Peer-review started: November 9, 2017
First decision: December 4, 2017
Revised: December 21, 2017
Accepted: January 15, 2018
Article in press: January 15, 2018
Published online: January 27, 2018
Processing time: 77 Days and 21.9 Hours
Inflammation and tumorigenesis are tightly linked pathways impacting cancer development. Inflammasomes are key signalling platforms that detect pathogenic microorganisms, including hepatitis C virus (HCV) infection, and sterile stressors (oxidative stress, insulin resistance, lipotoxicity) able to activate pro-inflammatory cytokines interleukin-1β and IL-18. Most of the inflammasome complexes that have been described to date contain a NOD-like receptor sensor molecule. Redox state and autophagy can regulate inflammasome complex and, depending on the conditions, can be either pro- or anti-apoptotic. Acute and chronic liver diseases are cytokine-driven diseases as several proinflammatory cytokines (IL-1α, IL-1β, tumor necrosis factor-alpha, and IL-6) are critically involved in inflammation, steatosis, fibrosis, and cancer development. NLRP3 inflammasome gain of function aggravates liver disease, resulting in severe liver fibrosis and highlighting this pathway in the pathogenesis of non-alcoholic fatty liver disease. On the other hand, HCV infection is the primary catalyst for progressive liver disease and development of liver cancer. It is well established that HCV-induced IL-1β production by hepatic macrophages plays a critical and central process that promotes liver inflammation and disease. In this review, we aim to clarify the role of the inflammasome in the aggravation of liver disease, and how selective blockade of this main pathway may be a useful strategy to delay fibrosis progression in liver diseases.
Core tip: Inflammasomes are newly recognized vital players in innate immunity. Several factors have been identified able to activate the NLRP3 inflammasome. Inappropriate activation of NLRP3 can contribute to the onset and progression of various diseases, particularly age-related diseases. It is well established that hepatitis C virus infection plays a critical role in the promotion of liver inflammation and disease, inducing the production of IL-1β and the activation of NLRP3. NLRP3 inflammasome gain of function aggravates liver disease, resulting in severe liver fibrosis and lately, hepatocellular carcinoma. In non-alcoholic fatty liver disease, the regulation of inflammation processes may prevent the progression of non-alcoholic steatohepatitis to fibrosis.
- Citation: Del Campo JA, Gallego P, Grande L. Role of inflammatory response in liver diseases: Therapeutic strategies. World J Hepatol 2018; 10(1): 1-7
- URL: https://www.wjgnet.com/1948-5182/full/v10/i1/1.htm
- DOI: https://dx.doi.org/10.4254/wjh.v10.i1.1
Hepatic inflammation is a common trigger of liver disease, and is considered the main driver of hepatic tissue damage, triggering the progression from non-alcoholic fatty liver disease (NAFLD) to severe fibrogenesis and, finally, hepatocellular carcinoma (HCC).
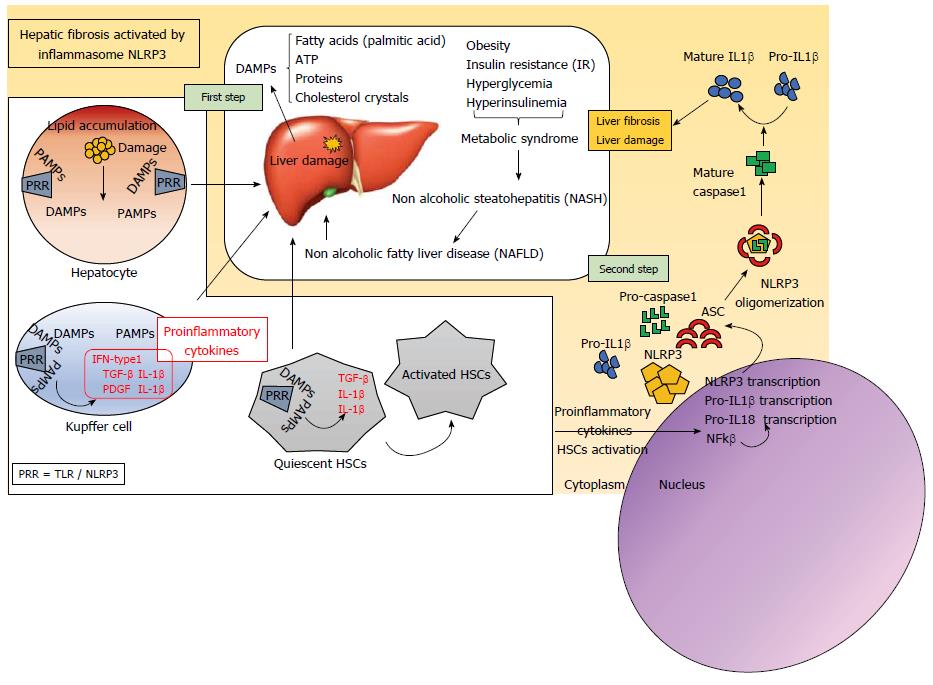
Liver diseases, whose etiology can be diverse, are becoming one of the most serious public health problems. The diseases usually occur in response to chronic hepatocellular injury caused mainly by the abuse of alcoholic intake, chronic infections such as those caused by the hepatitis C virus (HCV), bile duct damage, NAFLD or non-alcoholic steatohepatitis (NASH)[1]. NAFLD was defined for the first time in 1980[2] as an accumulation of fat (> 5%) in liver cells in the absence of excessive alcohol intake[3]. The disease affects more than 30% of the population of the western world, especially patients suffering from metabolic syndrome, obesity (76%), and type II diabetes (50%)[4]. The histological spectrum of NAFLD begins in a simple benign steatosis, evolving to a NASH. From this point, the consequent scarring and tissue replacement begins with type I collagen, developing fibrosis, cirrhosis and finally, in many cases complicating a HCC[3]. This pathogenesis, which is complex, is explained by two main impacts or damages to the liver tissue: on the one hand, a lipid accumulation in the hepatocytes, to which a second oxidative stress damage is added[5]. It is this second damage, which produces a lipotoxicit that triggers the inflammatory response by the release of danger-activated molecular patterns (DAMPS) and pathogenic-activated molecular patterns (PAMPs), such as lipopolysaccharide (LPS). Finally, they activate the innate immunity that causes the hepatic inflammation, being able to aggravate the process of fibrosis, cirrhosis, causing HCC.
That is why the knowledge of the relationship between liver disease and uncontrolled activation of the immune response, resulting in aggravating liver inflammation of disease progression, can be crucial for the design of therapeutic strategies blocking the immune response uncontrolled.
The pathogenesis of this disease is complex, and is explained by two major impacts or damage to the liver tissue: on the one hand, a lipid accumulation in the hepatocytes, to which a second oxidative stress damage is added[5].
The first of these is the abnormal accumulation of triglycerides in the hepatocyte, either due to a high intake of saturated fats and obesity, genetic deficiencies or insulin resistance (IR) due to hyperglycemia and hyperinsulinemia[6], what is known as metabolic syndrome. Hyperinsulinemia and increased hepatic glucose production induce the expression of the sterol regulatory element binding protein (SREBP-1c) and the carbohydrate response element binding protein (ChREBP), respectively, which activate in turn the transcription of most of the genes involved in the enzymatic machinery necessary for free fatty acids (FFA) synthesis, decreasing the beta oxidation thereof[7].
In addition, there are many mediating molecules such as peroxisome proliferator activating ligand receptors (PPAR), among which PPAR-γ, whose expression in conditions of liver damage is usually high, contribute to the accumulation of FFA[7]. The liver X receptor (LXR), another important mediator, activates genes involved in the synthesis of FFA, such as SREBP-1c and ChREBP, contributing to steatosis[8]. Finally, the AMP-activated protein kinase (AMPK) functions as a sensor of the energy levels of the cell, stimulating catabolic pathways such as mitochondrial beta oxidation, and inhibiting ATP-consuming processes, such as lipogenesis. All this is done by phosphorylating different proteins involved in these pathways, such as acetyl-CoA carboxylase (ACC), ChREBP and SREBP-1c, which end up being inhibited.
In the process of lipid accumulation, several molecules act as DAMPs and pathogen-activated molecular patterns (PAMPs), that is, molecules that trigger inflammation. These may include the fatty acids, adenosine triphosphate (ATP), uric acid or proteins derived from the extracellular matrix, among others. In addition, recent research suggests that in the liver tissue of humans and mice with steatohepatitis, cholesterol crystals are present within hepatocytes, and they can act as DAMPs.
A second impact that explains the characteristic histological lesions of NAFLD is oxidative stress and lipid peroxidation[5,9]. As a result of liver damage and liver inflammation, the inflammatory cells and the hepatocyte itself release cytokines, such as TNF-α and reactive oxygen species (ROS). These mediators can cause peroxidation of plasma and mitochondrial membranes, which leads to cell death due to necrosis or apoptosis[5,9]. All this activates endothelial cells of the liver, which increase the expression of cytokines, and finally activates the hepatic stellate cells (HSCs), producing a phenotypic change, associated with the acquisition of pro-fibrogenic and pro-inflammatory functions[10].
Significant accumulation of triglycerides and cholesterol in VLDL and LDL particles, added to the oxidative stress developed in the hepatocytes, produces the oxidation of the cholesterol linked to LDL, generating particles of LDL oxidase (oxLDL) that constitute an important risk factor of the disease[11]. Recent studies show that oxLDL also contribute to the process of cellular inflammation and apoptosis.
Excessive lipids accumulation leads to hepatocyte damage, activating an inflammatory response that aggravates the progress of the liver disease, and which, in turn, feeds back the activation of the inflammation[12].
The immune response of the liver is produced by the action of immune cells such as Kupffer cells, monocytes, neutrophils, dendritic cells (DCs), natural killer cells (NK), and NK T cells (NKT), which initiate and maintain the hepatic inflammation through the production of cytokines and chemokines, especially TNF and interleukin (IL) -1β, as well as reactive oxygen species[13].
The triggering of hepatic inflammation, as noted above, is caused by the accumulation of infectious and non-infectious material, which is released during cell damage and is recognized by pattern recognition receptors (PRRs). These PRRs include Toll-like receptors (TLRs), NOD-like receptors (NLRs), C-type lectin receptors (CLRs), and several other receptors[14].
The endogenous molecules produced by cellular damage or stress result in an inflammatory response (DAMPs). These molecules are very diverse and do not have a common structure between them. In the pathology of the liver disease and liver inflammation, induced-inflammation particles are included, such as cholesterol crystals. These cholesterol crystals produce the development, not only of the atheroma plaques, but of the inflammation, being present in humans and mice with NASH[15] candidates are free fatty acids (FFA) that are released during liver damage. A specific mechanism by which they act is to activate TLR4, while fetuin-A, a 64-kDa protein specific for hepatocytes, is required. It has been proven that the reduction of fetuin-A in mice with high fat diet results in a decrease of inflammatory signalling mediated by TLR4 in adipose tissue. Normally, in patients with NAFLD, the expression of fetuin-A is high[16]. Palmitic acid (PA) is another molecule that causes liver inflammation, and it has recently been linked to the TLR2 receptor. The palmitic acid ligand of TLR2 induces the activation of caspase- Other 1 and the release of IL-1α and IL-1β, having an evident role in the liver inflammatory process[17].
Many of these DAMPs activate PRR present in immune cells, of which the TLRs are the best characterized. In liver disease, saturation of fatty acids produces inflammation in the hepatocytes, which increases the induction of caspase-1 activation and the release of IL-1β. This results in a release of more DAMPs from the hepatocytes, generating a feedback that amplifies the inflammatory response.
Among the various NLR receptors, the NLRP3 inflammasome is the best characterized and associated with a wider range of diseases including infections, auto-inflammatory diseases and other autoimmune diseases. The NLRP3 inflammasome has the characteristic of forming a complex with apoptosis-associated speck-like protein to CARD (ASC), to activate caspase-1 and induce the maturation and secretion of important proinflammatory cytokines such as IL-1β and IL-18. Cytokines act directly against the damage and infection of the liver tissue[18].
Activation of the NLRP3 inflammasome is important in the inflammatory process, and occurs in two steps. The first includes the activation of TLRs by different molecules DAMPs and PAMPs (Figure 1). TLRs belong to the family of PRRs and their function is to maintain tissue homeostasis through the regulation of inflammatory responses. In the liver, TLRs are expressed in Kupffer cells, endothelial cells, DCs, epithelial biliary cells, HSCs and hepatocytes. Activated TLRs activate the cells and contribute to the release of cytokines that facilitate the progression of liver disease. There are at least 13 known TLRs, whose structure is characterized by having a leucine-rich repeat structure (LRR) in the extracellular domain and a Toll/IL-1 receptor (TIR) in its intracellular domain[19].
TLR4 has an interesting role in liver inflammation and fibrogenesis[20]. The Kupffer cells of the liver are the first to be attacked by the damage produced, and express TLR4 to which lipopolysaccharides (LPS) bind. This produces the activation of NF-kB, mitogen-activated protein kinase (MAPK)[21] extracellular signal-regulated kinase-1 (ERK1), p38, c-jun N-terminal kinase (JNK) and interferon regulatory factor 3(IRF3)[22], which finally triggers the production of proinflammatory cytokines and enhances IFN-β and STAT1 expression[23]. The proinflammatory stimulus facilitates hepatocyte damage, contributing to the secretion of profibrogenic cytokines such as transforming growth factor beta (TGF-β) and the platelet-derived growth factor (PDGF), promoting the activation of HSCs. This activation, in turn, up-regulates transcription of inflammasome-related components, including inactive NLRP3, pro-IL-1β and proIL-18[24,25].
The second step of inflammasome activation is the oligomerization of NLRP3 and subsequent assembly of NLRP3, ASC, and procaspase-1 into a complex (Figure 1). This triggers the transformation of procaspase-1 to caspase-1, as well as the production and secretion of mature IL-1β and IL-18[18].
After NLRs overexpression, members of this family play an important role in the formation of intracellular multiprotein complexes called inflammasomes. The union of microbial components or activators of the inflammasome (DAMPs and PAMPs) that enter the cytoplasm are detected by these cytosolic NLRs, activating the inflammasome formation. The inflammasome consists of an NLR protein, the adapter molecule ASC and procaspase-1, which is an effector molecule[26]. The formation of this complex serves to activate cysteine protease caspase-1, which, in turn, produces the maturation of proinflammatory cytokines, including IL-1b and IL-18, and the proteolytic inactivation of IL-33[27].
In short, and specifically in NAFLD, the saturated fatty acids from the excess lipid in the liver represent harmful endogenous molecules that finally induce the activation of the inflammasome. In addition, hepatocytes exposed to these saturated fatty acids release signals that trigger the activation of immune cells. Activation of the inflammasome activates caspase-1 in Kupffer cells, inducing proinflammatory signaling and activation of HSCs. In this way, collagen deposition occurs that triggers liver fibrosis[28] have shown that cholesterol crystals could be one pathway to activate the inflammasome in NASH. To test whether inflammasome blockade alters inflammatory recruitment, they used a drug called MCC950, which has already been shown to block NLRP3 activation, in an attempt to reduce liver injury in NASH. This drug partly reversed liver inflammation, particularly in obese diabetic mice.
Inflammatory signaling of the liver is regulated by cytokines capable of activating effector functions in immune cells. Kupffer cells are the first to detect the presence of PAMPs and DAMPs through TLRs, activating the release of cytokines such as TNF-α, IL-1 and IL-6, as well as chemokines chemokine (C-X-C motif) ligand 13 (CXCL13), chemokine (C-X-C motif) ligand 8 CXCL-8 and Chemokine (C-C motif) ligand 24 (CCL24), which initiate an acute phase inflammatory response. These cytokines can produce apoptosis of hepatocytes, steatosis and inflammation, including the onset of a fibrosis process after interaction with HSCs via TGF-β. Cytokines can also activate hepatic sinusoidal endothelial cells that are ultimately involved in the recruitment of neutrophils, monocytes and NKT cells, being a feature of acute liver damage. Neutrophils can be activated, and their phenotype changed, releasing ROS, defensins and other chemokines that attract more neutrophils and monocytes. Monocytes can be differentiated into TNF-α, IL-1β, granulocyte colony stimulating factor (G-CSF) and granulocyte-macrophage colony stimulating factor (GM-CSF) macrophages, increasing the life expectancy of these neutrophils[29,30]. Finally, the inflammation resolves with the apoptosis of the neutrophils, being these apoptotic neutrophils signals involved in the onset of phagocytosis and in the increase of IL-10 and TGF-β, cytokines related to the end of the inflammatory response and the repair of the tissue[31].
Mesenchymal stem cells (MSCs) are a heterogeneous subset of stromal stem cells with immunonodulatory characteristics. MSCs are considered to act through multiple mechanisms to coordinate a dynamic, integrated response to liver inflammation and fibrosis, which prevents the progressive distortion of hepatic architecture. Management of inflammatory patterns is crucial also in case of potential treatment of liver diseases with stem cells[32]. Adult stem cells have gained in attractiveness over embryonic stem cells for liver cell therapy due to their origin, multipotentiality, and the possibility of autologous transplantation.
Any of these necroinflammatory mechanisms described above can activate HSCs, the main ones involved in the process of hepatic fibrogenesis[33]. In normal liver, stellate cells are described as being in a quiescent state. Quiescent stellate cells represent 5%-8% of the total number of liver cells[34]. When the liver is damaged, stellate cells can change into an activated state. The activated stellate cell is characterized by proliferation, contractility, and chemotaxis. This state of the stellate cell is the main source of extracellular matrix production in liver injury[35].
The hepatic stellate cells are characterized by having in their cytoplasm small droplets of fat 1-2 microns in diameter that allow the storage of vitamin A, one of its main functions. Besides, they control intercellular communication through the release of mediators, and participate in the homeostasis of the MEC of the liver by the production of collagens and non-collagens, synthesis of metalloproteinases (MMP) that catabolize the components of the MEC, and synthesis of inhibitors of MPPs, also called tissue inhibitors of metalloproteinases (TIMP) that control the catalytic activity of MMPs to maintain a homeostasis of the MEC[36].
Therefore, after the chronic injury of the liver tissue, both the HSC and other cells producing the ECM undergo activation, a pathological process characterized by the loss of fat droplets, an increase in the number and size of the cells, and phenotypic transdifferentiation to proliferating, fibrogenic and contractile cells, which will be very similar to myofibroblasts. All this is mediated by different factors that induce cell proliferation, fibrogenic mediators such as TGF-β1 and IL-6, inducers of HSC contraction, such as endothelin-1, thrombin or angiotensin II and finally mediators of anti-inflammatory and anti-fibrogenic activity, such as IL-10 and interferon-γ (IFN-γ)[36].
Because of the activation of HSCs, phenotypic changes occur that affect the development of hepatic fibrosis, such as the production of collagen and non-collagen proteins of the MEC (collagen type I, type III, type IV, laminin, elastin, fibronectin and various proteoglycans) by the CEH[37].
Many evidences reported in the literature suggest that the activation of the NLRP3 inflammasome complex and the consequent generation of the acute inflammatory response in the liver, facilitates the progress of steatohepatitis to liver fibrosis, cirrhosis and finally, HCC development. However, recent studies show that the KO mice of NRLP6 and NLRP3 inflammasomes have a worse progression in the NAFLD/NASH disease. The absence of the inflammasome is associated with changes in the homeostasis of the intestinal microbiota, resulting in a strong hepatic steatosis and inflammation through TLR receptor agonists, such as TLR4, which allows the release of TNF-α which leads to the progression of NASH[38]. This demonstrates the complexity of the effects of the activation of the inflammasome, which can generate an acute inflammation or, conversely, be protective. However and in general terms, this activation is usually proinflammatory in the liver, and its inactivation and absence in relation to the microbiota should be carefully studied. Recently, Pierantonelli et al[39] have shown that the progression of liver fibrosis is associated with the downregulation of NLRP3 in the gut which, together with the current evidence of a strong correlation between intestinal changes (including modification of microbiota composition) and liver disease, makes the role of NLRP3 in the intestine extremely attractive as a protective factor. Finally, MCC950 has been proven as an effective NLRP3 inhibitor, being able to reduce liver injury and inflammation.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kanda T, Ozenirler S, Pellicano R, Sazci A S- Editor: Cui LJ L- Editor: A E- Editor: Li RF
| 1. | Younossi ZM, Loomba R, Anstee QM, Rinella ME, Bugianesi E, Marchesini G, Neuschwander-Tetri BA, Serfaty L, Negro F, Caldwell SH. Diagnostic Modalities for Non-alcoholic Fatty Liver Disease (NAFLD), Non-alcoholic Steatohepatitis (NASH) and Associated Fibrosis. Hepatology. 2017; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 309] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 2. | Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434-438. [PubMed] |
| 3. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2413] [Cited by in RCA: 2611] [Article Influence: 200.8] [Reference Citation Analysis (1)] |
| 4. | Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1127] [Cited by in RCA: 1330] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 5. | Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65:1038-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1490] [Cited by in RCA: 2104] [Article Influence: 233.8] [Reference Citation Analysis (1)] |
| 6. | Brunt EM, Wong VW, Nobili V, Day CP, Sookoian S, Maher JJ, Bugianesi E, Sirlin CB, Neuschwander-Tetri BA, Rinella ME. Nonalcoholic fatty liver disease. Nat Rev Dis Primers. 2015;1:15080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 610] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 7. | Hassan K, Bhalla V, El Regal ME, A-Kader HH. Nonalcoholic fatty liver disease: a comprehensive review of a growing epidemic. World J Gastroenterol. 2014;20:12082-12101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 129] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (3)] |
| 8. | Chisholm JW, Hong J, Mills SA, Lawn RM. The LXR ligand T0901317 induces severe lipogenesis in the db/db diabetic mouse. J Lipid Res. 2003;44:2039-2048. [PubMed] |
| 9. | Begriche K, Massart J, Robin MA, Bonnet F, Fromenty B. Mitochondrial adaptations and dysfunctions in nonalcoholic fatty liver disease. Hepatology. 2013;58:1497-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 456] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 10. | Diehl AM, Li ZP, Lin HZ, Yang SQ. Cytokines and the pathogenesis of non-alcoholic steatohepatitis. Gut. 2005;54:303-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 163] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 11. | Walenbergh SM, Koek GH, Bieghs V, Shiri-Sverdlov R. Non-alcoholic steatohepatitis: the role of oxidized low-density lipoproteins. J Hepatol. 2013;58:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Wree A, McGeough MD, Peña CA, Schlattjan M, Li H, Inzaugarat ME, Messer K, Canbay A, Hoffman HM, Feldstein AE. NLRP3 inflammasome activation is required for fibrosis development in NAFLD. J Mol Med (Berl). 2014;92:1069-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 400] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 13. | Tilg H, Diehl AM. Cytokines in alcoholic and nonalcoholic steatohepatitis. N Engl J Med. 2000;343:1467-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 688] [Cited by in RCA: 685] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 14. | Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4047] [Cited by in RCA: 4664] [Article Influence: 202.8] [Reference Citation Analysis (0)] |
| 15. | Ioannou GN, Haigh WG, Thorning D, Savard C. Hepatic cholesterol crystals and crown-like structures distinguish NASH from simple steatosis. J Lipid Res. 2013;54:1326-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 186] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 16. | Ix JH, Sharma K. Mechanisms linking obesity, chronic kidney disease, and fatty liver disease: the roles of fetuin-A, adiponectin, and AMPK. J Am Soc Nephrol. 2010;21:406-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 265] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 17. | Miura K, Yang L, van Rooijen N, Brenner DA, Ohnishi H, Seki E. Toll-like receptor 2 and palmitic acid cooperatively contribute to the development of nonalcoholic steatohepatitis through inflammasome activation in mice. Hepatology. 2013;57:577-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 230] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 18. | Ozaki E, Campbell M, Doyle SL. Targeting the NLRP3 inflammasome in chronic inflammatory diseases: current perspectives. J Inflamm Res. 2015;8:15-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 201] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 19. | Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5434] [Cited by in RCA: 6515] [Article Influence: 434.3] [Reference Citation Analysis (0)] |
| 20. | Iracheta-Vellve A, Petrasek J, Gyongyosi B, Satishchandran A, Lowe P, Kodys K, Catalano D, Calenda CD, Kurt-Jones EA, Fitzgerald KA. Endoplasmic Reticulum Stress-induced Hepatocellular Death Pathways Mediate Liver Injury and Fibrosis via Stimulator of Interferon Genes. J Biol Chem. 2016;291:26794-26805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 129] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 21. | Bian H, Li F, Wang W, Zhao Q, Gao S, Ma J, Li X, Ren W, Qin C, Qi J. MAPK/p38 regulation of cytoskeleton rearrangement accelerates induction of macrophage activation by TLR4, but not TLR3. Int J Mol Med. 2017;40:1495-1503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Hu W, Jain A, Gao Y, Dozmorov IM, Mandraju R, Wakeland EK, Pasare C. Differential outcome of TRIF-mediated signaling in TLR4 and TLR3 induced DC maturation. Proc Natl Acad Sci USA. 2015;112:13994-13999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 23. | Kanda T, Steele R, Ray R, Ray RB. Hepatitis C virus infection induces the beta interferon signaling pathway in immortalized human hepatocytes. J Virol. 2007;81:12375-12381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology. 2008;48:322-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 551] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 25. | Zhang B, Xu D, She L, Wang Z, Yang N, Sun R, Zhang Y, Yan C, Wei Q, Aa J. Silybin inhibits NLRP3 inflammasome assembly through the NAD + /SIRT2 pathway in mice with nonalcoholic fatty liver disease. FASEB J. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 26. | Dolunay A, Senol SP, Temiz-Resitoglu M, Guden DS, Sari AN, Sahan-Firat S, Tunctan B. Inhibition of NLRP3 Inflammasome Prevents LPS-Induced Inflammatory Hyperalgesia in Mice: Contribution of NF-κB, Caspase-1/11, ASC, NOX, and NOS Isoforms. Inflammation. 2017;40:366-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 27. | Csak T, Velayudham A, Hritz I, Petrasek J, Levin I, Lippai D, Catalano D, Mandrekar P, Dolganiuc A, Kurt-Jones E. Deficiency in myeloid differentiation factor-2 and toll-like receptor 4 expression attenuates nonalcoholic steatohepatitis and fibrosis in mice. Am J Physiol Gastrointest Liver Physiol. 2011;300:G433-G441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 198] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 28. | Mridha AR, Wree A, Robertson AAB, Yeh MM, Johnson CD, Van Rooyen DM, Haczeyni F, Teoh NC, Savard C, Ioannou GN. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J Hepatol. 2017;66:1037-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 820] [Article Influence: 102.5] [Reference Citation Analysis (0)] |
| 29. | Bhargava P, Lee CH. Role and function of macrophages in the metabolic syndrome. Biochem J. 2012;442:253-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 30. | Nallagangula KS, Nagaraj SK, Venkataswamy L, Chandrappa M. Liver fibrosis: a compilation on the biomarkers status and their significance during disease progression. Future Sci OA. 2017;4:FSO250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 31. | Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol. 2010;10:427-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 685] [Cited by in RCA: 770] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 32. | Fagoonee S, Famulari ES, Silengo L, Camussi G, Altruda F. Prospects for Adult Stem Cells in the Treatment of Liver Diseases. Stem Cells Dev. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Wu X, Wu X, Ma Y, Shao F, Tan Y, Tan T, Gu L, Zhou Y, Sun B, Sun Y. CUG-binding protein 1 regulates HSC activation and liver fibrogenesis. Nat Commun. 2016;7:13498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 34. | Geerts A. History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin Liver Dis. 2001;21:311-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 562] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 35. | Eng FJ, Friedman SL. Fibrogenesis I. New insights into hepatic stellate cell activation: the simple becomes complex. Am J Physiol Gastrointest Liver Physiol. 2000;279:G7-G11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 149] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 36. | Seki E, Schwabe RF. Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology. 2015;61:1066-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 734] [Article Influence: 73.4] [Reference Citation Analysis (0)] |
| 37. | Duarte S, Baber J, Fujii T, Coito AJ. Matrix metalloproteinases in liver injury, repair and fibrosis. Matrix Biol. 2015;44-46:147-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 260] [Cited by in RCA: 353] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 38. | Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1620] [Cited by in RCA: 1874] [Article Influence: 144.2] [Reference Citation Analysis (0)] |
| 39. | Pierantonelli I, Rychlicki C, Agostinelli L, Giordano DM, Gaggini M, Fraumene C, Saponaro C, Manghina V, Sartini L, Mingarelli E. Lack of NLRP3-inflammasome leads to gut-liver axis derangement, gut dysbiosis and a worsened phenotype in a mouse model of NAFLD. Sci Rep. 2017;7:12200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |