Published online Jan 27, 2018. doi: 10.4254/wjh.v10.i1.88
Peer-review started: October 2, 2017
First decision: November 27, 2017
Revised: December 6, 2017
Accepted: December 13, 2017
Article in press: December 13, 2017
Published online: January 27, 2018
Processing time: 120 Days and 22.3 Hours
To evaluate the efficacy of direct-acting antivirals (DAAs) in Kanto Rosai Hospital.
All patients with hepatitis C virus (HCV) who underwent DAA prescription were enrolled in this study. The present study was a single center retrospective analysis using patients infected with HCV genotype 1 or 2. Resistance analysis was performed by using direct sequencing and cycleave PCR in genotype 1 patients treated with interferon (IFN)-free DAA. The primary endpoint was sustained virologic response at 12 wk after therapy (SVR12).
A total of 117 patients participated in the study, including 135 with genotype 1 and 42 with genotype 2. Of the 135 patients with genotype 1, 16 received protease inhibitor + IFN + ribavirin and all achieved SVR. Of the 119 patients who received IFN-free DAA (in different combinations), 102 achieved SVR and 9 failed (7/9 were on daclatasvir/asunaprevir and 2/9 on ledipasvir/sofosbuvir). Efficacy analysis was done only for 43 patients who received daclatasvir/asunaprevir. From this analysis, Y93 resistance-associated substitutions were significantly correlated with SVR.
The SVR rate was 98% for genotype 1 and 100% for genotype 2. However, caution is needed for HCV NS5A resistance-associated substitutions that are selected by HCV NS5A inhibitors because cerebrovascular adverse events are induced by some DAA drugs.
Core tip: Direct-acting antivirals have been approved for the treatment of hepatitis C virus (HCV) genotype 1 and 2 infections in Japan since 2011. In the new era of DAA therapy, predictors who fail to respond to DAA might be compromised by resistance-associated substitutions. There have been few reports of daclatasvir/asunaprevir failure because daclatasvir/asunaprevir is limited in Japan. Therefore, it might be important to report these cases for future research and treatment of HCV.
- Citation: Kaneko R, Nakazaki N, Omori R, Yano Y, Ogawa M, Sato Y. Efficacy of direct-acting antiviral treatment for chronic hepatitis C: A single hospital experience. World J Hepatol 2018; 10(1): 88-94
- URL: https://www.wjgnet.com/1948-5182/full/v10/i1/88.htm
- DOI: https://dx.doi.org/10.4254/wjh.v10.i1.88
Hepatitis C is a worldwide health problem with 170 million carriers globally and 4 million new cases appearing per year[1]. Approximately 70% of hepatocellular carcinoma cases in Japan are attributable to hepatitis C virus (HCV) infection[2,3]. Since the late 1990s in Japan, the management of HCV infection has improved and there has been a decrease in the widespread use of non-sterile needles and blood transfusions[4-7]. Protease inhibitors such as simeprevir or telaprevir resulting in highly sustained virologic responses (SVRs) in HCV patients were introduced in 2011[8-10]. More recently, interferon (IFN)-free DAAs inhibiting key viral functions have become the mainstay of anti-HCV treatment[11-13]. Prior to the introduction of these therapeutic agents, IFN-based treatments were the standard therapy against HCV infection[14], despite the suboptimal SVR induced by this treatment (40%-50%). However, patients responding to IFN therapy and sustaining a loss of HCV RNA are generally regarded as being at low risk of developing liver cirrhosis or hepatocellular carcinoma (HCC)[4]. However, these continuous efforts and advances in anti-HCV therapy may influence improvements in the long-term outcome of patients with HCV.
In the new era of DAA therapy, the reason for patients’ failure in responding to DAAs might be related to the presence or development of resistance-associated substitutions (RASs)[15,16]. The aim of this study was to characterize the treatment response of new DAAs in patients infected with HCV.
Japanese patients aged 30-87 years with chronic HCV genotype 1 and genotype 2 infections and without decompensated cirrhosis were commenced with DAA treatment. Overall, 177 participants treated with telaprevir or simeprevir with pegylated (PEG)-IFN and ribavirin (RBV) or IFN-free DAA, and in whom SVR12 was judged between November 2012 and March 2017 at Kanto Rosai Hospital were included. Treatment-naïve and treatment-experienced patients were included.
Parameters were defined by standard laboratory techniques in Kanto Rosai Hospital. HCV NS5A RASs at Y93 and L31 were detected by commercial direct sequencing and cycleave PCR (SRL Laboratory, Tokyo, Japan) as well as PCR-invader methods (BML Laboratory, Tokyo, Japan). HCV RNA was measured by COBAS TaqMan PCR assay version 2.0 (Roche, Tokyo, Japan), with a lower limit of quantification of 25 IU/mL. For 10 patients who received either telaprevir or simeprevir with PEG-IFN treatment, the IL28B genotype was defined by PCR amplification and sequencing of the rs8099917, rs1188122 and rs88103142 nucleotide polymorphisms (SRL Laboratory). HCV core amino acids 70 and 99 were defined by PCR direct sequencing (LSI Laboratory, Tokyo, Japan). Liver cirrhosis was diagnosed by ultrasonography, computed tomography (CT), magnetic resonance imaging (MRI) or a liver biopsy.
The primary efficacy end point was the proportion of patients with undetectable HCV RNA at 12 wk post-treatment (SVR12).
Analyses were performed using STATA/MP14.0 software (Stata-Corp LP, College Station, TX, United States).
Before any study procedures were undertaken, informed consent was obtained from all patients. This study conformed to the ethical guidelines of the Declaration of Helsinki, and was approved by the ethics committee of Japan Organization of Occupational Health and Safety Kanto Rosai Hospital (2015-2017).
Among 177 cases, 16 patients with genotype 1 were assigned to telaprevir or simeprevir with PEG-IFN and RBV, and 119 were assigned to IFN-free DAA [daclatasvir/asunaprevir (DCV/ASV), ledipasvir/sofosbuvir (LDV/SOF), ombitasvir/paritaprevir/ritonavir (OBV/PTV/r)]. Forty-two patients were treated with SOF and RBV for genotype 2. The average age ± standard deviation of the patients was 67.8 ± 11.0 years. Of these, the group with the highest average age of 72.7 ± 8.3 years was prescribed DCV/ASV. The number and proportion of males and females were 79 (44.6%) and 98 (55.4%), respectively. There were 74 cases (46.2%) with cirrhosis, including 21 cases diagnosed pathologically and 45 (25.4%) patients who had experienced IFN-based treatment previously. Twenty-six (14.7%) patients had a history of curative HCC (Table 1).
| Parameter | Overall, n = 177 | Genotype 1 | Genotype 2 SOF/RBV n = 42 | ||||
| IFN/TVR/RBV n = 5 | IFN/SMV/RBV n = 11 | DCV/ASV n = 43 | LDV/SOF n = 66 | OBV/PTV/r n = 10 | |||
| Age, median1 | 67.8 (11.0) | 62.9 (8.7) | 60.2 (8.9) | 72.7 (8.3) | 66.0 (11.2) | 70.9 (6.5) | 67.5 (12.6) |
| > 65, n (%) | 118 (66.7) | 3 (60) | 4 (36.4) | 37 (88.1) | 39 (59.0) | 7 (70) | 29 (67.4) |
| Sex, n (%) | |||||||
| Male | 79 (44.6) | 3 (60) | 7 (63.6) | 14 (32.6) | 31 (47.0) | 4 (40) | 20 (47.6) |
| Female | 98 (55.4) | 2 (40) | 4 (36.4) | 29 (67.4) | 35 (53.0) | 6 (60) | 22 (52.4) |
| HCV RNA, median Log10 LGE1 | 6.1 (0.8) | 6.5 (0.56) | 6.2 (1.1) | 6.30 (0.5) | 6.16 (0.6) | 5.4 (0.9) | 5.8 (0.9) |
| > 100000 IU/mL, n (%) | 109 (61.6) | 4 (80) | 9 (81.8) | 32 (76.2) | 43 (0.7) | 2 (20) | 19 (45.2) |
| Cirrhosis present, n (%) | |||||||
| Yes | 74 (41.8) | 0 (0) | 0 (0) | 34 (79.0) | 29 (44.0) | 3 (30) | 8 (18.6) |
| No | 103 (58.2) | 5 (100) | 11 (100) | 9 (20.1) | 37 (56.0) | 7 (70) | 34 (81.4) |
| HCV treatment history, n (%) | |||||||
| Naïve | 132 (74.6) | 1 (20) | 2 (18.2) | 25 (58.1) | 63 (95.5) | 9 (90) | 32 (76.2) |
| Prior IFN-based treatment | 45 (25.4) | 4 (80) | 9 (81.8) | 18 (41.8) | 3 (4.5) | 1 (1) | 10 (23.8) |
| History of HCC, n (%) | |||||||
| Yes | 26 (14.7) | 1 (20) | 0 (0) | 19 (44.1) | 3 (4.5) | 0 (0) | 3 (9) |
| No | 151 (85.3) | 4 (80) | 11 (100) | 24 (55.8) | 63 (95.5) | 10 (100) | 39 (90.7) |
| Laboratory values | |||||||
| Baseline platelet count, mean (× 104/μL)1 | 15.1 (6.5) | 15.4 (3.4) | 15.1 (6.2) | 11.5 (5.8) | 15.5 (6.5) | 18.0 (5.96) | 17.6 (6.0) |
| Baseline ALT level, mean (IU/L)1 | 51.2 (37.3) | 41.8 (9.7) | 50.1 (50.5) | 53.1 (27.8) | 60.3 (45.2) | 39.9 (26.8) | 38.9 (28.6) |
| Baseline AFP level, mean (ng/mL)1 | 12.1 (17.6) | 5.6 (1.6) | 7.18 (9.1) | 23.4 (27.2) | 8.99 (11.6) | 9.9 (11.6) | 6.8 (6.9) |
Among 16 patients with IFN-based protease inhibitor treatment, 10 were tested for the polymorphism NS5A region of IL28B, and HCV core amino acids 70 and 91. In both treatment groups, patients with the mutation who were predicted to have a low treatment response were included (Table 2).
SVR12 was achieved in 167 of 177 (94.4%) patients. All 16 who received protease inhibitor with PEG-IFN and RBV (5 with teraprevir, 11 with simeprevir) achieved SVR12. All 42 patients with genotype 2 who received the treatment with SOF with RBV achieved SVR12. There was no case of relapse to the date of this paper. The response rate of the IFN-free DAA regimen (DCV/ASV, LDV/SOF, OBV/PTV/r) is shown in Table 3. Of the 43 patients who were treated with DCV/ASV, 1 patient broke through and 6 relapsed. Of the 66 patients on LDV/SOF, 2 relapsed and 2 had severe adverse events, including subarachnoid hemorrhage and cerebral hemorrhage. Although medication was stopped at 8 wk and 6 wk after prescription, SVR was achieved. Two patients also relapsed with LDV/SOF treatment. Of the 10 patients who have been on OBV/PTV/r, 1 was lost to follow-up.
| Response | Overall, n = 119 | Genotype 1 | Genotype 2 SOF + RBV n = 42 | ||
| DCV/ASV n = 43 | LDV/SOF n = 66 | OBV/PTV/r n = 10 | |||
| HCV RNA < LLOQ during treatment1, n (%) | 119 (100) | 41 (100) | 66 (100) | 9 (90)3 | 42 (100) |
| HCV RNA < LLOQ after end of treatment1, n (%) | 118 (98.3) | 42 (97.6) | 66 (100) | 9 (90)3 | 42 (100) |
| SVR122, n (%) | 109 (91.6) | 35 (83.3) | 64 (97) | 9 (90)3 | 42 (100) |
| On-treatment failure, n (%) | 1 (0.8) | 1 (2.3) | 0 (0) | 0 (0) | 0 (0) |
| Relapse, n (%) | 8 (6.7) | 6 (16.7) | 2 (3) | 0 (0) | 0 (0) |
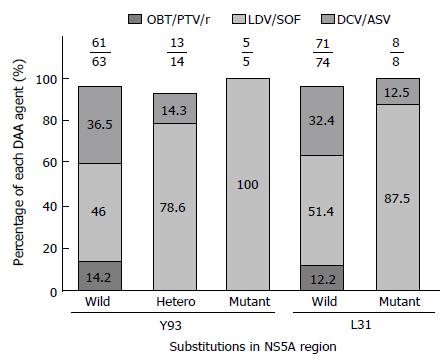
NS5A RASs were analyzed in 82 patients with IFN-free DAA treatment (Figure 1). Of these, 2 relapsed patients with wild-type Y93 and 1 with Y93 hetero were treated with DCV/ASV. Three relapsed patients with wild-type L31 were also treated with DCA/ASV. Another 6 patients that failed to achieve SVR with DAA treatment had not obtained NS5A RASs prior to treatment. Of the 9 failure patients, 7 were diagnosed as cirrhosis before DAA treatment, and 4 had a history of curative HCC (Table 4).
| Patient No. | Sex | Age in yr | LC1 | HCC2 | Initial DAA | NS5A RASs | Second DAA | Second result | ||
| Before DAA | After DAA (invader) | After DAA (cycleave) | ||||||||
| 1 | Female | 73 | No | No | DCV/ASV | NA | Y93H L31F Q54H A92V | Y93 mutant L31 mutant | LDV/SOF/RBV | SVR |
| 2 | Female | 77 | Yes | Yes | DCV/ASV | NA | Y93H L31M Q24Q/R | Y93 mutant L31 mutant | LDV/SOF/RBV | Relapse |
| 3 | Female | 71 | Yes | No | DCV/ASV | NA | NA | Y93 wild-type L31mutant | LDV/SOF/RBV | SVR |
| 4 | Female | 78 | Yes | No | DCV/ASV | NA | NA | Y93 mutant L31 mutant | LDV/SOF/RBV | SVR |
| 5 | Male | 74 | Yes | Yes | DCV/ASV | NA | Y93H L31V Q54y Q62D | Y93 mutant L31 mutant | LDV/SOF | SVR |
| 6 | Female | 83 | Yes | Yes | DCV/ASV | Y93Y/H L31L | Y93H L31M L31V | Y93 mutant L31 wild-type | No | NA |
| 7 | Male | 71 | Yes | No | DCV/ASV | Y93Y L31L | NA | Y93 wild-type L31 mutant | DCV-TRIO | Undergoing |
| 8 | Female | 66 | No | No | LDV/SOF | Y93Y L31L | NA | Failure | Waiting | NA |
| 9 | Male | 78 | Yes | Yes | LDV/SOF | NA | NA | Failure | Waiting | NA |
Patients who failed to respond to the initial IFN-free DAA regimen were given second-line therapies. Four patients were enrolled to LDV/SOF with RBV therapy in another hepatitis core hospital in Kanagawa prefecture and SVR was achieved in 3 of these patients, with 1 relapsing. One patient treated with LDV/SOF achieved SVR. One patient is now undergoing daclatasvir/asunaprevir/beclabuvir (known as DCV-TRIO) treatment (Table 4).
Of the 25 patients having HCC history and treated with IFN-free DAA, 4 had recurrence to date. Of these, 2 came back with extremely rapid growth of HCC.
Multivariable logistic regression for SVR factors using patients with DCV/ASV treatment was performed using two models. Regression using all baseline variables as covariates (model 1) showed HCV RNA levels were independently associated with SVR. Model 2 was built with suspected variables from DAA failure patients (Table 5) and showed that only Y93 RAS was associated with SVR (Table 5).
| Odds ratio | 95%CI | P-value | |
| Model 1: All variables | |||
| Platelet count | 0.00 | -0.01-0.27 | 0.71 |
| AFP level | 0.00 | -0.00-0.01 | 0.44 |
| ALT level | 0.00 | -0.00-0.01 | 0.31 |
| HCV RNA level | 0.26 | 0.02-0.45 | 0.04a |
| Age | 0.02 | -0.01-0.04 | 0.14 |
| Sex | -0.13 | -0.39-0.12 | 0.28 |
| Y93 | 0.23 | -0.31-0.77 | 0.38 |
| L31 | -0.17 | -1.05-0.70 | 0.68 |
| History of HCC | -0.29 | -0.68-0.92 | 0.13 |
| Cirrhosis | -0.30 | -0.38-0.26 | 0.67 |
| Prior IFN | -0.15 | -0.41-0.99 | 0.21 |
| Model 2: Limited suspicious covariates | |||
| Age | 0.00 | -0.13-0.14 | 0.93 |
| Y93 | 0.48 | 0.08-0.87 | 0.02a |
| L31 | -0.42 | -1.09-0.24 | 0.2 |
| Cirrhosis | -0.15 | -0.37-0.08 | 0.19 |
This study of patients with HCV infection demonstrated that high SVR rates can be achieved with DAA regimens including IFN-based protease inhibitor and IFN-free DAAs. DAAs conferred good effectiveness and safety for both treatment-naïve patients and previously treated cases.
Until recently, PEG-IFN combined with RBV therapy was the only antiviral drug regimen capable of terminating HCV infection[8]. However, SVR was only achieved in about 50% of treated patients[17-19]. Many DAAs have been designed to improve this situation[20]. To activate the IFN pathway, telaprevir, boceprevir and simeprevir were introduced as 1st and 2nd generation HCV protease inhibitors[8-10,20]. However, these agents increase the risk of adverse events, such as anemia, renal failure and severe drug rash. In the initial IFN-free regimen, DCV/ASV eliminated IFN-related toxicity and achieved a SVR24 rate of 84% in chronic hepatitis C patients and 90.9% in liver cirrhosis cases in Japan[21]. The SVR12 rate of LDV/SOF was 100%[12] and for OBV/PTV/r it was 98%[22] in genotype 1 HCV. SOF/RBV and OBV/PTV/r have been approved for genotype 2 HCV, which accounts for up to 30% of chronic HCV infection and which is increasing in prevalence in Japan[23]. Although OBV/PTV/r was limited to use for genotype 2b, the SVR rate was 95-98% when RBV was used[23-25]. The use of IFN-free DAA enables the treatment of IFN ineligible/intolerant individuals with HCV infection.
A low rate of virological failure in genotype 1 was observed in patients with baseline Y93 or L31 variants in NS5A receiving DCV/ASV or OBV/PTV/r treatment[13,22]. It has been reported that pretreatment with NS5A RASs did not impact LDV/SOF therapy[26].
Moreover, there have been few reports of DCV/ASV failure because DCV/ASV is limited in Japan. Therefore, it might be important to report these cases for future research and treatment of HCV.
In the present report, the SVR rate of each therapy in Kanto Rosai Hospital was similar to previous reports[12,13,21,23,27]. In genotype 1 patients, 7 failures with DCV/ASV and 2 with LDV/SOF were reported. Among these, 7 patients were diagnosed cirrhosis and 4 patients who had a history of HCC were also reported. Y93 RAS was correlated to SVR failure in DCV/ASV cases. In 2 relapsers with LDV/SOF, DAA RAS could not detected. Subsequently, it was revealed that the core genotype of HCV was 1a and 2a in these patients.
We experienced 2 patients with subarachnoid hemorrhage and cerebral hemorrhage, and these discontinued LDV/SOF therapy. They were 51- and 68-year-old females without cirrhosis and other medical history. In 2016, postmarketing surveillance data were reported in Japan, and 31 cases of severe cerebrovascular disease were reported[28]. As far as we know, there is no detailed report about cerebrovascular adverse reaction. Therefore, the physiological mechanism underlying the cerebrovascular adverse events is unclear. Caution is needed when prescribing LDV/SOF therapy.
Two patients had aggressive and rapid HCC recurrence after treatment with DAA. The assumption that the use of DAAs may induce HCC relapse had been reported[29]. The surveillance of HCC must be taken strictly after DAA treatment in patients with prior HCC.
Recent reports demonstrated that the SVR rate was only 69% for salvage therapy for patients who failed to respond to NS5A inhibitors[30]. Prior DCV/ASV treatment is associated with a failure of LDV/SOF for multiple HCV NAS5A RASs[30,31].
We could not treat patients with LDV/SOF and RBV simultaneously because this treatment regimen has not been approved for general insurance. However, the ratio of SVR increased to 75% in initial DAA failure patients, even though multiple NS5A RASs were observed.
The achievement of an SVR of 100% for overall patients with HCV infection may be accomplished in the future.
This study had some limitations. First, data for RASs were not available for all cases. Due to the small sample size, the power of the multiple regression analysis remains low. Second, because this was a study from one hospital, the total number of treatment cases was small. Third, because DCV/ASV has only been approved in Japan, there are some limitations regarding the generalizability of the results. However, this study provides some important knowledge about HCV treatment.
In conclusion, DAA treatment for HCV infection is highly effective in Kanto Rosai Hospital. However, caution is needed for HCV NS5A RASs that are selected by HCV NS5A inhibitors because cerebrovascular adverse events are induced by some DAA drugs.
In a previous study, it was shown that resistance-associated substitutions (RASs) were predictors of direct-acting antiviral (DAA) failure. No significant adverse effect was reported in the DAA treatment in clinical trials. In this study, the prestudy hypothesis was that another predictor might exist concerning about DAA failure. Another hypothesis was that more severe adverse effects must occur in the real world because patients conditions were more severe than those of clinical trials.
DAAs have been approved for the treatment of hepatitis C virus (HCV) genotype 1 and 2 infections in Japan since 2011. In the new era of DAA therapy, predictors who fail to respond to DAA might be compromised by RASs. There have been few reports of daclatasvir/asunaprevir (DCV/ASV) failure because DCV/ASV is limited in Japan. Therefore, it might be important to report these cases for future research and treatment of HCV.
All patients with HCV infection who underwent DAA prescription were enrolled in this study. Overall, 177 participants treated with DAAs and in whom sustained virologic response at 12 wk after therapy (SVR12) was judged between November 2012 and March 2017 at Kanto Rosai Hospital were included.
HCV patients who underwent DAA prescription were enrolled in this study. Resistance analysis was performed by using direct sequencing and cycleave PCR. Multiple regression analysis was performed to evaluate factors related to loss of HCV RNA.
In total, 117 patients participated in the study, including 135 with genotype 1 and 42 with genotype 2. Of the 135 patients with genotype 1, 16 received protease inhibitor + interferon + ribavirin and all achieved SVR. Of the 119 patients who received interferon-free DAA (in different combinations), 102 achieved SVR while 9 failed, including 7/9 who were on DCV/ASV and 2/9 who were on ledipasvir/sofosbuvir. Efficacy analysis was done only for 42 patients who received DCV/ASV. From this analysis, Y93 RASs were significantly correlated with SVR.
The SVR rate was 98% for genotype 1 and 100% for genotype 2. NS5A RASs are most likely to affect the outcomes of DAA therapy in our facility.
The SVR rate was 98% for genotype 1 and 100% for genotype 2. However, caution is needed for HCV NS5A RASs that are selected by HCV NS5A inhibitors because cerebrovascular adverse events are induced by some DAA drugs.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Hann HW, Kao JT, Rezaee-Zavareh MS, Toyoda T S- Editor: Kong JX L- Editor: Filipodia E- Editor: Li D
| 1. | Ray Kim W. Global epidemiology and burden of hepatitis C. Microbes Infect. 2002;4:1219-1225. [PubMed] |
| 2. | Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011;17:107-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 919] [Cited by in RCA: 945] [Article Influence: 67.5] [Reference Citation Analysis (2)] |
| 3. | Zhu RX, Seto WK, Lai CL, Yuen MF. Epidemiology of Hepatocellular Carcinoma in the Asia-Pacific Region. Gut Liver. 2016;10:332-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 266] [Cited by in RCA: 362] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 4. | Nishiguchi S, Kuroki T, Nakatani S, Morimoto H, Takeda T, Nakajima S, Shiomi S, Seki S, Kobayashi K, Otani S. Randomised trial of effects of interferon-alpha on incidence of hepatocellular carcinoma in chronic active hepatitis C with cirrhosis. Lancet. 1995;346:1051-1055. [PubMed] |
| 5. | Tanaka H, Imai Y, Hiramatsu N, Ito Y, Imanaka K, Oshita M, Hijioka T, Katayama K, Yabuuchi I, Yoshihara H. Declining incidence of hepatocellular carcinoma in Osaka, Japan, from 1990 to 2003. Ann Intern Med. 2008;148:820-826. [PubMed] |
| 6. | Umemura T, Ichijo T, Yoshizawa K, Tanaka E, Kiyosawa K. Epidemiology of hepatocellular carcinoma in Japan. J Gastroenterol. 2009;44 Suppl 19:102-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 156] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 7. | Goh GB, Chang PE, Tan CK. Changing epidemiology of hepatocellular carcinoma in Asia. Best Pract Res Clin Gastroenterol. 2015;29:919-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 114] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 8. | Kumada H, Toyota J, Okanoue T, Chayama K, Tsubouchi H, Hayashi N. Telaprevir with peginterferon and ribavirin for treatment-naive patients chronically infected with HCV of genotype 1 in Japan. J Hepatol. 2012;56:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 231] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 9. | Hayashi N, Izumi N, Kumada H, Okanoue T, Tsubouchi H, Yatsuhashi H, Kato M, Ki R, Komada Y, Seto C. Simeprevir with peginterferon/ribavirin for treatment-naive hepatitis C genotype 1 patients in Japan: CONCERTO-1, a phase III trial. J Hepatol. 2014;61:219-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 10. | Izumi N, Hayashi N, Kumada H, Okanoue T, Tsubouchi H, Yatsuhashi H, Kato M, Ki R, Komada Y, Seto C. Once-daily simeprevir with peginterferon and ribavirin for treatment-experienced HCV genotype 1-infected patients in Japan: the CONCERTO-2 and CONCERTO-3 studies. J Gastroenterol. 2014;49:941-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 11. | Pawlotsky JM. NS5A inhibitors in the treatment of hepatitis C. J Hepatol. 2013;59:375-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 149] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 12. | Mizokami M, Yokosuka O, Takehara T, Sakamoto N, Korenaga M, Mochizuki H, Nakane K, Enomoto H, Ikeda F, Yanase M. Ledipasvir and sofosbuvir fixed-dose combination with and without ribavirin for 12 weeks in treatment-naive and previously treated Japanese patients with genotype 1 hepatitis C: an open-label, randomised, phase 3 trial. Lancet Infect Dis. 2015;15:645-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 301] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 13. | Suzuki Y, Ikeda K, Suzuki F, Toyota J, Karino Y, Chayama K, Kawakami Y, Ishikawa H, Watanabe H, Hu W. Dual oral therapy with daclatasvir and asunaprevir for patients with HCV genotype 1b infection and limited treatment options. J Hepatol. 2013;58:655-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 210] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 14. | Izumi N. Diagnostic and treatment algorithm of the Japanese society of hepatology: a consensus-based practice guideline. Oncology. 2010;78 Suppl 1:78-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Itakura J, Kurosaki M, Takada H, Nakakuki N, Matsuda S, Gondou K, Asano Y, Hattori N, Itakura Y, Tamaki N. Naturally occurring, resistance-associated hepatitis C virus NS5A variants are linked to interleukin-28B genotype and are sensitive to interferon-based therapy. Hepatol Res. 2015;45:E115-E121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Kinugasa H, Ikeda F, Takaguchi K, Mori C, Matsubara T, Shiraha H, Takaki A, Iwasaki Y, Toyooka S, Yamamoto K. Low frequency of drug-resistant virus did not affect the therapeutic efficacy in daclatasvir plus asunaprevir therapy in patients with chronic HCV genotype-1 infection. Antivir Ther. 2016;21:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Jr . Haussinger D, Diago M, Carosi G, Dhumeaux D, Craxi A, Lin A, Hoffman J, Yu J. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4847] [Cited by in RCA: 4747] [Article Influence: 206.4] [Reference Citation Analysis (0)] |
| 18. | Hadziyannis SJ, Sette H, Jr . Morgan TR, Balan V, Diago M, Marcellin P, Ramadori G, Bodenheimer H, Jr., Bernstein D, Rizzetto M, Zeuzem S, Pockros PJ, Lin A, Ackrill AM. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346-355. [PubMed] |
| 19. | Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-965. [PubMed] |
| 20. | Asselah T, Marcellin P. New direct-acting antivirals’ combination for the treatment of chronic hepatitis C. Liver Int. 2011;31 Suppl 1:68-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 21. | Kumada H, Suzuki Y, Ikeda K, Toyota J, Karino Y, Chayama K, Kawakami Y, Ido A, Yamamoto K, Takaguchi K. Daclatasvir plus asunaprevir for chronic HCV genotype 1b infection. Hepatology. 2014;59:2083-2091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 468] [Cited by in RCA: 453] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 22. | Kumada H, Chayama K, Rodrigues L, Jr . Suzuki F, Ikeda K, Toyoda H, Sato K, Karino Y, Matsuzaki Y, Kioka K, Setze C, Pilot-Matias T, Patwardhan M, Vilchez RA, Burroughs M, Redman R. Randomized phase 3 trial of ombitasvir/paritaprevir/ritonavir for hepatitis C virus genotype 1b-infected Japanese patients with or without cirrhosis. Hepatology. 2015;62:1037-1046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 134] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 23. | Omata M, Nishiguchi S, Ueno Y, Mochizuki H, Izumi N, Ikeda F, Toyoda H, Yokosuka O, Nirei K, Genda T. Sofosbuvir plus ribavirin in Japanese patients with chronic genotype 2 HCV infection: an open-label, phase 3 trial. J Viral Hepat. 2014;21:762-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 163] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 24. | Shafran SD, Shaw D, Charafeddine M, Agarwal K, Foster GR, Abunimeh M, Pilot-Matias T, Pothacamury RK, Fu B, Cohen E. Efficacy and safety results of patients with HCV genotype 2 or 3 infection treated with ombitasvir/paritaprevir/ritonavir and sofosbuvir with or without ribavirin (QUARTZ II-III). J Viral Hepat. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Schnell G, Tripathi R, Krishnan P, Beyer J, Reisch T, Irvin M, Dekhtyar T, Setze C, Rodrigues-Jr L, Alves K. Resistance characterization of hepatitis C virus genotype 2 from Japanese patients treated with ombitasvir and paritaprevir/ritonavir. J Med Virol. 2018;90:109-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Mizokami M, Dvory-Sobol H, Izumi N, Nishiguchi S, Doehle B, Svarovskaia ES, De-Oertel S, Knox S, Brainard DM, Miller MD. Resistance Analyses of Japanese Hepatitis C-Infected Patients Receiving Sofosbuvir or Ledipasvir/Sofosbuvir Containing Regimens in Phase 3 Studies. J Viral Hepat. 2016;23:780-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Backus LI, Belperio PS, Shahoumian TA, Loomis TP, Mole LA. Real-world effectiveness of ledipasvir/sofosbuvir in 4,365 treatment-naive, genotype 1 hepatitis C-infected patients. Hepatology. 2016;64:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 161] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 28. | Giliead. Post-marketing surveillance of ledipasvir/sofosbuvir 2015-2016. Available from: https://www.harvoni.jp/~/media/files/gilead/harvoni/proper/hvn_post_marketing_surveillance_final_report.pdf?la=ja-jp. |
| 29. | Reig M, Marino Z, Perello C, Inarrairaegui M, Ribeiro A, Lens S, Diaz A, Vilana R, Darnell A, Varela M. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol. 2016;65:719-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 803] [Article Influence: 89.2] [Reference Citation Analysis (0)] |
| 30. | Akuta N, Sezaki H, Suzuki F, Fujiyama S, Kawamura Y, Hosaka T, Kobayashi M, Kobayashi M, Saitoh S, Suzuki Y. Ledipasvir plus sofosbuvir as salvage therapy for HCV genotype 1 failures to prior NS5A inhibitors regimens. J Med Virol. 2017;89:1248-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | Iio E, Shimada N, Takaguchi K, Senoh T, Eguchi Y, Atsukawa M, Tsubota A, Abe H, Kato K, Kusakabe A. Clinical evaluation of sofosbuvir/ledipasvir in patients with chronic hepatitis C genotype 1 with and without prior daclatasvir/asunaprevir therapy. Hepatol Res. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |