Published online Jan 27, 2018. doi: 10.4254/wjh.v10.i1.73
Peer-review started: November 22, 2017
First decision: December 7, 2017
Revised: December 15, 2017
Accepted: January 9, 2018
Article in press: January 9, 2018
Published online: January 27, 2018
Processing time: 65 Days and 9.9 Hours
To explore the relationship between collagen proportionate area (CPA) and portal hypertension-related clinical manifestations in alcoholic liver disease (ALD).
Retrospective study with chart review of patients with ALD adressed to our center between January 2012 and December 2013 for a transjugular liver biopsy (TJLB) and hepatic hemodynamic study. Patients were included if they met the following criteria: (1) Medical indication for a liver biopsy in the setting of ALD; (2) recent (< 15 d) clinical, radiological, endoscopic and biological data available; and (3) estimated follow-up of at least 6 mo. Liver tissue from cirrhotic subjects obtained from transjugular liver biopsies was stained with PicroSirius red and computer-assisted digital image analysis to determine fibrosis density using CPA was performed.
We included 61 patients with alcoholic ALD, subdivided in 41 active alcohol drinkers and 20 durably abstinent patients. Nine healthy liver donors served as controls. Mean CPA in patients with ALD was 7.1%, with no difference between active drinkers and abstinent patients (P = 0.17). Using a fibrosis density cutoff of 5%, we observed a positive correlation between high fibrosis density and the hepatic venous pressure gradient (HVPG) only in active drinkers (P = 0.02). At 12-mo of follow-up, in the group of active alcohol drinkers, patients reaching a composite outcome showed a higher HVPG value as compared to those who did not (18.5 mmHg vs 14.5 mmHg P < 0.04) whereas CPA values were similar (6.9% vs 11%, P = 0.23).
In active alcoholic ALD, CPA correlates to portal pressure but only HVPG predicts clinical events, pointing to the role of alcohol as a modulator of portal hypertension.
Core tip: This is the first study exploring the relationships between fibrosis density assessed by collagen proportionate area (CPA) in liver biopsy, hepatic venous pressure gradient (HVPG) and development of clinical manifestations of portal hypertension in patients with chronic advanced alcoholic liver disease addressed for liver investigations. The results suggest a positive correlation between high fibrosis density and the HVPG only in active drinkers. HVPG, but not CPA, predicts clinical events in active alcohol drinkers pointing to the role of alcohol as a modulator of portal hypertension.
- Citation: Restellini S, Goossens N, Clément S, Lanthier N, Negro F, Rubbia-Brandt L, Spahr L. Collagen proportionate area correlates to hepatic venous pressure gradient in non-abstinent cirrhotic patients with alcoholic liver disease. World J Hepatol 2018; 10(1): 73-81
- URL: https://www.wjgnet.com/1948-5182/full/v10/i1/73.htm
- DOI: https://dx.doi.org/10.4254/wjh.v10.i1.73
Determination of liver fibrosis is crucial in the management of patients with chronic liver disease[1]. Fibrosis is associated with the development of portal hypertension (PHT) which has a strong negative impact on patients’ outcome and survival[2]. The diagnosis of liver fibrosis is often made at an advanced stage in the population of excessive drinkers, when they come to medical attention for portal-hypertensive complications[3,4]. The development of fibrosis is of particular interest in alcoholic liver disease (ALD) as compared to other etiologies, with regards to the pattern of distribution (pericellular, centrilobular, periportal)[5,6], the large amount of fibrosis[7] and the typical histological lesions (including steatohepatitis) reported to accelerate disease progression[8].
Traditionally, liver biopsy is the gold standard for the detection and staging of liver fibrosis. However, recent advances in non-invasive strategies, including serum markers, transient elastography and other elasticity-based radiological techniques are now able to provide reliable information on liver fibrosis (no significant fibrosis vs advanced fibrosis or cirrhosis) and may avoid unnecessary liver biopsy in a subset of patients with viral[9] or alcohol related liver disease[10]. However, when a liver biopsy is indicated, the transjugular route allows to obtain both a liver tissue specimen and the measurement of the hepatic venous pressure gradient (HVPG) which is the reference method to measure portal pressure in the clinical setting[11]. This parameter is a predictor of clinical decompensation and PHT-related complications when equal or superior to 10 mmHg[11], and may be influenced by factors including histological lesions[12] and alcohol intake[13]. Thus, both architectural distortion of liver lobule by fibrosis and dynamic components participate to intrahepatic resistance to blood flow[14] and are associated with increased HVPG value in patients with ALD.
Fibrosis in a liver biopsy can be assessed by histological staging systems that are based on a semi-quantitative evaluation and provide information on the importance of architectural changes. The optimal histological staging system for ALD is not universally accepted. Common tools such as Ishak and METAVIR have been mostly developed in chronic viral hepatitis in which fibrosis predominates around the portal tracts. Thus, application of a score used in non-alcoholic fatty liver disease might be more appropriate in patients with ALD, as both diseases share several similarities in terms of pathogenic mechanisms and morphological alterations.
The quantitative measurement of liver fibrosis by a computer-assisted digital image analysis of a liver tissue specimen collagen proportionate area (CPA) overcomes the limitations of semiquantitative scores, demonstrated a good correlation with HVPG and noninvasive markers of fibrosis[15], and is of prognostic significance. These results, however, have been mostly obtained in HCV-related liver disease[16,17], and very few data are available in ALD. Therefore, we undertook the present study to explore the relationships between fibrosis density in liver biopsy, HVPG and development of clinical manifestations of PHT in patients with chronic advanced alcoholic liver disease (cALD) addressed for liver investigations. We aimed to better understand the relative prognostic contribution of HVPG and liver fibrosis quantification in subjects with advanced ALD.
Consecutive patients with ALD adressed to the Gastroenterology and Hepatology Division of Geneva University Hospitals between January 2012 and December 2013 for a transjugular liver biopsy (TJLB) and hepatic hemodynamic study were eligible for this study. Both active alcoholic patients and abstinent patients were eligible for inclusion. Abstinent patients were defined as patients who did no drink any glass of alcohol for the last 6 mo before the inclusion. Abstinence or relapse status was self reported. Patients were included if they met the following criteria: (1) Medical indication for a liver biopsy in the setting of ALD; (2) recent (< 15 d) clinical, radiological, endoscopic and biological data available; and (3) estimated follow-up of at least 6 mo. Complete portal vein thrombosis, multifocal hepatocellular carcinoma and coexistent sepsis were considered as exclusion criteria. A group of healthy candidates for living donation, who underwent a protocol TJLB, served as controls. It is our policy to perform a liver biopsy early in patients eligible for living donation in agreement with our institutional ethical committee.
Liver function tests and clinical symptoms used for the determination of both Child-Pugh and model for end-stage liver disease (MELD) scores were recorded at baseline. During a 12-mo follow-up, liver related death or liver transplantation, as well as clinically relevant episodes including ascitic decompensation, overt episodes of hepatic encephalopathy (HE) and PHT-related bleeding were carefully documented and considered composite events. Information regarding alcohol consumption or abstinence (given by family or relatives, or extrapolated from biological samples (blood or urine alcohol, liver tests) was extracted from patients file.
Two trained operators (LS and NG) experts in TJLB performed all the procedures using a standard procedure and material that included both a TJL-101-ET needle set (Cook Europe, Bjaeverskov, Denmark) and an 8F curved catheter (Cordis Europa, Amsterdam, The Netherlands). Under light sedation, the right jugular vein was punctured and the catheter introduced in the right hepatic vein under radiological guidance. The catheter was wedged into a small hepatic venule in order to block blood flow, followed by injection of iodinated contrast media to verify the proper position of the catheter in a wedge position. A stable tracing on the monitor was required to accept the measure as valid, while taking the mid-chest as the external zero reference. The free hepatic venous pressure was measured while the catheter was floating in the hepatic vein close to the ostium of the vena cava. The mean value of at least two measurements of wedge and free pressures recorded in different vascular territories in the right liver lobe were kept for analysis. The difference between wedge- and free hepatic venous pressure, named the HVPG, is the parameter used to assess PHT in a clinical setting[11].
Liver stiffness, expressed in kilopascals (kPa), was measured by transient elastometry using the Fibroscan (Echosens®, Paris, France) in patients without ascites, following the recommended criteria for valid measurements[9]. The value measured in advanced fibrosis or cirrhosis are in the range of 15 kPa or higher, with both the M and XL probes providing values of similar performance[18]. The examinators performing liver stiffness measurements (SR, NG, LS) were not aware of HVPG values.
Liver biopsy samples were formalin fixed, paraffin embedded, and serial sections were stained with hematoxylin and eosin, Masson Trichrome and PicroSirius red. The histopathological specimens were thoroughly examined by an expert in liver pathology (LRB) using standard high-power field views, as previously described[19]. We analyzed the presence and severity of the following features, using a scoring system derived from a recent publication on non alcoholic fatty liver[20]: Steatosis (above 33% of hepatocytes: marked steatosis), ballooning (> 2 enlarged hepatocytes with clear reticular cytoplasm on high power field (× 49): Marked ballooning degeneration), and inflammation (< 2 foci of inflammatory cells in the lobule: mild inflammation; > 2 foci: marked inflammation). A detailed fibrosis evaluation was not performed as all patients presented at an advanced stage of ALD that reached the stage of cirrhosis. The examiner was blinded to patients’ hemodynamic and liver stiffness data.
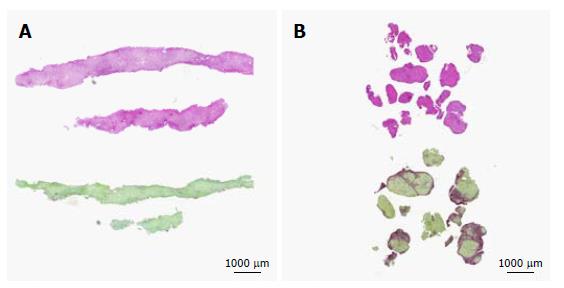
The PicroSirius red tissue section was used for CPA using digital image analysis, as reported[16]. Images of the entire specimen were acquired with a digital camera (Leica DC 300 F) connected to a high resolution DMRBE Leica microscope, using a × 10 objective and a × 10 magnification lens. Collagen proportionate area was subsequently quantified using the Qwin Leica Q550IW software (Meyer Instr. TX, United States) which is particularly suited for quantification of microscopic alterations. This fine quantification method allowed us to determine the percentage of PicroSirius red positive area in the biopsy specimen, expressed as 0%-5%, 5%-10%, 10%-20% and > 20%, and represented the fibrosis density (Figure 1). Significant fibrosis was defined as CPA > 5%. This procedure was performed by two trained investigators (SR and NG) under the supervision of the pathologist (LRB).
Variables are reported as median and interquartile range (IQR). Comparisons between groups were performed using the Wilcoxon signed rank, chi-square or Fischer’s exact tests as appropriate. Correlations between variables were evaluated by Spearman correlation. The results are presented as odds ratios (OR) with 95%CI. A P value < 0.05 was considered statistically significant. Statistical analyses were performed with the program R (R Foundation for Statistical Computing, version 3.0).
The study population consisted of 9 healthy subjects and 61 patients with cALD, divided in 20 patients with longstanding abstinence from alcohol (> 6 mo), and 41 patients who consumed regularly alcohol. The patients’ clinical characteristics are described in Table 1. They were predominantly male, with Child-Pugh B cirrhosis, the majority of patients presenting with clinically significant PHT manifest as ascites and esophageal varices, and a history of HE. The mean HVPG value was 18 mmHg in both groups. Only few patients benefited from a transient elastography (n = 12, 12/41 patients in the group of active alcohol drinkers, and 0/20 in the abstinent group) precluding any comparison. This was mostly due to technical limitations related to the presence of ascites or poor quality of measures. Overall, in this population with cALD at a cirrhotic stage, abstinent and active alcohol drinkers presented similar clinical characteristics with regard to the degree of liver failure, HVPG and clinical manifestations of PHT. Only serum albumin was decreased in active alcohol consumers vs abstinent patients (23 ± 2 gr/L vs 29.5 ± 1.8 gr/L, P = 0.01).
| Variable | Controls (n = 9) | Alcohol abstinents (n = 20) | Active alcohol drinkers (n = 41) | P value (abstinent vs active alcohol drinkers) |
| Age (yr) | 38.2 (35-49) | 59.5 (55-65) | 56.9 (52-63) | 0.40 |
| Male sex | 6 (67%) | 16 (80%) | 33 (80%) | 1.0 |
| Ascites | 0 | 11 (61%) | 23 (64%) | 1.0 |
| HE | 0 | 3 (16%) | 7 (21%) | 1.0 |
| Esophageal varices | 0 | 15 (83%) | 25 (69%) | 0.30 |
| HVPG (mmHg) | 2 (2-3) | 18 (16-19) | 18 (14-20) | 0.90 |
| Liver stiffness (kPa) | 3.8 (3-3.8) | Not available | 38.6 (14.6-68.2) | - |
| Platelet count (G/L) | 286 (170-340) | 44.5 (20-55) | 33.5 (20-53) | 0.40 |
| Child-Pugh score | - | 9.5 (8-10) | 9 (8-11) | 0.94 |
| MELD score | - | 14 (12-19) | 19 (11-22) | 0.50 |
At 12 mo, survival without liver transplantation was 84%. During follow-up, 7 patients died of liver-related causes (4/20 in active alcohol drinkers and 3/41 in abstinent patients), and 3 patients from the abstinent group underwent liver transplantation. A return to regular, moderate alcohol consumption (20-30 gr/d) was reported in the group of patients who qualified as abstinent at baseline. All but 3 patients from the active alcohol drinkers group persisted in a regular alcohol consumption (30-50 g/d) during follow-up.
At 12 mo, a composite clinical outcome including ascitic decompensation, portal hypertensive bleeding, or episode of overt HE was reported in 32 patients, with ascites requiring large volume paracentesis being the most prevalent complication. A transjugular intrahepatic shunt (TIPS) procedure was performed in 5 patients, all from the active alcohol group to treat manifestations of PHT.
Liver biopsy was successful in all patients. It yielded material that measured 8.1 mm (6.8-14.2) in length that was sufficient for an accurate histological diagnosis and additional liver tissue studies, as previously reported[19,21]. In all patients, fragmented material showing diffuse architectural changes with nodular formation and extensive fibrosis were consistent with the diagnosis of cirrhosis. Results of histological evaluation are presented in Table 2. Marked steatosis and ballooned hepatocytes were more prominent in the subgroup of active alcohol drinkers as compared to abstinent patients, an observation which is consistent with published data[22]. Mild lobular inflammation composed in a majority of mononuclear cells was present in all patients with cALD. One patient from the active alcohol group demonstrated marked inflammation without reaching the histological criteria for alcoholic hepatitis.
| Variable | Controls (n = 9) | Alcohol abstinents (n = 20) | Active alcohol drinkers (n = 41) | P value (abstinent vs active alcohol drinkers) |
| Cirrhosis | 0 (9%) | 20 (100%) | 41 (100%) | 1.0 |
| Marked steatosis | 0 (0%) | 1 (5%) | 21 (51%) | 0.001 |
| Marked ballooned hepatocytes | 0 (0%) | 5 (26%) | 25 (64%) | 0.01 |
| Marked inflammation | 0 (0%) | 0 (0%) | 1 (2%) | 0.9 |
| Fibrosis density (%) | 0.7 (0.2-1.5) | 3.8 (1.1-11.8) | 8.2 (3.8-14.1) | 0.17 |
| Fibrosis category | 0.48 | |||
| 0%-5% | 9 | 11 | 15 | |
| 5%-10% | 0 | 3 | 9 | |
| 10%-20% | 0 | 3 | 12 | |
| > 20% | 0 | 3 | 5 |
Determination of fibrosis density by CPA could be performed in all patients and controls, demonstrating, as expected, higher values in patients as compared to controls (Figure 1B). The inter-rater agreement (SR and NG) was good with a kappa index of 0.9. In patients with ALD, the fibrosis density tended to be higher in active alcohol drinkers as compared to alcohol abstinent patients, but without reaching a statistically significant level [8.2% (3.4-14.1) vs 3.8% (1.-11.8), P = 0.17, Table 2]. In the subgroup of active alcohol users, using a cut-off value of 5% of fibrosis density (derived from the median value in the whole patient group), patients with low fibrosis (< 5%) had a lower value of HVPG as compared to those with high fibrosis (> 5%) (16 ± 1.9 mmHg vs 19 ± 2 mmHg, P < 0.01, Table 3 and Figure 1B). Within subjects with active or abstinent alcohol abuse, in multivariate analysis including HVPG, drinking status and sex, only HVPG was independently associated with fibrosis density [OR 1.2 per unit increase in HVPG, 95%CI (1.1-1.4), P = 0.01].
| Variable | Fibrosis density ≤ 5% (n = 26) | Fibrosis density > 5% (n = 35) | P value |
| Active alcohol | 15 (58%) | 26 (74%) | 0.27 |
| Age (yr) | 58 (55-64) | 57 (52-63) | 0.4 |
| Male sex | 24 (92%) | 25 (71%) | 0.06 |
| Ascites | 12 (55%) | 22 (69%) | 0.4 |
| HE | 7 (32%) | 3 (10%) | 0.07 |
| Esophageal varices | 15 (65%) | 25 (81%) | 0.2 |
| HVPG (mmHg) | 16 (11-18) | 19 (17-20) | 0.01 |
| Liver stiffness (kPa) | 19 (12-42) | 57 (34-72) | 0.5 |
| Platelet count (G/L) | 37 (20-52) | 38 (20-55) | 0.7 |
| MELD score | 14 (11-19) | 18 (13-22) | 0.2 |
| Histology | |||
| Marked steatosis | 11 (42%) | 11 (31%) | 0.4 |
| Marked ballooned hepatocytes | 9 (36%) | 21 (64%) | 0.06 |
| Marked inflammation | 0 (0%) | 1 (3%) | 0.9 |
Except for a trend towards more ballooned hepatocytes in the high fibrosis group, other histological features were similar with regards to fibrosis density in the liver biopsy.
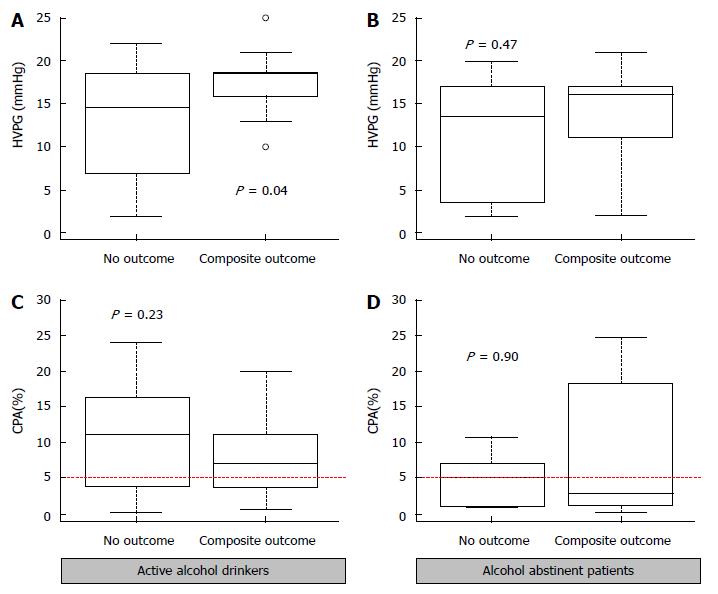
In the whole group, fibrosis density measured by CPA correlated with HVPG using a 5% cut-off value (Figure 2A). In a subgroup analysis, this correlation was conserved only in active drinkers (see Figure 2B). Figure 3 and Supplementary Table 1 illustrate the relationship between CPA, HVPG and clinical outcome in the subgroups of abstinent and active alcohol drinkers. In the group of active drinkers, patients who reached a composite outcome showed a higher HVPG value as compared to those who did not [19 (16-19) mmHg vs 15 (7.5-18) mmHg P = 0.04]. Such a difference could not be observed using the CPA [6.9 (3.9-11) % vs 11 (4.0-16) %, P = 0.23]. In the group of patients abstinent from alcohol, neither HVPG (P = 0.47) nor CPA (P = 0.90) were associated with the development of a composite clinical outcome during follow-up.
The severity of histological features was not related to CPA or HVPG values, and were not associated with the development of a complication of PHT.
In the group of patients with chronic advanced alcoholic liver disease presenting with moderate to severe liver insufficiency and clinical manifestations of PHT, we demonstrate a clear relationship between portal pressure and the precise quantification of liver fibrosis by CPA in transjugular liver biopsies. In multivariate analysis, only HVPG was independently associated with fibrosis density (OR 1.2 per unit increase in HVPG, 95% CI [1.1-1.4], P = 0.01). In this apparently uniform group of alcoholics, we could objectivate differences in the level of HVPG between active drinkers and abstinent patients according to the density of liver fibrosis. Finally, high HVPG in active alcohol drinkers (but not in abstinent patients) was associated with future complications of PHT during follow-up.
The superiority of quantification of liver collagen over semi-quantitative methods to predict clinical outcome has been mostly demonstrated in chronic liver diseases of various etiologies and degrees of liver failure[16]. This approach is of interest to assess fibrosis in liver tissue obtained by needle biopsy, a situation where the number and size of nodules and fibrous septa may be difficult to evaluate with regards to the small size of the specimen. Although biopsies obtained by transjugular route are smaller in size and more fragmented as compared to percutaneous sampling, this method allows an accurate histological diagnosis[21] and a simultaneous measurement of the HVPG. We believe that our results are valid, as we also provide data in healthy subjects submitted to the same CPA and HVPG measures demonstrating striking differences, as expected.
To the best of our knowledge, this is the first study to explore the relationship between CPA and HVPG in a well characterized and homogeneous group of patients with ALD at an advanced stage, and to provide a separate analysis between abstinent and actively drinking subjects. The specificity of ALD as compared to chronic liver diseases of other etiologies includes an elevated density of liver fibrosis[7] and the strong influence of alcohol on PHT complications[13,23]. The former relates to the important collagen deposition and dense perivenular pattern of fibrosis typical of ALD[7], and the latter is associated with the increased intrahepatic resistance promoted by cofactors such as endothelial dysfunction, inflammation and marked steatosis associated with acute alcohol intake[24]. CPA positively correlates with HVPG only in active drinkers. Our findings suggest that active alcohol consumption may influence portal hemodynamic in addition to existing architectural changes due to cirrhosis. Accordingly, an oral administration of 0.5 gr/kg of ethanol increases both HVPG and azygos blood flow in patients with alcoholic cirrhosis and may precipitate variceal bleeding[13]. This observation is consistent with the higher value of HVPG in active alcohol drinkers who developed a clinical complication of PHT during follow-up as compared to those without clinical decompensation[25]. We were not able to identify an influence of histological lesions on parameters such as CPA and HVPG. We speculate that major architectural changes of cirrhosis present in all patients may have blunted the possible role of lesions such as marked steatosis or inflammation on the HVPG.
In view of these results, what would be the use of CPA as a fine quantitative method of measuring fibrosis density in liver biopsy of ALD patients? The diagnosis of cirrhosis is best defined on histological criteria, and subclassification of fibrosis by semi-quantitative scores allows to grade the severity of the disease[26]. Direct quantification of collagen by CPA is another way to provide a detailed analysis of fibrosis and to predict clinical outcomes in patients with cirrhosis of mixed etiologies[16]. In our patients with cirrhotic ALD, high CPA values did not correlate with clinical events. Thus, in this situation, measurement of fibrosis density adds no clinically relevant information. Determination of the amount of fibrosis at an earlier stage of perisinusoidal collagen deposition could: (1) bring valuable prognostic information alone or in association with existing scores; (2) be closely correlated to liver stiffness as a non invasive monitoring method[15]; and (3) provide a promising research tool to monitor the possible regression of fibrosis.
The strength of this study includes a well characterized population of patients with chronic advanced ALD submitted to both hemodynamic and histological baseline evaluation and close follow-up during 12 mo. Nevertheless, we acknowledge that our study suffers from several limitations. First, we limited our study to patients with advanced ALD all at a cirrhotic stage, without providing data on the entire clinical spectrum of ALD. However, we decided to focus on cirrhotic subjects as they are the highest risk of clinical events and HVPG measurement have mostly been validated as prognostic factors in this population Secondly, only a minority of patients had liver stiffness measurement precluding any comparisons with CPA and HVPG. However, the usefulness of liver stiffness is limited in this population as ascites, a frequent complication of cirrhosis, limits the performance of liver stiffness measurement. Third, information on abstinence was self-reported leading to a risk of information bias that could possibly preclude association between HVPG/CPA and clinical outcomes in abstinent patients.
In conclusion, quantification of fibrosis on transjugular liver biopsy in advanced ALD correlated to portal pressure in the subgroup of active drinkers. The HVPG, but not CPA, predicts clinical events in active alcohol drinkers pointing to the role of alcohol as an important modulator of PHT.
Fibrosis staging in a liver biopsy is based on a semi-quantitative evaluation by the pathologist however inter-observer concordance may be a limiting factor. The quantitative measurement of liver fibrosis by a computer-assisted digital image analysis of a liver tissue specimen collagen proportionate area (CPA) overcomes some limitations of semiquantitative scores.
Previous studies have shown a good correlation between CPA and hepatic venous pressure gradient (HVPG) and association of CPA with prognosis. However, these results have been mostly obtained in HCV-related liver disease, and very few data are available in alcoholic liver disease (ALD).
The objective of the study was to explore the relationships between fibrosis density in liver biopsy, HVPG and development of clinical manifestations of portal hypertension (PHT) in patients with chronic advanced alcoholic liver disease (cALD) addressed for liver investigations. We aimed to better understand the relative prognostic contribution of HVPG and CPA in subjects with advanced ALD.
We conduced a retrospective study with chart review of patients with ALD adressed to our center between January 2012 and December 2013 for a transjugular liver biopsy (TJLB) and hepatic hemodynamic study. Patients were included if they met the following criteria: (1) Medical indication for a liver biopsy in the setting of ALD; (2) recent (< 15 days) clinical, radiological, endoscopic and biological data available; (3) estimated follow-up of at least 6 mo. Liver tissue from cirrhotic subjects obtained from transjugular liver biopsies was stained with PicroSirius red and computer-assisted digital image analysis to determine fibrosis density using CPA was performed.
We included 61 patients with alcoholic ALD, subdivided in 41 active alcohol drinkers and 20 durably abstinent patients. Nine healthy liver donors served as controls. Mean CPA in patients with ALD was 7.1%, with no difference between active drinkers and abstinent patients (P = 0.17). Using a fibrosis density cutoff of 5%, we observed a positive correlation between high fibrosis density and the hepatic venous pressure gradient (HVPG) only in active drinkers (P = 0.02). At 12-month of follow-up, in the group of active alcohol drinkers, patients reaching a composite outcome showed a higher HVPG value as compared to those who did not (18.5 mmHg vs 14.5 mmHg P < 0.04) whereas CPA values were similar (6.9% vs 11%, P = 0.23).
This is the first study exploring the relationships between fibrosis density assessed by CPA in liver biopsy, HVPG and development of clinical manifestations of portal hypertension in patients with ALD addressed for liver investigations. The results of this study suggest a positive correlation between high fibrosis density (using a fibrosis density cutoff of 5%), and HVPG only in active drinkers. At 12-mo of follow-up, in the group of active alcohol drinkers, patients reaching a composite outcome showed a higher HVPG value as compared to those who did not. Therefore, HVPG, but not CPA, predicts clinical events in active alcohol drinkers pointing to the role of alcohol as a modulator of portal hypertension.
The validation of CPA as a quantitative method of measuring fibrosis density in liver biopsy of ALD patients requires further investigations in order to determine a correlation with clinical events, in particular overall and liver-related mortality in various subgroup of patients with difference etiologies of liver disease.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Switzerland
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B, B
Grade C (Good): 0
Grade D (Fair): D, D, D
Grade E (Poor): 0
P- Reviewer: Carvalho-Filho RJ, Gorrell MD, Kohla MAS, Namisaki T, Safer AM, Vanni E, Wang H S- Editor: Kong JX L- Editor: A E- Editor: Li RF
| 1. | Trautwein C, Friedman SL, Schuppan D, Pinzani M. Hepatic fibrosis: Concept to treatment. J Hepatol. 2015;62:S15-S24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Ripoll C, Bañares R, Rincón D, Catalina MV, Lo Iacono O, Salcedo M, Clemente G, Núñez O, Matilla A, Molinero LM. Influence of hepatic venous pressure gradient on the prediction of survival of patients with cirrhosis in the MELD Era. Hepatology. 2005;42:793-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 177] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 3. | Rehm J, Samokhvalov AV, Shield KD. Global burden of alcoholic liver diseases. J Hepatol. 2013;59:160-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Lackner C, Bataller R, Burt A, Miquel R, Schuppan D, Tiniakos D, Trauner M. Fibrosis evaluation by transient elastography in alcoholic liver disease: Is the histological scoring system impacting cutoff values? Hepatology. 2017;65:1758-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Savolainen V, Perola M, Lalu K, Penttilä A, Virtanen I, Karhunen PJ. Early perivenular fibrogenesis--precirrhotic lesions among moderate alcohol consumers and chronic alcoholics. J Hepatol. 1995;23:524-531. [PubMed] [DOI] [Full Text] |
| 6. | Michalak S, Rousselet MC, Bedossa P, Pilette C, Chappard D, Oberti F, Gallois Y, Calès P. Respective roles of porto-septal fibrosis and centrilobular fibrosis in alcoholic liver disease. J Pathol. 2003;201:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Hall A, Germani G, Isgrò G, Burroughs AK, Dhillon AP. Fibrosis distribution in explanted cirrhotic livers. Histopathology. 2012;60:270-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Mathurin P, Beuzin F, Louvet A, Carrié-Ganne N, Balian A, Trinchet JC, Dalsoglio D, Prevot S, Naveau S. Fibrosis progression occurs in a subgroup of heavy drinkers with typical histological features. Aliment Pharmacol Ther. 2007;25:1047-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Castera L. Noninvasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology. 2012;142:1293-1302.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Lombardi R, Buzzetti E, Roccarina D, Tsochatzis EA. Non-invasive assessment of liver fibrosis in patients with alcoholic liver disease. World J Gastroenterol. 2015;21:11044-11052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Merkel C, Montagnese S. Hepatic venous pressure gradient measurement in clinical hepatology. Dig Liver Dis. 2011;43:762-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Poynard T, Degott C, Munoz C, Lebrec D. Relationship between degree of portal hypertension and liver histologic lesions in patients with alcoholic cirrhosis. Effect of acute alcoholic hepatitis on portal hypertension. Dig Dis Sci. 1987;32:337-343. [PubMed] |
| 13. | Luca A, García-Pagán JC, Bosch J, Feu F, Caballería J, Groszmann RJ, Rodés J. Effects of ethanol consumption on hepatic hemodynamics in patients with alcoholic cirrhosis. Gastroenterology. 1997;112:1284-1289. [PubMed] [DOI] [Full Text] |
| 14. | Hu LS, George J, Wang JH. Current concepts on the role of nitric oxide in portal hypertension. World J Gastroenterol. 2013;19:1707-1717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Chen SH, Peng CY, Lai HC, Chang IP, Lee CJ, Su WP, Lin CH, Kao JT, Chuang PH. Head-to-Head Comparison between Collagen Proportionate Area and Acoustic Radiation Force Impulse Elastography in Liver Fibrosis Quantification in Chronic Hepatitis C. PLoS One. 2015;10:e0140554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Tsochatzis E, Bruno S, Isgro G, Hall A, Theocharidou E, Manousou P, Dhillon AP, Burroughs AK, Luong TV. Collagen proportionate area is superior to other histological methods for sub-classifying cirrhosis and determining prognosis. J Hepatol. 2014;60:948-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 17. | Manousou P, Burroughs AK, Tsochatzis E, Isgro G, Hall A, Green A, Calvaruso V, Ma GL, Gale J, Burgess G. Digital image analysis of collagen assessment of progression of fibrosis in recurrent HCV after liver transplantation. J Hepatol. 2013;58:962-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | de Lédinghen V, Wong VW, Vergniol J, Wong GL, Foucher J, Chu SH, Le Bail B, Choi PC, Chermak F, Yiu KK. Diagnosis of liver fibrosis and cirrhosis using liver stiffness measurement: comparison between M and XL probe of FibroScan®. J Hepatol. 2012;56:833-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Spahr L, Rubbia-Brandt L, Frossard JL, Giostra E, Rougemont AL, Pugin J, Fischer M, Egger H, Hadengue A. Combination of steroids with infliximab or placebo in severe alcoholic hepatitis: a randomized controlled pilot study. J Hepatol. 2002;37:448-455. [PubMed] [DOI] [Full Text] |
| 20. | Bedossa P, Poitou C, Veyrie N, Bouillot JL, Basdevant A, Paradis V, Tordjman J, Clement K. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology. 2012;56:1751-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 644] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 21. | Kalambokis G, Manousou P, Vibhakorn S, Marelli L, Cholongitas E, Senzolo M, Patch D, Burroughs AK. Transjugular liver biopsy--indications, adequacy, quality of specimens, and complications--a systematic review. J Hepatol. 2007;47:284-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 262] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 22. | Elphick DA, Dube AK, McFarlane E, Jones J, Gleeson D. Spectrum of liver histology in presumed decompensated alcoholic liver disease. Am J Gastroenterol. 2007;102:780-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Liao WC, Hou MC, Chang CJ, Lee FY, Lin HC, Lee SD. Potential precipitating factors of esophageal variceal bleeding: a case-control study. Am J Gastroenterol. 2011;106:96-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Francque S, Laleman W, Verbeke L, Van Steenkiste C, Casteleyn C, Kwanten W, Van Dyck C, D’Hondt M, Ramon A, Vermeulen W. Increased intrahepatic resistance in severe steatosis: endothelial dysfunction, vasoconstrictor overproduction and altered microvascular architecture. Lab Invest. 2012;92:1428-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Bolognesi M, Verardo A, Di Pascoli M. Peculiar characteristics of portal-hepatic hemodynamics of alcoholic cirrhosis. World J Gastroenterol. 2014;20:8005-8010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 26. | Kim MY, Cho MY, Baik SK, Park HJ, Jeon HK, Im CK, Won CS, Kim JW, Kim HS, Kwon SO. Histological subclassification of cirrhosis using the Laennec fibrosis scoring system correlates with clinical stage and grade of portal hypertension. J Hepatol. 2011;55:1004-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |