Published online Jan 27, 2018. doi: 10.4254/wjh.v10.i1.166
Peer-review started: September 15, 2017
First decision: October 31, 2017
Revised: November 6, 2017
Accepted: December 28, 2017
Article in press: December 28, 2017
Published online: January 27, 2018
Processing time: 106 Days and 18 Hours
Primary hepatic angiosarcoma is the most common malignant mesenchymal tumor of the liver. It has a poor prognosis and various appearances on magnetic resonance (MR) images. We report a case of hepatic angiosarcoma with a characteristic appearance on gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid (Gd-EOB-DTPA)-enhanced MR imaging in the hepatobiliary phase. A 72-year-old man was admitted with a complaint of abdominal pain. Gd-EOB-DTPA-enhanced MR imaging revealed a liver tumor that showed slight hyperintensity in the hepatobiliary phase. These findings suggested Gd-EOB-DTPA uptake in the tumor. An autopsy revealed the solid proliferation and sinusoidal spreading of hepatic angiosarcoma cells. Immunohistochemistry indicated that the tumor was negative for OATP1B3. Gd-EOB-DTPA uptake in the liver tumor in the hepatobiliary phase suggested sinusoidal tumor invasion with residual normal hepatocytes.
Core tip: Hepatic angiosarcoma has various appearances on computed tomography and magnetic resonance (MR) images. In the context of cirrhosis, hepatic angiosarcoma often cannot be readily distinguished from hepatocellular carcinoma. We present contrast uptake in primary hepatic angiosarcoma on gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced MR imaging in the hepatobiliary phase, and contrast uptake suggested sinusoidal tumor invasion with residual normal hepatocytes. This finding may assist physicians in the diagnosis of future cases of hepatic angiosarcoma.
- Citation: Hayashi M, Kawana S, Sekino H, Abe K, Matsuoka N, Kashiwagi M, Okai K, Kanno Y, Takahashi A, Ito H, Hashimoto Y, Ohira H. Contrast uptake in primary hepatic angiosarcoma on gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging in the hepatobiliary phase. World J Hepatol 2018; 10(1): 166-171
- URL: https://www.wjgnet.com/1948-5182/full/v10/i1/166.htm
- DOI: https://dx.doi.org/10.4254/wjh.v10.i1.166
Primary hepatic angiosarcoma is the most common malignant mesenchymal tumor of the liver but accounts for only 2% of primary hepatic tumors[1-4]. It has a poor prognosis, and most patients die within one year of diagnosis[3]. Although various environmental carcinogens are known causes of hepatic angiosarcoma, other possible major causes of this disease remain unknown[2]. Hepatic angiosarcoma has various appearances on computed tomography (CT) and magnetic resonance (MR) images[5,6]. In the context of cirrhosis, hepatic angiosarcoma often cannot be readily distinguished from hepatocellular carcinoma (HCC)[7]. The usefulness of MR images for detecting HCC is widely known, especially with respect to gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid (Gd-EOB-DTPA)-enhanced MR imaging in the hepatobiliary phase. Here, we report a case of hepatic angiosarcoma with a characteristic appearance on Gd-EOB-DTPA-enhanced MR imaging in the hepatobiliary phase.
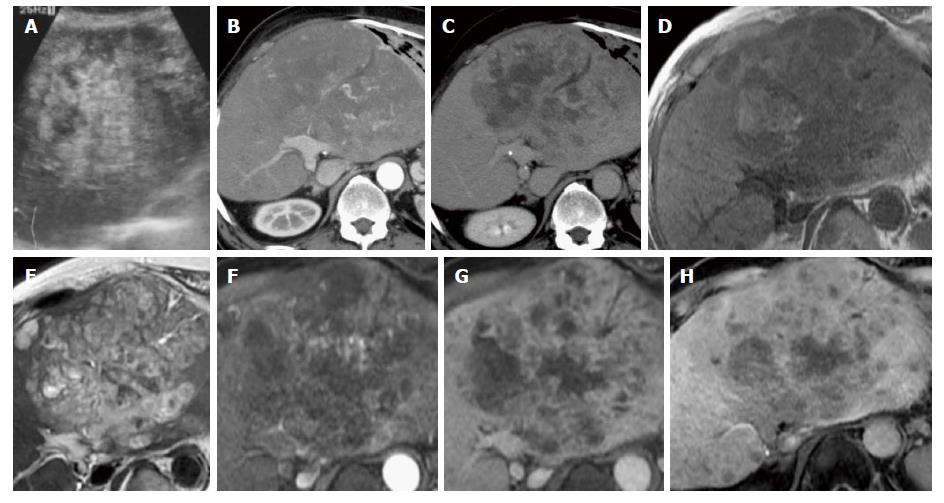
A 72-year-old man visited our institution due to the onset of abdominal pain that had begun one month previously. Abdominal ultrasonography revealed a heterogeneous hyperechoic tumor in the left hepatic lobe (Figure 1A), and the patient was admitted to our hospital. He had a history of resection of the right hepatic lobe due to HCC (T2N0M0) with hepatitis B virus-related liver cirrhosis 18 years previously. After this resection, no recurrence was detected on unenhanced CT or ultrasound images until his most recent check-up, which occurred during the previous year. He did not receive antiviral therapy for hepatitis B virus, such as interferon or nucleotide analogues. The patient consumed 360 mL of Japanese sake (containing 40 g of ethanol) per day prior to the hepatic resection and was a non-smoker. He did not have a history of environmental carcinogen exposure. His BMI was 25.9. His abdomen was soft and flat with upper abdominal tenderness. The following blood test results were obtained at admission: white blood cells, 8300/μL; hemoglobin, 10.1 g/dL; platelets, 12.3 × 104/μL; albumin, 3.0 g/dL; total bilirubin, 1.5 mg/dL; aspartate aminotransferase, 118 U/L; alanine aminotransferase, 85 U/L; alkaline phosphatase, 553 U/L; γ-glutamyl transpeptidase, 212 U/L; C-reactive protein, 17.08 mg/dL; alpha-fetoprotein, 3.3 ng/mL; des-gamma-carboxy prothrombin, 29 mAU/mL; carcinoembryonic antigen, 4.1 ng/mL; carbohydrate antigen 19–9, 19.2 U/mL; soluble interleukin-2 receptor, 925 U/mL; hepatitis B surface antigen, negative; hepatitis B surface antibody, positive; and hepatitis C virus antibody, negative.
Dynamic contrast-enhanced CT images showed a 16 cm × 10 cm tumor in the left hepatic lobe and multiple nodules (Figure 1B and C). The tumor was not enhanced in the arterial phase. Gd-EOB-DTPA-enhanced MR imaging was then performed (Figure 1D-H). T1-weighted images revealed a dominant tumor with low intensity that contained focal areas of high intensity suggestive of hemorrhage. The dominant tumor had high intensity in T2-weighted images and diffusion-weighted images and did not show enhancement in the arterial phase. In the hepatobiliary phase, the tumor showed slightly elevated intensity, a finding that suggested slight uptake of Gd-EOB-DTPA in the tumor. Based on these findings, we considered the possibility that the tumor was derived from hepatocytes. Given our results, it was difficult to discriminate between HCC and another type of malignant tumor.
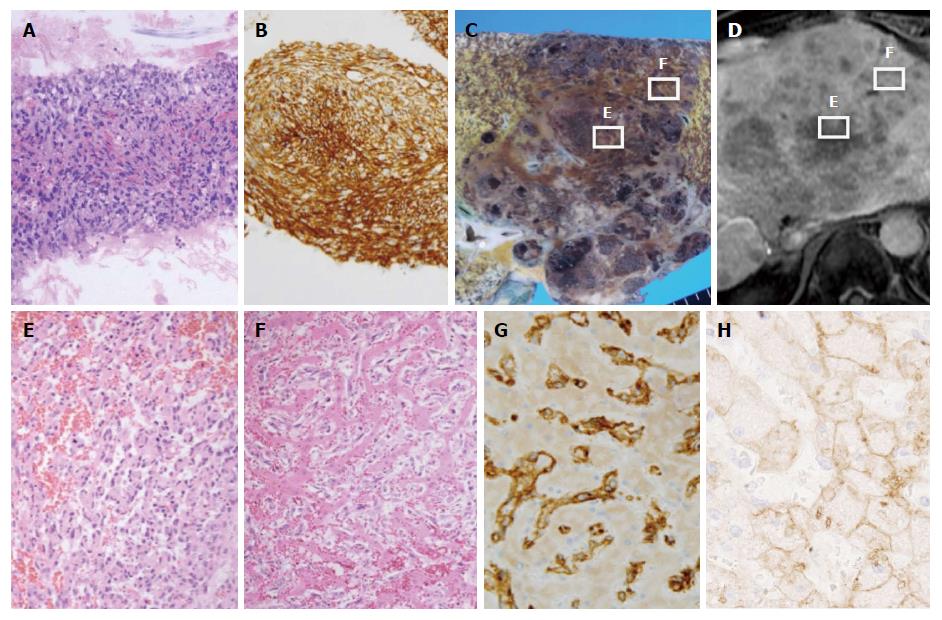
One day after admission, a liver tumor biopsy was performed that revealed solid proliferation of spindle cells with enlarged and hyperchromatic nuclei. These spindle cells had an intracytoplasmic lumen with erythrocytes, suggesting endothelial differentiation. With respect to immunohistochemistry, tumor cells were positive for CD34 but negative for CK7 and HepPar 1 (Figure 2A and B). These results were consistent with hepatic angiosarcoma. After admission, the patient experienced worsening liver and renal failure, and his state of consciousness deteriorated. He died from multiple organ failure nine days after admission, and an autopsy was performed.
Macroscopy indicated that the liver was enlarged and weighed 2,440 g. A large tumor and many satellite nodules were observed in the liver (Figure 2C); Figure 2B presents Gd-EOB-DTPA-enhanced MR images in the hepatobiliary phase in almost the same plane as the images in Figure 2C. The boundary between the tumor and surrounding liver tissue was clear. The tumor had a cavernous pattern, and necrosis was present. On microscopy, similarly to the biopsy specimen, the tumor showed the solid proliferation of atypical spindle cells (Figure 2E). Necrosis and hemorrhage were also observed. Spindle cells tended to shift to atypical endothelial cells that had large, irregularly shaped, hyperchromatic nuclei. Atypical endothelial cells had regularly infiltrated into the sinusoid and replaced sinusoidal cells in a broad range of hepatic parenchyma; as a result, hepatic cell cords remained in the tumor (Figure 2F). In addition, in hepatic parenchyma outside of the tumor, atypical endothelial cells had often infiltrated and replaced sinusoidal cells to form ill-defined foci that were difficult to identify via macroscopy. With respect to immunohistochemistry, tumor cells were positive for CD34, CD31, and vimentin but negative for Factor VIII, FLI1, HepPar 1, Arginase-1, and Glypican-3 (Figure 2G shows CD34 staining). In addition, tumor cells were negative for OATP1B3, whereas hepatic cells around the spindle cells were positive for OATP1B3 (Figure 2H). A diagnosis of hepatic angiosarcoma was confirmed. Only approximately 30% of the liver tissue remained, and much of this tissue was compressed by the tumor. The remaining liver tissue appeared to be dysfunctional. There were no microvascular thrombi, and no evidence suggested disseminated intravascular coagulation. No recurrent HCC or angiosarcoma metastatic lesions were found. The cause of death was confirmed to be liver failure due to the progression of hepatic angiosarcoma.
In this case, the hepatic angiosarcoma showed slightly elevated intensity on Gd-EOB-DTPA-enhanced MR imaging in the hepatobiliary phase. These MR images suggested uptake of Gd-EOB-DTPA in the mass. A comparison of MR imaging results in the hepatobiliary phase with microscopic findings at autopsy indicates that the area of the tumor with Gd-EOB-DTPA uptake exhibited tumor cells spreading in hepatic sinusoids that contained residual normal hepatocytes. In contrast, the area of the tumor with no uptake of Gd-EOB-DTPA showed massive tumor cell proliferation. There were few normal hepatocytes.
CT or MR images of hepatic angiosarcoma have shown various appearances. Multiphase contrast-enhanced CT and MR images showed the masses to have heterogeneous and progressive enhancement[7]. On contrast-enhanced CT images, tumor nodules showed hypoattenuating and contained focal areas of enhancement. The attenuation of many foci of enhancement was less than that of the aorta but greater than that of the hepatic parenchyma. The tumor nodules demonstrated heterogeneous enhancement that suggested central necrosis and fibrotic change. On MR T1-weighted images, the nodules were of low intensity but contained focal areas of high intensity, suggesting hemorrhage[7]. In the setting of cirrhosis, lack of tumor washout and vascular invasion argue against multifocal HCC[5]. A previous case report described Gd-EOB-DTPA-enhanced MR imaging of hepatic angiosarcoma[6]. In that report, the hepatic angiosarcoma was entirely hypointense in the hepatobiliary phase. There are many reports describing the radiological findings of HCC. The presence of arterial hypervascularity and washout are considered to be typical imaging features of classical HCC[8]. On the other hand, well-differentiated and poorly differentiated HCC often showed atypical enhancement patterns, such as hypovascularity in the arterial phase[9]. HCC generally can be seen as hypointense in the hepatobiliary phase of Gd-EOB-DTPA-enhanced MR imaging[10]. A minority of HCC tumors showed iso- or hyperintensity because of preserved OATP expression[11]. In the present case, the dominant mass showed hypovascularity in the arterial phase of MR or CT images and slight hyperintensity in the hepatobiliary phase of Gd-EOB-DTPA-enhanced MR imaging. Furthermore, this patient had a history of HCC. Diagnosis was difficult with MRI or CT findings alone.
Gd-EOB-DTPA-enhanced MR imaging has been recognized as a useful imaging technique for diagnosing liver tumors. A prior study found that for HCC, Gd-EOB-DTPA uptake was determined by OATP1B3 expression[12]. The degree of enhancement in Gd-EOB-DTPA-enhanced MR images in the hepatobiliary phase has been positively correlated with OATP1B3 expression levels[13]. A case of pseudolymphoma of the liver with partial uptake of Gd-EOB-DTPA in the hepatobiliary phase has also been reported[14]. In that case, infiltration of lymphoid cells was seen along the hepatic sinusoid, leaving some hepatocytes intact. In our case, the tumor cells microscopically showed a sinusoidal spreading pattern, and numerous viable hepatic cells remained. Furthermore, staining indicated that tumor cells were negative for OATP1B3 but that hepatic cells were positive for OATP1B3. We speculated that the reason for Gd-EOB-DTPA uptake in the mass was that tumor cells coexisted with hepatic cells. In this case, the findings of slight Gd-EOB-DTPA uptake in the liver tumor in the hepatobiliary phase may suggest the proliferation of malignant tumor cells in the sinusoids and the presence of hepatocytes.
The clinical behavior of hepatic angiosarcoma is extremely aggressive, and this disease has a poor prognosis[3,15]. Although various treatments for patients with hepatic angiosarcoma have been reported, chemotherapy, hepatic resection, and liver transplantation have all been found to have limited effects[16-18]. However, there have been reports of long-term survival after hepatic resection[19,20]. Early diagnosis of hepatic angiosarcoma is an important consideration when recommending surgical treatment for this disease. An association between liver cirrhosis and hepatic angiosarcoma has been shown. Even if patients have a history of treatment for HCC, it is necessary to consider hepatic angiosarcoma as a possible diagnosis.
The described case involved primary hepatic angiosarcoma that developed after the resection of HCC. To the best of our knowledge, there have been no reports describing the occurrence of HCC and hepatic angiosarcoma in the same patient. HCC with sarcomatous change has been observed in patients with a history of treatment for HCC or liver cirrhosis[21]. In the current case, we diagnosed primary hepatic angiosarcoma because HCC components were not observed and because tumor cells expressed neither HepPar 1 nor Arginase-1, which are lineage markers of hepatic cells, in immunohistochemical assessments.
We have reported a case involving primary hepatic angiosarcoma that developed after HCC resection; this tumor had slightly elevated intensity on Gd-EOB-DTPA-enhanced MR imaging. The appearance of uptake of Gd-EOB-DTPA in a liver tumor in the hepatobiliary phase may suggest the presence of a sinusoidal tumor spreading to the remaining hepatic cells. Our findings may assist physicians in the diagnosis of future cases of hepatic angiosarcoma.
A 72-year-old man visited our institution due to the onset of abdominal pain.
Computed tomography (CT) and magnetic resonance (MR) images revealed a liver tumor.
Hepatocellular carcinoma and another type of malignant tumor of the liver.
Laboratory tests demonstrated liver enzyme elevation. Alpha-fetoprotein, des-gamma-carboxy prothrombin, carcinoembryonic antigen and carbohydrate antigen were within normal range.
Gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced (Gd-EOB-DTPA -enhanced) MR imaging revealed a liver tumor that showed slight hyperintensity in the hepatobiliary phase.
Atypical endothelial cells had regularly infiltrated into the sinusoid and replaced sinusoidal cells in a broad range of hepatic parenchyma; as a result, hepatic cell cords remained in the tumor. Pathological findings were consistent with hepatic angiosarcoma.
After admission, the patient experienced worsening liver and renal failure. He died from multiple organ failure nine days after admission.
Hepatic angiosarcoma has various appearances on CT and MR images, but contrast uptake in primary hepatic angiosarcoma on Gd-EOB-DTPA-enhanced MR imaging in the hepatobiliary phase has not been reported.
There are no non-standard terms used in this manuscript.
The authors present this case to share important knowledge for hepatic angiosarcoma diagnosis.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Adela Maria Streba LAM, Tsoulfas G S- Editor: Cui LJ L- Editor: A E- Editor: Wang CH
| 1. | Alrenga DP. Primary angiosarcoma of the liver. Review article. Int Surg. 1975;60:198-203. [PubMed] |
| 2. | Ishak K, Peters R, editors . Mesenchymal tumor of the liver. Hepatocellular carcinoma. New York: Wiley 1976; 247-308. |
| 3. | Locker GY, Doroshow JH, Zwelling LA, Chabner BA. The clinical features of hepatic angiosarcoma: a report of four cases and a review of the English literature. Medicine (Baltimore). 1979;58:48-64. [PubMed] |
| 4. | Buetow PC, Buck JL, Ros PR, Goodman ZD. Malignant vascular tumors of the liver: radiologic-pathologic correlation. Radiographics. 1994;14:153-166; quiz 167-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 79] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Pickhardt PJ, Kitchin D, Lubner MG, Ganeshan DM, Bhalla S, Covey AM. Primary hepatic angiosarcoma: multi-institutional comprehensive cancer centre review of multiphasic CT and MR imaging in 35 patients. Eur Radiol. 2015;25:315-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Kamatani T, Iguchi H, Okada T, Yamazaki H, Tsunoda H, Watanabe M, Oda M, Ohbu M, Yokomori H. Co-registered positron emission tomography/computed tomography and gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid magnetic resonance imaging features of multiple angiosarcoma of the liver. Hepatol Res. 2014;44:E297-E303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Koyama T, Fletcher JG, Johnson CD, Kuo MS, Notohara K, Burgart LJ. Primary hepatic angiosarcoma: findings at CT and MR imaging. Radiology. 2002;222:667-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 141] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 8. | Bota S, Piscaglia F, Marinelli S, Pecorelli A, Terzi E, Bolondi L. Comparison of international guidelines for noninvasive diagnosis of hepatocellular carcinoma. Liver Cancer. 2012;1:190-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | Lee JH, Lee JM, Kim SJ, Baek JH, Yun SH, Kim KW, Han JK, Choi BI. Enhancement patterns of hepatocellular carcinomas on multiphasicmultidetector row CT: comparison with pathological differentiation. Br J Radiol. 2012;85:e573-e583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Ichikawa T, Sano K, Morisaka H. Diagnosis of Pathologically Early HCC with EOB-MRI: Experiences and Current Consensus. Liver Cancer. 2014;3:97-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 11. | Park HJ, Choi BI, Lee ES, Park SB, Lee JB. How to Differentiate Borderline Hepatic Nodules in Hepatocarcinogenesis: Emphasis on Imaging Diagnosis. Liver Cancer. 2017;6:189-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 12. | Narita M, Hatano E, Arizono S, Miyagawa-Hayashino A, Isoda H, Kitamura K, Taura K, Yasuchika K, Nitta T, Ikai I. Expression of OATP1B3 determines uptake of Gd-EOB-DTPA in hepatocellular carcinoma. J Gastroenterol. 2009;44:793-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 279] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 13. | Kitao A, Zen Y, Matsui O, Gabata T, Kobayashi S, Koda W, Kozaka K, Yoneda N, Yamashita T, Kaneko S. Hepatocellular carcinoma: signal intensity at gadoxetic acid-enhanced MR Imaging--correlation with molecular transporters and histopathologic features. Radiology. 2010;256:817-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 281] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 14. | Osame A, Fujimitsu R, Ida M, Majima S, Takeshita M, Yoshimitsu K. Multinodular pseudolymphoma of the liver: computed tomography and magnetic resonance imaging findings. Jpn J Radiol. 2011;29:524-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Weitz J, Klimstra DS, Cymes K, Jarnagin WR, D’Angelica M, La Quaglia MP, Fong Y, Brennan MF, Blumgart LH, Dematteo RP. Management of primary liver sarcomas. Cancer. 2007;109:1391-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 122] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Kim HR, Rha SY, Cheon SH, Roh JK, Park YN, Yoo NC. Clinical features and treatment outcomes of advanced stage primary hepatic angiosarcoma. Ann Oncol. 2009;20:780-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 17. | Kojiro M, Nakashima T, Ito Y, Ikezaki H, Mori T, Kido C. Thorium dioxide-related angiosarcoma of the liver. Pathomorphologic study of 29 autopsy cases. Arch Pathol Lab Med. 1985;109:853-857. [PubMed] |
| 18. | Orlando G, Adam R, Mirza D, Soderdahl G, Porte RJ, Paul A, Burroughs AK, Seiler CA, Colledan M, Graziadei I. Hepatic hemangiosarcoma: an absolute contraindication to liver transplantation--the European Liver Transplant Registry experience. Transplantation. 2013;95:872-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Timaran CH, Grandas OH, Bell JL. Hepatic angiosarcoma: long-term survival after complete surgical removal. Am Surg. 2000;66:1153-1157. [PubMed] |
| 20. | Ozden I, Bilge O, Erkan M, Cevikbaş U, Acarli K. Five years and 4 months of recurrence-free survival in hepatic angiosarcoma. J Hepatobiliary Pancreat Surg. 2003;10:250-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |