohen et al[13] found that long-term alcohol exposure can lead to apoptosis of liver cells, and the degree of apoptosis is positively correlated with liver injury. However, whether short-term alcohol exposure can cause hepatocyte apoptosis was not known. Further research found that short-term alcohol exposure did not cause hepatocyte apoptosis, but it did promote the deposition of complement C3b and the expression of inflammatory cytokines (tumor necrosis factor and IL6). After the Cq gene was knocked out, the expression of inflammatory cytokines was significantly reduced compared to that in wild-type animals[12,13]. Experiments by Païdassi et al[36] and Lu et al[37] supported these observations.
Complement C5, a core component of the complement activation pathway, is involved in the occurrence and development of alcoholic hepatitis, in addition to fatty liver[6,8,38]. Bavia et al[38] documented this in a hepatitis model induced by alcohol; they found that values of pro-inflammatory cytokines (IL-6, IFN-γ, IL-1β, and others) in B6C5+ mice were significantly higher than those in B6C5- mice, and anti-inflammatory factors (IL10 and IL17) were secreted significantly more in B6C5- mice. These findings illustrated that activated C5 induced the expression of proinflammatory cytokines after alcohol exposure. Up-regulated expression of pro-inflammatory cytokines (IL-6, IFN-γ, IL-1β, and others) aids the body’s defense against pathogenic microorganisms, but it also participates in the pathogenesis of alcoholic fatty liver and alcoholic hepatitis[8,39-41].
Intrahepatic inflammatory reaction and a decrease in structural integrity of hepatic sinusoidal endothelial cells after long-term alcohol exposure are important inducements to liver injury. Sinusoidal endothelial cells express C5R1, which is the foundation of C5 activation-induced alcoholic hepatic fibrosis[42]. In recent years, the pathogenesis of alcoholic hepatic fibrosis has attracted worldwide attention , but the cause of the fibrosis is still not fully defined[43-45]. According to published reports[46,47], complement C3, C4 and activation of the MBL pathway are involved in the development of fibrosis. Bavia et al[38] using the mouse model of ALD, found that values of TGF-β, which promotes hepatic fibrosis, were significantly higher in B6C5+ mice than in B6C5- mice[38,48]. Hillebradt et al[49] found that the C5 gene was involved in the regulation of hepatic fibrosis on human chromosome, and further study found that C5- mice had decreased hepatic fibrosis. Thus, the evidence indicates that activation of complement C5 may promote hepatic fibrosis. Exploration of the relationship between complement activation and alcoholic hepatic fibrosis, and of possible intervention in ALD by reversing the progression of hepatic fibrosis in its early stage, seems worthwhile goals.
Kupffer cells, located in liver sinusoids, are an important part of the mononuclear phagocyte system. Alcohol exposure in the early stage can promote apoptosis of Kupffer cells, but longer exposure usually is needed[13,50,51]. Ethanol-induced activation of complement component C1q at the early stage of ALD promotes the release of inflammatory cytokines from Kupffer cells, which further promote alcoholic liver injury[51-56]. Furthermore, Kupffer cells can express C3R and C5R, then induce prostaglandin release and synthesis of pro-inflammatory cytokines[57-60]. However, in certain pathological conditions, activated C5 combines with C5R, inducing the upregulation of fibrinogen on Kupffer cells, an interaction that is believed to lead to hepatic fibrosis[22,61]. In addition, alcohol-induced upregulation of CD14 leads to Kupffer cells combining with lipopolysaccharide, which induces liver damage through the activation of TLR4 in Kupffer cells and inflammatory signaling pathways; these events can further aid in the development of hepatic fibrosis or cirrhosis[62]. Thus, Kupffer cells seem to be extensively involved in the development of ALD[63-66].
Reducing inflammatory reactions by inhibiting amplification of the complement cascade and blocking the combination of complement with the corresponding complement receptors are being pursued worldwide. Excessive activation of complement can be inhibited by self-regulation of the body (Table 2). For example, the complement regulatory protein decay accelerating factor (DAF) can inhibit C3, C5 convertase, thereby inhibiting amplification of the complement cascade. The complement regulatory protein Crry can cooperate with DAF and factor H to accelerate dissociation of C3 and C5 convertase and to cleave C3b and C4b, so that the cells avoid being attacked by autologous complement[67-69]. Deficiency of CD55/DAF and complement regulatory factors aggravate liver injury[8,11], whereas factor H can control the activity and stability of C3 convertase via binding with C3b[70,71]. Also, defects in the factor H gene can cause persistent activation of complement pathways and trigger various diseases[72-75]. By contrast, factor H-related proteins (FHRs), including FHR1-5, can either promote or inhibit complement activation. The degree of complement activation depends on the homeostasis between factor H and FHR[71]. However, the relationship between factor H and ALD has not been clarified and needs further research. McCullough et al[76] found FD-dependent amplification of complement is an adaptive response that promotes hepatic healing and recovery in response to chronic ethanol. In other complement regulatory activities, CD59, protein S and clusterin inhibit the formation of the MAC through limiting the binding of complement C9[77-81]. Membrane cofactor protein (MCP) and factor I can inhibit cells from binding with C3b and C4b[82,83].
| Type of regulators | Regulators | Functions |
| Complement regulatory protein | DAF/CD55, Crry, FH, FI | Inhibit C3, C5 convertase |
| CD59, protein S, clusterin | Inhibit MAC | |
| Complement inhibitor | C1-INH | Inhibit C1r, C1s |
| Targeted inhibitor | h5G1, 1-ScFv | Inhibit C5 activation |
| RNA aptamer | Specifically bind C1q, C5 | |
Specific epitope structures of complement, such as anti-complement antibody, complement antisense strand, and complement mutants[84-91] have been invented, with the intent of inhibiting complement activation. In addition, complement inhibitors and RNA aptamer are being used to inhibit progression of complement-related diseases[92,93], and C1-INH and CR1 have been used in the treatment of ALD and other diseases[10].
Mounting evidence indicates that complement activation is involved in the development of ALD at all its stages - fatty liver, alcoholic hepatitis, and fibrosis/cirrhosis. Moreover, all three pathways of complement activation (classical, MBL, and alternative) promote the development of ALD. Therapeutic strategies, using various measures to inhibit complement activation, might prevent the development of ALD. Thorough understanding of the relationships between complement activation and ALD may aid in developing new approaches for the treatment of ALD.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Marcos M, Metin U, Morales-González JA, Kollmann D S- Editor: Ma YJ L- Editor: Ma JY E- Editor: Bian YN
| 1. | Warren KR, Murray MM. Alcoholic liver disease and pancreatitis: global health problems being addressed by the US National Institute on Alcohol Abuse and Alcoholism. J Gastroenterol Hepatol. 2013;28 Suppl 1:4-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Yang Y. Mannose Binding Lectin and kidney disease. Int J Nephrol Urol. 2010;30:241-244. [DOI] [Full Text] |
| 3. | Borza DB. Glomerular basement membrane heparan sulfate in health and disease: A regulator of local complement activation. Matrix Biol. 2017;57-58:299-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Russkamp NF, Ruemmler R, Roewe J, Moore BB, Ward PA, Bosmann M. Experimental design of complement component 5a-induced acute lung injury (C5a-ALI): a role of CC-chemokine receptor type 5 during immune activation by anaphylatoxin. FASEB J. 2015;29:3762-3772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Reichhardt MP, Meri S. Intracellular complement activation-An alarm raising mechanism? Semin Immunol. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 6. | Shen H, French BA, Liu H, Tillman BC, French SW. Increased activity of the complement system in the liver of patients with alcoholic hepatitis. Exp Mol Pathol. 2014;97:338-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | He S, Atkinson C, Evans Z, Ellett JD, Southwood M, Elvington A, Chavin KD, Tomlinson S. A role for complement in the enhanced susceptibility of steatotic livers to ischemia and reperfusion injury. J Immunol. 2009;183:4764-4772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Pritchard MT, McMullen MR, Stavitsky AB, Cohen JI, Lin F, Edward Medof M, Nagy LE. Differential contributions of C3, C5, and decay-accelerating factor to ethanol-induced fatty liver in mice. Gastroenterology. 2007;132:1117-1126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Stewart S, Jones D, Day CP. Alcoholic liver disease: new insights into mechanisms and preventative strategies. Trends Mol Med. 2001;7:408-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 137] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Järveläinen HA, Väkevä A, Lindros KO, Meri S. Activation of complement components and reduced regulator expression in alcohol-induced liver injury in the rat. Clin Immunol. 2002;105:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Bykov I, Junnikkala S, Pekna M, Lindros KO, Meri S. Complement C3 contributes to ethanol-induced liver steatosis in mice. Ann Med. 2006;38:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Cohen JI, Roychowdhury S, DiBello PM, Jacobsen DW, Nagy LE. Exogenous thioredoxin prevents ethanol-induced oxidative damage and apoptosis in mouse liver. Hepatology. 2009;49:1709-1717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Cohen JI, Roychowdhury S, McMullen MR, Stavitsky AB, Nagy LE. Complement and alcoholic liver disease: role of C1q in the pathogenesis of ethanol-induced liver injury in mice. Gastroenterology. 2010;139:664-674, 674.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 14. | Orman ES, Odena G, Bataller R. Alcoholic liver disease: pathogenesis, management, and novel targets for therapy. J Gastroenterol Hepatol. 2013;28 Suppl 1:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 180] [Article Influence: 15.0] [Reference Citation Analysis (2)] |
| 15. | Zhao J. The metabolism and harm of alcohol. Keji & Shenghuo. 2011;6:195-198. |
| 16. | Kawaratani H, Tsujimoto T, Douhara A, Takaya H, Moriya K, Namisaki T, Noguchi R, Yoshiji H, Fujimoto M, Fukui H. The effect of inflammatory cytokines in alcoholic liver disease. Mediators Inflamm. 2013;2013:495156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 181] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 17. | Cohen JI, Chen X, Nagy LE. Redox signaling and the innate immune system in alcoholic liver disease. Antioxid Redox Signal. 2011;15:523-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Leung TM, Nieto N. CYP2E1 and oxidant stress in alcoholic and non-alcoholic fatty liver disease. J Hepatol. 2013;58:395-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 386] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 19. | Cohen JI, Nagy LE. Pathogenesis of alcoholic liver disease: interactions between parenchymal and non-parenchymal cells. J Dig Dis. 2011;12:3-9. [RCA] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Nagy LE. The Role of Innate Immunity in Alcoholic Liver Disease. Alcohol Res. 2015;37:237-250. [PubMed] |
| 21. | Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785-797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2932] [Cited by in RCA: 2751] [Article Influence: 183.4] [Reference Citation Analysis (0)] |
| 22. | Qin X, Gao B. The complement system in liver diseases. Cell Mol Immunol. 2006;333-340. |
| 23. | Vieyra MB, Heeger PS. Novel aspects of complement in kidney injury. Kidney Int. 2010;77:495-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | He W. Medical immunology. Beijing: People’s Medical Publishing House 2010; 76-91. |
| 25. | Roerecke M, Nanau R, Rehm J, Neuman M. Ethnicity matters: A Systematic Review and Meta-Analysis of the Non-Linear Relationship Between Alcohol Consumption and Prevalence and Incidence of Hepatic Steatosis. EBioMedicine. 2016;8:317-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Liu L, Zhao X, Wang Q, Sun X, Xia L, Wang Q, Yang B, Zhang Y, Montgomery S, Meng H. Prosteatotic and Protective Components in a Unique Model of Fatty Liver: Gut Microbiota and Suppressed Complement System. Sci Rep. 2016;6:31763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 27. | Wlazlo N, van Greevenbroek MM, Ferreira I, Jansen EH, Feskens EJ, van der Kallen CJ, Schalkwijk CG, Bravenboer B, Stehouwer CD. Activated complement factor 3 is associated with liver fat and liver enzymes: the CODAM study. Eur J Clin Invest. 2013;43:679-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Copenhaver M, Yu CY, Hoffman RP. Complement Components, C3 and C4, and the Metabolic Syndrome. Curr Diabetes Rev. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 29. | Gupta A, Rezvani R, Lapointe M, Poursharifi P, Marceau P, Tiwari S, Tchernof A, Cianflone K. Downregulation of complement C3 and C3aR expression in subcutaneous adipose tissue in obese women. PLoS One. 2014;9:e95478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Maslowska M, Wang HW, Cianflone K. Novel roles for acylation stimulating protein/C3adesArg: a review of recent in vitro and in vivo evidence. Vitam Horm. 2005;70:309-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Kalant D, Phélis S, Fielding BA, Frayn KN, Cianflone K, Sniderman AD. Increased postprandial fatty acid trapping in subcutaneous adipose tissue in obese women. J Lipid Res. 2000;41:1963-1968. [PubMed] |
| 32. | Bavia L, de Castro ÍA, Massironi SM, Isaac L. Basal physiological parameters of two congenic mice strains: C5 deficient C57BL/6 and C5 sufficient A/J. Immunol Lett. 2014;159:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Bavia L, de Castro ÍA, Isaac L. C57BL/6 and A/J Mice Have Different Inflammatory Response and Liver Lipid Profile in Experimental Alcoholic Liver Disease. Mediators Inflamm. 2015;2015:491641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Shi W, Zhu Q, Gu J, Liu X, Lu L, Qian X, Shen J, Zhang F, Li G. Anti-IL-17 antibody improves hepatic steatosis by suppressing interleukin-17-related fatty acid synthesis and metabolism. Clin Dev Immunol. 2013;2013:253046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 35. | Hu X, Wang Y, Hao LY, Liu X, Lesch CA, Sanchez BM, Wendling JM, Morgan RW, Aicher TD, Carter LL. Corrigendum: Sterol metabolism controls TH17 differentiation by generating endogenous RORγ agonists. Nat Chem Biol. 2015;11:741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Païdassi H, Tacnet-Delorme P, Garlatti V, Darnault C, Ghebrehiwet B, Gaboriaud C, Arlaud GJ, Frachet P. C1q binds phosphatidylserine and likely acts as a multiligand-bridging molecule in apoptotic cell recognition. J Immunol. 2008;180:2329-2338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 207] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 37. | Lu JH, Teh BK, Wang Ld, Wang YN, Tan YS, Lai MC, Reid KB. The classical and regulatory functions of C1q in immunity and autoimmunity. Cell Mol Immunol. 2008;5:9-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 38. | Bavia L, de Castro ÍA, Cogliati B, Dettoni JB, Alves VA, Isaac L. Complement C5 controls liver lipid profile, promotes liver homeostasis and inflammation in C57BL/6 genetic background. Immunobiology. 2016;221:822-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 39. | Petrasek J, Bala S, Csak T, Lippai D, Kodys K, Menashy V, Barrieau M, Min SY, Kurt-Jones EA, Szabo G. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest. 2012;122:3476-3489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 587] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 40. | Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1734] [Cited by in RCA: 1848] [Article Influence: 115.5] [Reference Citation Analysis (0)] |
| 41. | MacLaren R, Cui W, Cianflone K. Adipokines and the immune system: an adipocentric view. Adv Exp Med Biol. 2008;632:1-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 42. | Jiang XL, Cohen JI, Nagy LE. Pathogenesis of Alcoholic Liver Disease:Interactions between Parenchymal and Non-parenchymal Cells. Weichangbing Xue. 2011;16:131-134. [DOI] [Full Text] |
| 43. | Leeming DJ, Veidal SS, Karsdal MA, Nielsen MJ, Trebicka J, Busk T, Bendtsen F, Krag A, Møller S. Pro-C5, a marker of true type V collagen formation and fibrillation, correlates with portal hypertension in patients with alcoholic cirrhosis. Scand J Gastroenterol. 2015;50:584-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 44. | Jeong WI, Gao B. Innate immunity and alcoholic liver fibrosis. J Gastroenterol Hepatol. 2008;23 Suppl 1:S112-S118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 45. | Duddempudi AT. Immunology in alcoholic liver disease. Clin Liver Dis. 2012;16:687-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 46. | Baumann M, Witzke O, Canbay A, Patschan S, Treichel U, Gerken G, Philipp T, Kribben A. Serum C3 complement concentrations correlate with liver function in patients with liver cirrhosis. Hepatogastroenterology. 2004;51:1451-1453. [PubMed] |
| 47. | Chong WP, To YF, Ip WK, Yuen MF, Poon TP, Wong WH, Lai CL, Lau YL. Mannose-binding lectin in chronic hepatitis B virus infection. Hepatology. 2005;42:1037-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 48. | Karkampouna S, Ten Dijke P, Dooley S, Julio MK. TGFβ signaling in liver regeneration. Curr Pharm Des. 2012;18:4103-4113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 49. | Hillebrandt S, Wasmuth HE, Weiskirchen R, Hellerbrand C, Keppeler H, Werth A, Schirin-Sokhan R, Wilkens G, Geier A, Lorenzen J. Complement factor 5 is a quantitative trait gene that modifies liver fibrogenesis in mice and humans. Nat Genet. 2005;37:835-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 206] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 50. | Deaciuc IV, Fortunato F, D’Souza NB, Hill DB, McClain CJ. Chronic alcohol exposure of rats exacerbates apoptosis in hepatocytes and sinusoidal endothelial cells. Hepatol Res. 2001;19:306-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 51. | Miller AM, Wang H, Park O, Horiguchi N, Lafdil F, Mukhopadhyay P, Moh A, Fu XY, Kunos G, Pacher P. Anti-inflammatory and anti-apoptotic roles of endothelial cell STAT3 in alcoholic liver injury. Alcohol Clin Exp Res. 2010;34:719-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 52. | Bajtay Z, Józsi M, Bánki Z, Thiel S, Thielens N, Erdei A. Mannan-binding lectin and C1q bind to distinct structures and exert differential effects on macrophages. Eur J Immunol. 2000;30:1706-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 53. | Roychowdhury S, McMullen MR, Pritchard MT, Hise AG, van Rooijen N, Medof ME, Stavitsky AB, Nagy LE. An early complement-dependent and TLR-4-independent phase in the pathogenesis of ethanol-induced liver injury in mice. Hepatology. 2009;49:1326-1334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 54. | Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 729] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 55. | Fallowfield JA, Mizuno M, Kendall TJ, Constandinou CM, Benyon RC, Duffield JS, Iredale JP. Scar-associated macrophages are a major source of hepatic matrix metalloproteinase-13 and facilitate the resolution of murine hepatic fibrosis. J Immunol. 2007;178:5288-5295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 375] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 56. | Nieto N. Oxidative-stress and IL-6 mediate the fibrogenic effects of [corrected] Kupffer cells on stellate cells. Hepatology. 2006;44:1487-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 189] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 57. | Helmy KY, Katschke KJ Jr, Gorgani NN, Kljavin NM, Elliott JM, Diehl L, Scales SJ, Ghilardi N, van Lookeren Campagne M. CRIg: a macrophage complement receptor required for phagocytosis of circulating pathogens. Cell. 2006;124:915-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 431] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 58. | Hinglais N, Kazatchkine MD, Mandet C, Appay MD, Bariety J. Human liver Kupffer cells express CR1, CR3, and CR4 complement receptor antigens. An immunohistochemical study. Lab Invest. 1989;61:509-514. [PubMed] |
| 59. | Kumar V, Ali SR, Konrad S, Zwirner J, Verbeek JS, Schmidt RE, Gessner JE. Cell-derived anaphylatoxins as key mediators of antibody-dependent type II autoimmunity in mice. J Clin Invest. 2006;116:512-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 60. | Schieferdecker HL, Rothermel E, Timmermann A, Götze O, Jungermann K. Anaphylatoxin C5a receptor mRNA is strongly expressed in Kupffer and stellate cells and weakly in sinusoidal endothelial cells but not in hepatocytes of normal rat liver. FEBS Lett. 1997;406:305-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 61. | Schlaf G, Schmitz M, Heine I, Demberg T, Schieferdecker HL, Götze O. Upregulation of fibronectin but not of entactin, collagen IV and smooth muscle actin by anaphylatoxin C5a in rat hepatic stellate cells. Histol Histopathol. 2004;19:1165-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 62. | Guo SP, Zhang WF, Gong JP. Research advance in the origin and immunological function of kupffer cells. Sheng Li Ke Xue Jin Zhan. 2016;47:57-60. [PubMed] |
| 63. | Enomoto N, Ikejima K, Yamashina S, Hirose M, Shimizu H, Kitamura T, Takei Y, Sato And N, Thurman RG. Kupffer cell sensitization by alcohol involves increased permeability to gut-derived endotoxin. Alcohol Clin Exp Res. 2001;25:51S-54S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 83] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 64. | Wheeler MD, Thurman RG. Up-regulation of CD14 in liver caused by acute ethanol involves oxidant-dependent AP-1 pathway. J Biol Chem. 2003;278:8435-8441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 65. | Schieferdecker HL, Schlaf G, Jungermann K, Götze O. Functions of anaphylatoxin C5a in rat liver: direct and indirect actions on nonparenchymal and parenchymal cells. Int Immunopharmacol. 2001;1:469-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 66. | Reid DT, Reyes JL, McDonald BA, Vo T, Reimer RA, Eksteen B. Kupffer Cells Undergo Fundamental Changes during the Development of Experimental NASH and Are Critical in Initiating Liver Damage and Inflammation. PLoS One. 2016;11:e0159524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 117] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 67. | Barata L, Miwa T, Sato S, Kim D, Mohammed I, Song WC. Deletion of Crry and DAF on murine platelets stimulates thrombopoiesis and increases factor H-dependent resistance of peripheral platelets to complement attack. J Immunol. 2013;190:2886-2895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 68. | Laskowski J, Renner B, Le Quintrec M, Panzer S, Hannan JP, Ljubanovic D, Ruseva MM, Borza DB, Antonioli AH, Pickering MC. Distinct roles for the complement regulators factor H and Crry in protection of the kidney from injury. Kidney Int. 2016;90:109-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 69. | Liu F, Wu L, Wu G, Wang C, Zhang L, Tomlinson S, Qin X. Targeted mouse complement inhibitor CR2-Crry protects against the development of atherosclerosis in mice. Atherosclerosis. 2014;234:237-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 70. | Vernon KA, Ruseva MM, Cook HT, Botto M, Malik TH, Pickering MC. Partial Complement Factor H Deficiency Associates with C3 Glomerulopathy and Thrombotic Microangiopathy. J Am Soc Nephrol. 2016;27:1334-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 71. | Medjeral-Thomas N, Pickering MC. The complement factor H-related proteins. Immunol Rev. 2016;274:191-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 72. | Faber C, Williams J, Juel HB, Greenwood J, Nissen MH, Moss SE. Complement factor H deficiency results in decreased neuroretinal expression of Cd59a in aged mice. Invest Ophthalmol Vis Sci. 2012;53:6324-6330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 73. | Wilson V, Darlay R, Wong W, Wood KM, McFarlane J, Schejbel L, Schmidt IM, Harris CL, Tellez J, Hunze EM. Genotype/phenotype correlations in complement factor H deficiency arising from uniparental isodisomy. Am J Kidney Dis. 2013;62:978-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 74. | Schejbel L, Schmidt IM, Kirchhoff M, Andersen CB, Marquart HV, Zipfel P, Garred P. Complement factor H deficiency and endocapillary glomerulonephritis due to paternal isodisomy and a novel factor H mutation. Genes Immun. 2011;12:90-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 75. | Blackall DP, Marques MB. Hemolytic uremic syndrome revisited: Shiga toxin, factor H, and fibrin generation. Am J Clin Pathol. 2004;121 Suppl:S81-S88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 76. | McCullough RL, McMullen MR, Sheehan MM, Poulsen KL, Roychowdhury S, Chiang DJ, Pritchard MT, Caballeria J, Nagy LE. Complement Factor D protects mice from ethanol-induced inflammation and liver injury. Am J Physiol Gastrointest Liver Physiol. 2018;315:G66-G79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 77. | Wu G, Hu W, Shahsafaei A, Song W, Dobarro M, Sukhova GK, Bronson RR, Shi GP, Rother RP, Halperin JA. Complement regulator CD59 protects against atherosclerosis by restricting the formation of complement membrane attack complex. Circ Res. 2009;104:550-558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 78. | Wickham SE, Hotze EM, Farrand AJ, Polekhina G, Nero TL, Tomlinson S, Parker MW, Tweten RK. Mapping the intermedilysin-human CD59 receptor interface reveals a deep correspondence with the binding site on CD59 for complement binding proteins C8alpha and C9. J Biol Chem. 2011;286:20952-20962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 79. | Huang Y, Fedarovich A, Tomlinson S, Davies C. Crystal structure of CD59: implications for molecular recognition of the complement proteins C8 and C9 in the membrane-attack complex. Acta Crystallogr D Biol Crystallogr. 2007;63:714-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 80. | Podack ER, Müller-Eberhard HJ. Binding of desoxycholate, phosphatidylcholine vesicles, lipoprotein and of the S-protein to complexes of terminal complement components. J Immunol. 1978;121:1025-1030. [PubMed] |
| 81. | McDonald JF, Nelsestuen GL. Potent inhibition of terminal complement assembly by clusterin: characterization of its impact on C9 polymerization. Biochemistry. 1997;36:7464-7473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 69] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 82. | Chu Q, Liu X, Xiang Y, Liu QW. Study on the structure and function of complement C3. Zhongguo Mianyixue Zazhi. 2014;30:549-553. [DOI] [Full Text] |
| 83. | Liu Q, Xing G. Complement regulatory proteins and IgA nephropathy. Zhongguo Shiyong Yike. 2013;40:122-124. [DOI] [Full Text] |
| 84. | de Haas CJ, Veldkamp KE, Peschel A, Weerkamp F, Van Wamel WJ, Heezius EC, Poppelier MJ, Van Kessel KP, van Strijp JA. Chemotaxis inhibitory protein of Staphylococcus aureus, a bacterial antiinflammatory agent. J Exp Med. 2004;199:687-695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 315] [Cited by in RCA: 338] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 85. | Fujita E, Farkas I, Campbell W, Baranyi L, Okada H, Okada N. Inactivation of C5a anaphylatoxin by a peptide that is complementary to a region of C5a. J Immunol. 2004;172:6382-6387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 86. | Guo RF, Ward PA. Role of C5a in inflammatory responses. Annu Rev Immunol. 2005;23:821-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 755] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 87. | Nikiforovich GV, Baranski TJ. Structural models for the complex of chemotaxis inhibitory protein of Staphylococcus aureus with the C5a receptor. Biochem Biophys Res Commun. 2009;390:481-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 88. | Otto M, Hawlisch H, Monk PN, Müller M, Klos A, Karp CL, Köhl J. C5a mutants are potent antagonists of the C5a receptor (CD88) and of C5L2: position 69 is the locus that determines agonism or antagonism. J Biol Chem. 2004;279:142-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 89. | Li BS, Cui SH, Lv FL, Chen Y, Li YC, Hu CX, Chen CY. Protective effects of complementary peptide of anaphylatoxin C5a on lung injury of experimental sepsis mouse. Disan Junyi Daxue Xuebao. 2006;28:4. [DOI] [Full Text] |
| 90. | Wu Z, Lv F, Lv C, Wu YZ. Blocking effects of anti-sense peptide of C5a on the adhesion between pulmonary vascular endothelial cell and neutrophil. Zhongduo Mianyixue Zazhi. 2003;19:5. |
| 91. | Li L, Chen L, Zang J, Tang X, Liu Y, Zhang J, Bai L, Yin Q, Lu Y, Cheng J. C3a and C5a receptor antagonists ameliorate endothelial-myofibroblast transition via the Wnt/β-catenin signaling pathway in diabetic kidney disease. Metabolism. 2015;64:597-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 120] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 92. | Lee JH, Kim H, Ko J, Lee Y. Interaction of C5 protein with RNA aptamers selected by SELEX. Nucleic Acids Res. 2002;30:5360-5368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 93. | Biesecker G, Dihel L, Enney K, Bendele RA. Derivation of RNA aptamer inhibitors of human complement C5. Immunopharmacology. 1999;42:219-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 102] [Article Influence: 3.9] [Reference Citation Analysis (0)] |