Revised: September 10, 2009
Accepted: September 17, 2009
Published online: October 31, 2009
AIM: To clarify short- and long-term outcomes of combined resection of liver with major vessels in treating colorectal liver metastases.
METHODS: Clinicopathologic data were evaluated for 312 patients who underwent 371 liver resections for metastases from colorectal cancer. Twenty-five patients who underwent resection and reconstruction of retrohepatic vena cava, major hepatic veins, or hepatic venous confluence during hepatectomies were compared with other patients, who underwent conventional liver resections.
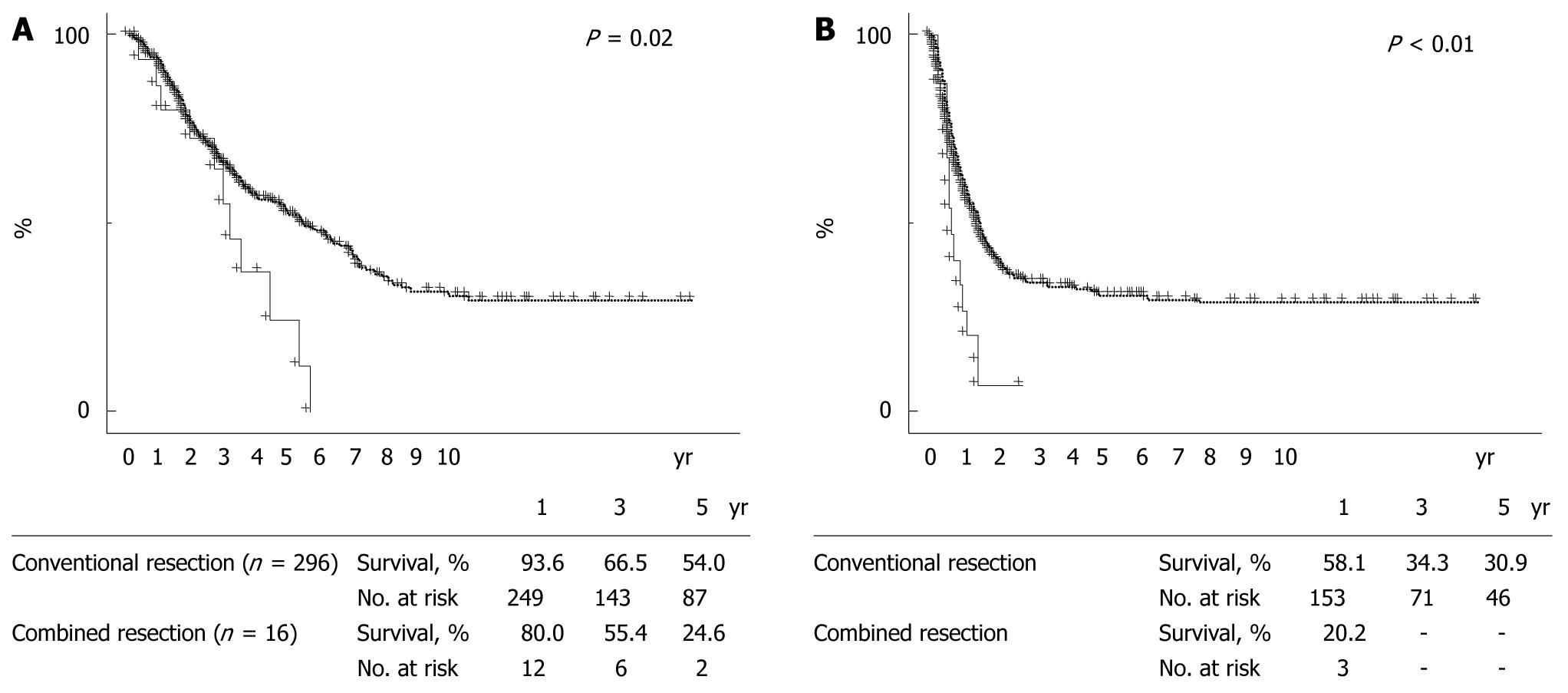
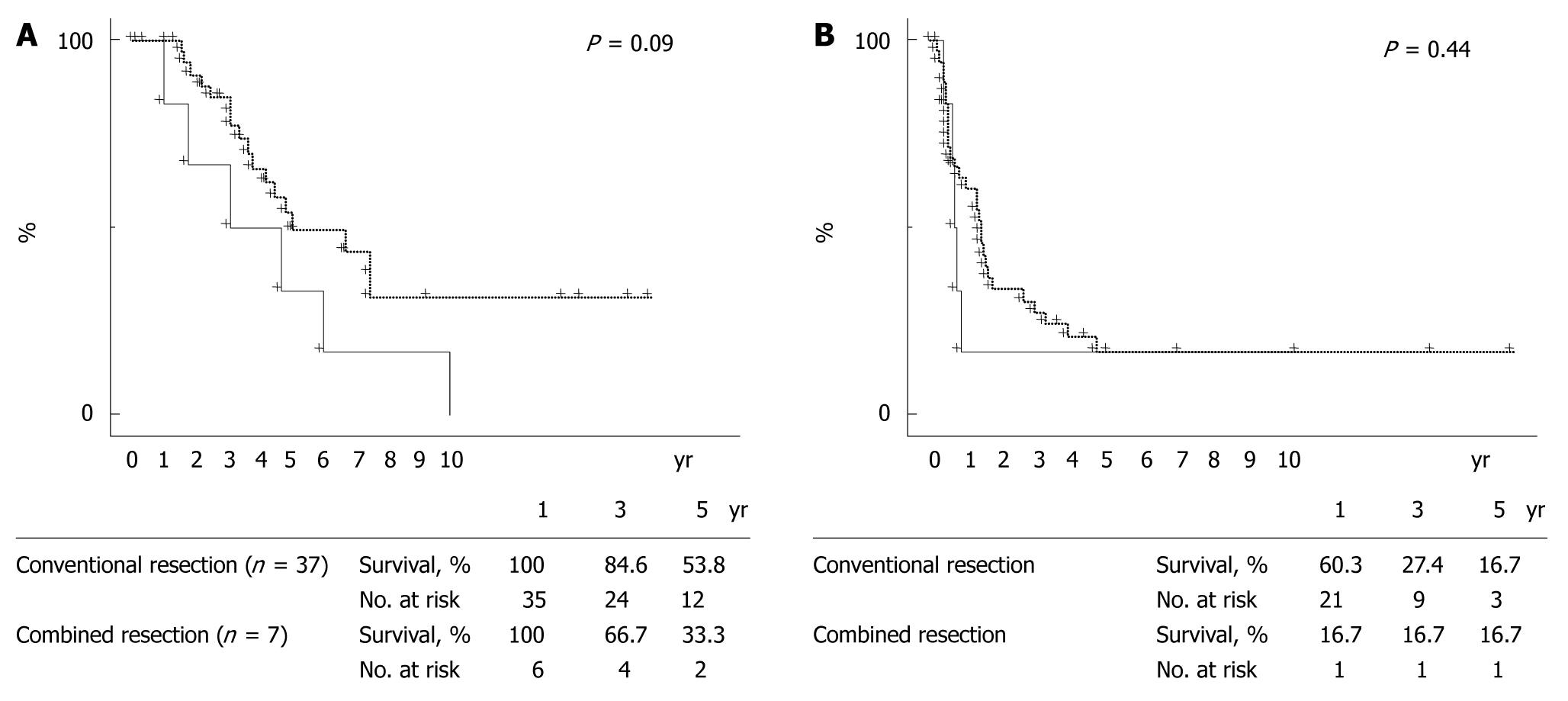
RESULTS: Morbidity was 20% (75/371) and mortality was 0.3% (1/312) in all patients after hepatectomy. Hepatic resection combined with major-vessel resection/reconstruction could be performed with acceptable morbidity (16%) and no mortality. By multivariate analysis, repeat liver resection (relative risk or RR, 5.690; P = 0.0008) was independently associated with resection/reconstruction of major vessels during hepatectomy, as were tumor size exceeding 30 mm (RR, 3.338; P = 0.0292) and prehepatectomy chemotherapy (RR, 3.485; P = 0.0083). When 312 patients who underwent a first liver resection for initial liver metastases were divided into those with conventional resection (n = 296) and those with combined resection of liver and major vessels (n = 16), overall survival and disease-free rates were significantly poorer in the combined resection group than in the conventional resection group (P = 0.02 and P < 0.01, respectively). A similar tendency concerning overall survival was observed for conventional resection (n = 37) vs major-vessel resection combined with liver resection (n = 7) performed as a second resection following liver recurrences (P = 0.09). Combined major-vessel resection at first hepatectomy (not performed; 0.512; P = 0.0394) and histologic major-vessel invasion at a second hepatectomy (negative; 0.057; P = 0.0005) were identified as independent factors affecting survival by multivariate analysis.
CONCLUSION: Hepatic resection including major-vessel resection/reconstruction for colorectal liver metastases can be performed with acceptable operative risk. However, such aggressive approaches are beneficial mainly in patients responding to effective prehepatectomy chemotherapy.
- Citation: Tanaka K, Matsuyama R, Takeda K, Matsuo K, Nagano Y, Endo I. Aggressive liver resection including major-vessel resection for colorectal liver metastases. World J Hepatol 2009; 1(1): 79-89
- URL: https://www.wjgnet.com/1948-5182/full/v1/i1/79.htm
- DOI: https://dx.doi.org/10.4254/wjh.v1.i1.79
Liver resections can be performed with increasing safety for metastatic liver cancer as a result of improved techniques and perioperative care. Major technical complications and fatal liver failure after hepatic resection have become rare. Classically, most reported surgical experience has involved patients with a small number of metastatic lesions in a distribution confined to the hemiliver, but recent advances involving surgical techniques and perioperative care have extended indications for hepatectomy in treatment of colorectal cancer metastases. While extensive hepatectomy, multiple partial liver resections, or both often are necessary to curatively resect aggressive and advanced metastases in the liver, these strategies all involve considerable reduction of hepatic mass, which can lead to clinical decompensation including hepatic insufficiency. Curative resection therefore is not always possible in such patients, despite modern hepatic surgical techniques.
Planned 2-stage hepatectomy, portal vein embolization (PVE), and hepatectomy together with local ablation have been studied as effective ways to completely remove diffuse liver metastases from colorectal cancer[1-4] while preserving functional remnant liver volume and broadening indications for curative resection in these patients. Another strategy is hepatectomy combined with major blood vessel resection and reconstruction. Advanced liver metastases occasionally invade major blood vessels such as the inferior vena cava (IVC), major hepatic veins, or hepatic venous confluence. Complete removal of such tumors requires patients to undergo vascular resection and reconstruction. In the past, involvement of the IVC has been considered a contraindication to resection of advanced liver tumors, because surgical risks were high and long-term prognosis was poor. Presently, liver resection combined with IVC resection and reconstruction has been reported to be a feasible procedure that can be performed with acceptable operative risk and improved long-term outcome in selected patients[5]. However, no definite consensus on long-term survival benefit of such challenging procedures has yet been reached.
In the present study, we retrospectively analyzed patients treated at our institution to estimate efficacy of hepatectomy combined with major blood vessel resection and reconstruction.
From April 1992 to March 2009, a total of 394 liver resections for colorectal liver metastases were performed for 334 patients at our Department of Gastroenterological Surgery, Yokohama City University Graduate School of Medicine. A second liver resection was performed in 45 patients with liver recurrence with or without extrahepatic metastases. A third hepatectomy for a second liver recurrence was performed in 11 patients; fourth hepatectomy for third recurrence in 3; and fifth hepatectomy for fourth recurrence in 1. Among the 394 resections, 23 (22 first resections, 1 s resection) were excluded either because curative hepatectomy could not be undertaken or concomitant extrahepatic tumor precluded R0 resection despite curative liver resection. Data from the remaining 312 patients with 371 liver resections were included in the analysis. The mean follow-up duration for these 312 patients after initial liver resection was 49 mo (median, 35; range, 1 to 221). Among these patients, resection and reconstruction of retrohepatic vena cava, major hepatic veins, or hepatic venous confluence was performed during hepatectomy in 25.
Preoperative staging included a physical examination, measurement of serum carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 19-9, colonoscopy, barium enema, abdominal ultrasonography, abdominal computed tomography (CT), and chest imaging by routine chest radiography or CT. Imaging by positron-emission tomography was introduced for preoperative staging after 2002.
Hepatectomy was not necessarily performed according to anatomic principles of resection; the guiding aim was assurance of tumor-free margins. To determine whether or not a hepatectomy procedure was acceptable for a given patient, we employed a prediction score (PS) introduced by Yamanaka et al[6] calculated using the formula; PS = -84.6 + 0.933a + 1.11b + 0.999c. The three variables designated by letter were; a, resection fraction (%) calculated from CT volumetry; b, indocyanine green retention rate at 15 min; c, patient age. A PS less than 50 indicated that a given hepatectomy would be acceptable. When a single-stage combined resection was precluded by insufficient estimated postoperative liver volume, excessive indocyanine green retention rate, or patient age considerations[6] a different strategy was adopted. In such cases PVE, 2-stage hepatectomy, or resection and reconstruction of major vessels during a hepatectomy planned to maximally preserve functional liver parenchyma was performed.
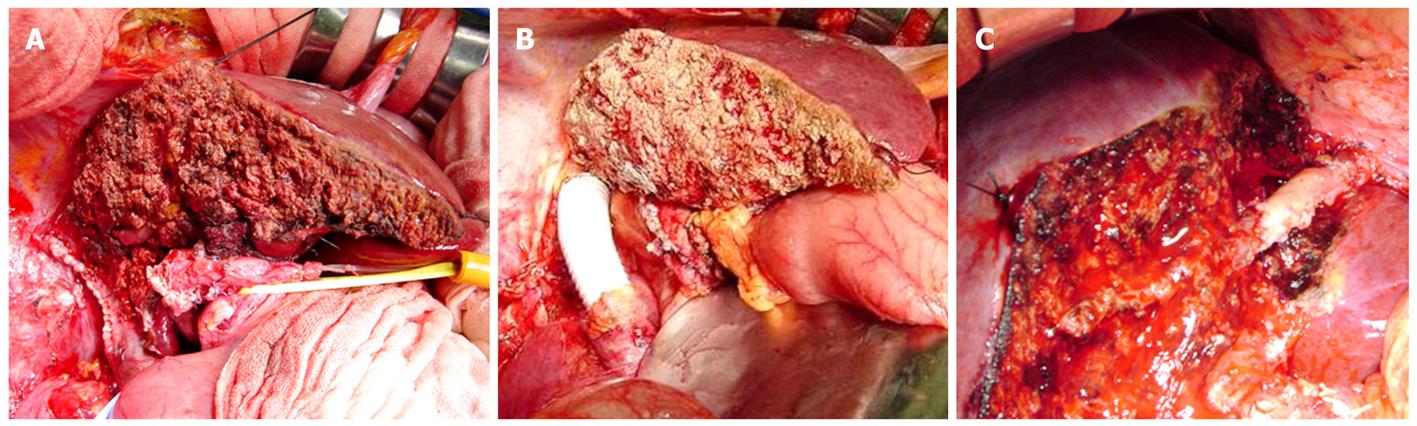
Resection and reconstruction of major vessels were performed as described below. When tumor involvement of the IVC was slight, control of the IVC during resection of the involved portion was achieved simply by placing a vascular clamp in a position tangential to the vena cava; a primary IVC repair then was performed with lateral venorrhaphy, taking care not to narrow the IVC excessively (Figure 1A). Larger resections of the IVC that could not be repaired primarily were reconstructed with synthetic (Figure 1B)[7] or autogenous grafts[8], using venovenous bypass with an active centrifugal force pump if necessary. When the tumor had infiltrated the proximal side of a major hepatic vein or the hepatic venous confluence entering the IVC but extent of tumor involvement of the vein was 2 cm or less, end-to-end anastomosis was carried out. When resection exceeding 2 cm was needed, an autogenous graft of portal vein within resected liver parenchyma was normally used (Figure 1C).
Intraoperative ultrasonography was used to identify any occult tumors not detected preoperatively, and to confirm relationships between tumors and vasculobiliary structures. Parenchymal dissection was performed using ultrasonic dissectors. When necessary, the liver pedicle was clamped intermittently in cycles including 15 min of clamping and 5 min of reperfusion. The Brisbane 2000 terminology of the International Hepato-Pancreato-Biliary Association was used to categorize operative procedures[9].
Any extrahepatic metastases were resected whenever possible, as decided on a case-by-case basis. For resectable metastases in both liver and lung, liver resection and primary tumor resection were performed prior to pulmonary resection, aiming to eliminate the liver as a source of potentially disseminating neoplastic cells. When liver metastases were associated with extrahepatic intra-abdominal metastases, both were resected at the same time.
Principles underlying selection criteria for resection of recurrent hepatic metastases were the same as those for initial hepatectomy. Technical considerations predominated in surgical decisions regarding feasibility of repeat hepatic resection. Since quality and quantity of remaining hepatic parenchyma were highly important factors, patients were excluded from repeat hepatic resection when the PS was greater than 50[6].
Some patients initially deemed to have unresectable liver involvement or patients with marginally resectable metastases (4 or more lesions distributed in 2 lobes; massive tumors; or unfavorably located tumors) underwent prehepatectomy chemotherapy. However, as the choice of treatment depended on several factors including initial assessment of resectability, treatment plans were made on a case-by-case basis. Treatment consisted of infusions into the hepatic artery (HAI) with a combination of 5-fluorouracil (5-FU), l-folinic acid (FA), and cisplatin (CDDP); systemic chemotherapy with 5-FU and FA with or without oxaliplatin or irinotecan; or a combination of both hepatic artery and systemic routes.
After resection for initial liver metastases, liver recurrence, or extrahepatic recurrence, adjuvant chemotherapy was carried out by HAI or intravenously, generally with 5-FU and FA and with or without addition of CDDP or irinotecan.
Among postoperative complications, hyperbilirubinemia was defined as a serum bilirubin concentration on postoperative day 7 of 3 mg/dL or greater. Biliary fistula was diagnosed when bile drainage from the abdominal wound or drain was apparent, with a total bilirubin concentration in the drainage fluid of more than 5 mg/mL or 3 times the serum concentration. Intra-abdominal abscess or liver stump abscess was confirmed by percutaneous drainage. Any medical problems that delayed postoperative recovery and prolonged hospital stay (e.g. ischemic heart disease) also were defined as postoperative morbidity.
Patients underwent monthly follow-up evaluation at our outpatient clinic. Data were obtained and recorded from each patient’s clinical record. Long-term outcome was ascertained through clinical follow-up, tumor registry follow-up, and contact with the patient, family, or referring physician when necessary. No patients were lost to follow-up. Serum CEA was measured every month, CT was performed every 3 mo, and a chest roentgenogram was obtained every 6 mo for 5 years after the most recent operation.
Statistical comparisons of baseline data were performed by the Mann-Whitney U test, the χ2 test, or Fisher’s exact test. Survival rates were calculated by the Kaplan-Meier method. Independent predictors of resection and reconstruction of major vessels being undertaken during hepatectomy were identified by multivariate analysis using multiple logistic regression. Multivariate regression analysis for identifying prognosticators was carried out by a proportional hazard method using a Cox model. Differences between survival curves were analyzed by the log-rank test. A difference was considered significant when the two-sided P value was below 0.05.
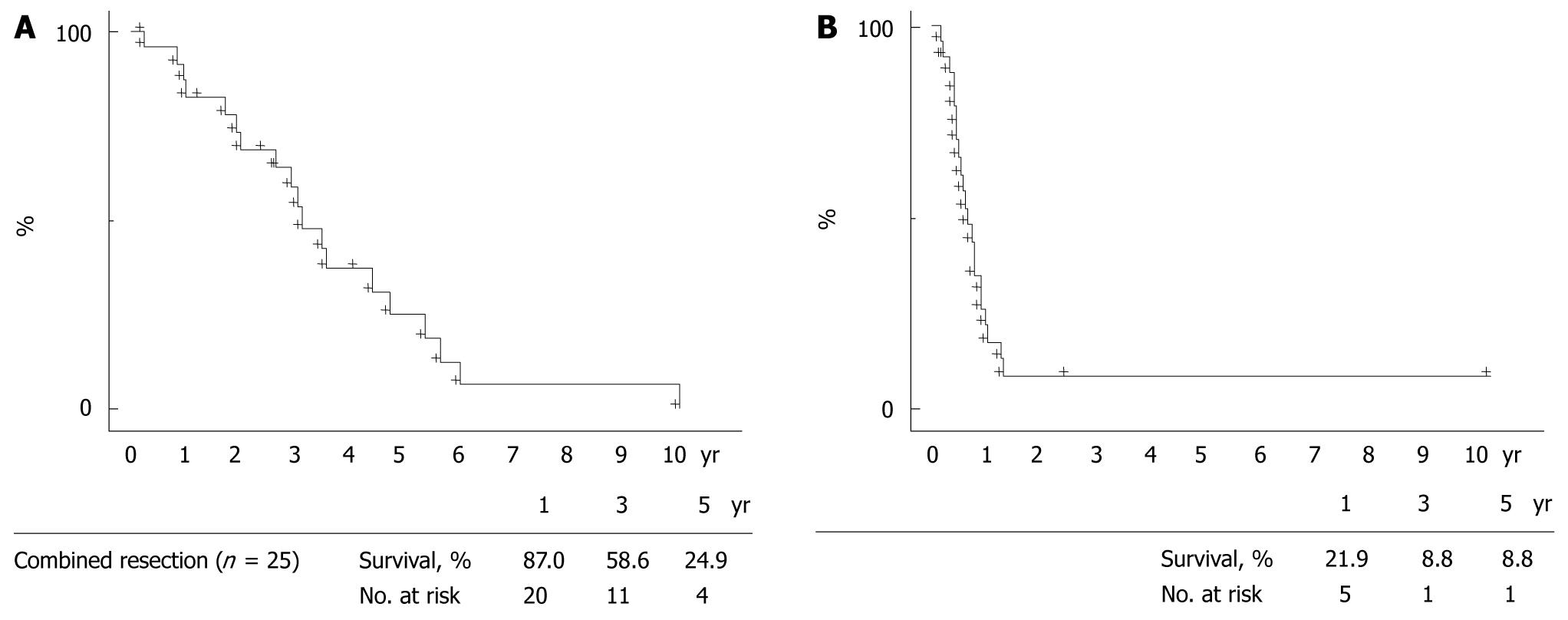
Vascular resection/reconstruction was performed on the IVC alone (n = 19), on the IVC including the confluence of the left hepatic vein (n = 1), on the middle hepatic vein (n = 3), and on the right hepatic vein (n = 2). In the 20 patients with IVC resection, direct suturing of the IVC was performed in 17 patients, an autogenous pericardial patch was applied in 1 patient, and the IVC segment was replaced by a synthetic graft (Gore-Tex; W. L. Gore & Assoc., USA) in 2 patients. All 3 patients with resection of the middle hepatic vein underwent reconstruction of the hepatic vein using a portion of the portal vein within the resected specimen. Vascular continuity was reestablished by end-to-end anastomosis in 1 patient with right hepatic vein resection and by a pericardial patch graft in the other. The patient whose IVC resection/reconstruction used a synthetic graft required venovenous bypass. Patient characteristics and outcomes are shown in Table 1. Negative resection margins were achieved in 15 of these 25 patients. Direct invasion of the IVC wall or major hepatic veins was confirmed histologically in 12 patients. Operative feasibility, hospital stays, and postoperative complications are shown in Table 2. No patients died within 60 d of hepatectomy. Morbidity occurred in 16% (4/25), and 1 patient had both severe ascites and hyperbilirubinemia. Preserved vascular patency was demonstrated by contrast-enhanced CT images approximately 1 mo after resection in all 25 patients with resection/reconstruction of major vessels during hepatectomy. Their 1-, 3-, and 5-year overall survival rates after hepatectomy were 87.0%, 58.6%, and 24.9%, respectively; disease-free rates at these time points were 21.9%, 8.8%, and 8.8%, respectively. mean ± SE and median for survival time in months were 45 ± 7 and 39; mean and median disease-free months respectively were 9 ± 1 and 8 (Figure 2).
| Patient No. | Resected vessel (s) | Reconstruction | No. of Hx | Age | Gender | Tumor distribution | No. of tumors | Maximum size (mm) | Resection margin | Outcome month | Status |
| 1 | IVC | Primary closure | 1 | 72 | F | Bilobar | 2 | 42 | Negative | 45 | DDT |
| 2 | IVC | Primary closure | 1 | 70 | M | Unilobar | 2 | 15 | Negative | 67 | DDT |
| 3 | IVC | Primary closure | 1 | 61 | M | Unilobar | 1 | 33 | Positive | 12 | DDT |
| 4 | IVC | Primary closure | 1 | 64 | M | Bilobar | 5 | 58 | Positive | 39 | DDT |
| 5 | IVC | Primary closure | 1 | 70 | F | Bilobar | 3 | 75 | Negative | 33 | DDT |
| 6 | IVC | Primary closure | 1 | 54 | M | Bilobar | 27 | 75 | Positive | 4 | DDT |
| 7 | IVC | Primary closure | 1 | 61 | F | Bilobar | 6 | 110 | Positive | 37 | DDT |
| 8 | IVC | Primary closure | 1 | 72 | M | Bilobar | 5 | 20 | Negative | 11 | DDT |
| 9 | IVC | Primary closure | 1 | 69 | F | Unilobar | 1 | 35 | Negative | 34 | NED |
| 10 | IVC | Primary closure | 1 | 71 | M | Unilobar | 1 | 55 | Positive | 31 | NED |
| 11 | IVC | Primary closure | 1 | 68 | F | Unilobar | 2 | 74 | Positive | 4 | NED |
| 12 | IVC | Graft replacement | 1 | 70 | F | Bilobar | 6 | 63 | Negative | 16 | AWD |
| 13 | IVC | Primary closure | 2 | 73 | M | Unilobar | 1 | 40 | Negative | 125 | DOD |
| 14 | IVC | Primary closure | 2 | 47 | F | Bilobar | 4 | 38 | Negative | 75 | DOD |
| 15 | IVC | Primary closure | 2 | 65 | M | Unilobar | 1 | 42 | Positive | 38 | DDT |
| 16 | IVC | Primary closure | 2 | 61 | M | Unilobar | 1 | 29 | Negative | 22 | DDT |
| 17 | IVC | Patch closure | 2 | 53 | F | Bilobar | 3 | 40 | Negative | 59 | DDT |
| 18 | IVC | Graft replacement | 2 | 56 | M | Unilobar | 1 | 50 | Negative | 3 | NED |
| 19 | IVC | Primary closure | 3 | 57 | F | Unilobar | 1 | 36 | Negative | 25 | DDT |
| 20 | IVC-LHV | Primary closure | 3 | 54 | F | Unilobar | 1 | 23 | Positive | 45 | DDT |
| 21 | MHV | Graft replacement | 1 | 62 | M | Bilobar | 4 | 45 | Positive | 55 | DDT |
| 22 | MHV | Graft replacement | 1 | 60 | M | Bilobar | 2 | 56 | Negative | 52 | AWD |
| 23 | MHV | Graft replacement | 2 | 80 | M | Unilobar | 1 | 45 | Negative | 13 | DDT |
| 24 | RHV | End-to-end | 1 | 60 | F | Unilobar | 1 | 19 | Negative | 24 | DDT |
| 25 | RHV | Patch closure | 1 | 55 | M | Bilobar | 5 | 17 | Positive | 71 | DDT |
| Variable | Patients (n = 25) |
| Resected liver volume (g) | 386 ± 242 |
| (median, range) | (360, 21-972) |
| Operative time, mean ± SE (min) | 488 ± 130 |
| (median, range) | (460, 270-735) |
| Total blood loss, mean ± SE (L) | 1.6 ± 1.3 |
| (median, range) | (1.4, 0.3-5.7) |
| Patients transfused | 17 (68%) |
| Hospital stay, in days, mean ± SE | 19 ± 8 |
| (median, range) | (18, 10-41) |
| Morbidity | 4 (16%) |
| Ascites | 2 |
| Hyperbilirubinemia | 1 |
| Bile leakage | 1 |
| Intra-abdominal abscess | 1 |
Univariate analysis identified initial vs repeat hepatectomy (P < 0.01), maximum size of metastases (P < 0.01), and prehepatectomy chemotherapy (P < 0.01) as significant predictors of resection/reconstruction (Table 3). Multivariate analysis including factors for which univariate analysis yielded P values below 0.1 (initial vs repeat hepatectomy, maximum size of metastases, prehepatectomy chemotherapy, primary Dukes stage, extent of hepatectomy, and PVE following hepatectomy) identified 3 factors independently associated with resection/reconstruction of major vessels during hepatectomy: repeat liver resection (relative risk or RR, 5.690; 95% CI, 2.053 to 15.765; P = 0.0008), maximum tumor diameter more than 30 mm (RR, 3.338; CI, 1.224 to 9.108; P = 0.0292), and prehepatectomy chemotherapy (RR, 3.485; CI, 1.379 to 8.807; P = 0.0083; Table 4).
| Variables | Conventional (n = 346) | Combined (n = 25) | P value | |
| n (%) | n (%) | |||
| Patient-related | ||||
| Age (yr) | ≤ 64 | 179 (52) | 14 (56) | 0.84 |
| ≥ 65 | 167 (48) | 11 (44) | ||
| Gender | Male | 209 (60) | 14 (56) | 0.68 |
| Female | 137 (40) | 11 (44) | ||
| Primary-related | ||||
| Site | Colon | 204 (59) | 19 (76) | 0.14 |
| Rectum | 142 (41) | 6 (24) | ||
| Histology | Moderate | 223 (64) | 14 (56) | 0.40 |
| Others | 123 (36) | 11 (44) | ||
| Dukes stage | A/B | 120 (35) | 4 (16) | 0.08 |
| C | 226 (65) | 21 (84) | ||
| Liver-related | ||||
| Hepatectomy | Initial | 296 (86) | 16 (64) | < 0.01 |
| Repeat | 50 (14) | 9 (36) | ||
| Distribution | Unilobar | 213 (62) | 12 (48) | 0.21 |
| Bilobar | 133 (38) | 13 (52) | ||
| Number | ≤ 2 | 222 (64) | 14 (56) | 0.52 |
| ≥ 3 | 124 (36) | 11 (44) | ||
| Maximum tumor size (mm) | ≤ 30 | 197 (57) | 6 (24) | < 0.01 |
| > 30 | 149 (43) | 19 (76) | ||
| Prehepatectomy CEA (ng/mL) | < 10 | 175 (54) | 17 (68) | 0.21 |
| ≥ 10 | 150 (46) | 8 (32) | ||
| Treatment-related | ||||
| Extent of hepatectomy | Major | 112 (32) | 13 (52) | 0.05 |
| Minor | 234 (68) | 12 (48) | ||
| PVE | Performed | 44 (13) | 7 (28) | 0.06 |
| Not performed | 302 (87) | 18 (72) | ||
| Staged procedure | Performed | 20 (6) | 1 (4) | > 0.99 |
| Not performed | 326 (94) | 24 (96) | ||
| Hepatectomy with ablation | Performed | 26 (8) | 4 (16) | 0.13 |
| Not performed | 320 (92) | 21 (84) | ||
| Prehepatectomy chemotherapy | Performed | 63 (18) | 11 (44) | < 0.01 |
| Not performed | 283 (82) | 14 (56) |
| Variables | RR | P value |
| Hepatectomy | ||
| Repeat | 5.690 (2.053-15.765) | 0.0008 |
| Maximum tumor size (mm) | ||
| > 30 | 3.338 (1.224-9.108) | 0.0186 |
| Prehepatectomy chemotherapy | ||
| Performed | 3.485 (1.379-8.807) | 0.0083 |
When 312 patients who underwent a first liver resection for initial liver metastases were divided into those with conventional resection (n = 296) and those with combined resection of liver and major vessels (n = 16), 2 patient- or tumor-related variables, maximum tumor diameter and prehepatectomy CEA, were significantly greater in the combined resection group than in the conventional resection group (P = 0.02 and P < 0.01, respectively; Table 5). When survival was compared between these groups, overall survival and disease-free rates were significantly poorer in the combined resection group than in the conventional resection group (P = 0.02 and P < 0.01, respectively, Figure 3). Univariate analysis of these 312 patients identified tumor distribution (P < 0.01), number of metastases (P < 0.01), maximum tumor size (P < 0.01), prehepatectomy CEA (P = 0.01), extrahepatic metastases (P < 0.01), extent of hepatectomy (P < 0.01), tumor-free margin (P < 0.01), PVE (P < 0.01), staged hepatectomy (P < 0.01), prehepatectomy chemotherapy (P = 0.01), adjuvant chemotherapy after resection (P = 0.02), and combined major-vessel resection (P = 0.02) as significant prognostic determinants of the initial resection (Table 6). Multivariate analysis, including factors identified as significant by univariate analysis, identified factors independently affecting survival as number of metastases (≤ 2; RR, 0.543; CI, 0.378 to 0.779; P = 0.0009), prehepatectomy CEA (< 10 ng/mL; RR, 0.683; CI, 0.485 to 0.961; P = 0.0288), extrahepatic metastases (none; RR, 0.549; CI, 0.358 to 0.842; P = 0.0060), staged hepatectomy not performed (RR, 0.481; CI, 0.273 to 0.848; P = 0.0114), use of adjuvant chemotherapy (RR, 0.539; CI, 0.335 to 0.866; P = 0.0107), and no combined major-vessel resection performed (0.512; CI, 0.271 to 0.968; P = 0.0394).
| Initial resection | Second resection | ||||||
| Variables | Conventional (n = 296) | Combined (n = 16) | P value | Conventional (n = 37) | Combined (n = 7) | P value | |
| Patient-related | |||||||
| Age, years | 64 | 66 | 0.6 | 63 | 61 | 0.91 | |
| (30-85) | (54-72) | (32-83) | (47-80) | ||||
| Gender | Male | 186 (63%) | 9 (56%) | 0.6 | 19 (51%) | 5 (71%) | 0.43 |
| Female | 110 (37%) | 7 (44%) | 18 (49%) | 2 (29%) | |||
| Primary-related | |||||||
| Site | Colon | 169 (57%) | 11 (69%) | 0.61 | 27 (73%) | 6 (86%) | 0.66 |
| Rectum | 127 (43%) | 5 (31%) | 10 (27%) | 1 (14%) | |||
| Dukes stage | A or B | 103 (35%) | 3 (19%) | 0.33 | 13 (35%) | 1 (14%) | 0.53 |
| C | 193 (65%) | 13 (81%) | 24 (65%) | 6 (86%) | |||
| Histology | Well | 99 (33%) | 7 (44%) | 0.62 | 11 (30%) | 2 (29%) | > 0.99 |
| Moderate | 184 (62%) | 8 (50%) | 26 (70%) | 5 (71%) | |||
| Others | 13 (4%) | 1 (6%) | - | - | |||
| Liver-related | |||||||
| Timing | Synchronous | 146 (49%) | 10 (63%) | 0.44 | |||
| Metachronous | 150 (51%) | 6 (38%) | |||||
| Distribution | Unilobar | 175 (59%) | 6 (38%) | 0.12 | 27 (73%) | 5 (71%) | > 0.99 |
| Bilobar | 121 (41%) | 10 (63%) | 10 (27%) | 2 (29%) | |||
| Number | 2 | 3.5 | 0.11 | 1 | 1 | 0.57 | |
| (1-38) | (1-27) | (1-7) | (1-4) | ||||
| Maximum tumor size (mm) | 28 | 50 | 0.02 | 29 | 40 | 0.05 | |
| (5-185) | (15-110) | (10-80) | (29-50) | ||||
| Extrahepatic disease | Present | 40 (14%) | 4 (25%) | 0.26 | 8 (22%) | 1 (14%) | > 0.99 |
| Prehepatectomy CEA (ng/mL) | 8.3 | 43.9 | < 0.01 | 8.6 | 21.5 | 0.45 | |
| (1-10 536) | (2-4498) | (3-360) | (2-559) | ||||
| Survival (%) | |||||
| Variables | n | 3 years | 5 years | P value | |
| Patient-related | |||||
| Age (yr) | ≤ 64 | 158 | 66.9 | 53.1 | 0.83 |
| ≥ 65 | 154 | 64.9 | 52.3 | ||
| Gender | Male | 195 | 68.2 | 53.0 | 0.77 |
| Female | 117 | 62.4 | 51.9 | ||
| Primary-related | |||||
| Site | Colon | 180 | 67.7 | 53.7 | 0.87 |
| Rectum | 132 | 63.5 | 50.9 | ||
| Histology | Moderate | 192 | 67.1 | 52.1 | 0.41 |
| Others | 120 | 64.1 | 53.5 | ||
| Dukes stage | A/B | 104 | 72.2 | 59.9 | 0.11 |
| C | 208 | 62.7 | 48.8 | ||
| Liver-related | |||||
| Timing | Synchronous | 156 | 63.7 | 48.5 | 0.17 |
| Metachronous | 156 | 68.1 | 56.5 | ||
| Distribution | Unilobar | 181 | 72.2 | 59.0 | < 0.01 |
| Bilobar | 131 | 57.2 | 43.6 | ||
| Number | ≤ 2 | 188 | 72.6 | 61.2 | < 0.01 |
| ≥ 3 | 124 | 55.6 | 39.1 | ||
| Maximum tumor size (mm) | ≤ 30 | 169 | 75.0 | 61.7 | < 0.01 |
| > 30 | 143 | 55.3 | 41.5 | ||
| Prehepatectomy CEA (ng/mL) | |||||
| < 10 | 158 | 72.6 | 60.8 | 0.01 | |
| ≥ 10 | 145 | 60.1 | 44.5 | ||
| Extrahepatic metastases | Present | 44 | 40.9 | 26.7 | < 0.01 |
| Absent | 268 | 70.6 | 57.3 | ||
| Treatment-related | |||||
| Extent of hepatectomy | Major | 113 | 57.9 | 39.8 | < 0.01 |
| Minor | 199 | 70.6 | 59.8 | ||
| Tumor-free margin | Not exposed | 256 | 70.5 | 58.7 | < 0.01 |
| Exposed | 56 | 46.6 | 27.9 | ||
| PVE | Performed | 49 | 51.0 | 37.4 | < 0.01 |
| Not performed | 263 | 68.8 | 55.2 | ||
| Staged procedure | Performed | 21 | 33.2 | 22.1 | < 0.01 |
| Not performed | 291 | 68.5 | 55.0 | ||
| Hepatectomy with ablation | Performed | 27 | 60.5 | 34.1 | 0.08 |
| Not performed | 285 | 66.5 | 54.2 | ||
| Prehepatectomy chemotherapy | |||||
| Performed | 68 | 56.0 | 35.1 | 0.01 | |
| Not performed | 244 | 68.4 | 56.0 | ||
| Adjuvant chemotherapy | Performed | 257 | 67.0 | 54.6 | 0.02 |
| Not performed | 55 | 63.1 | 42.0 | ||
| Combined resection | Performed | 16 | 55.4 | 24.6 | 0.02 |
| Not performed | 296 | 66.5 | 54.0 | ||
| Major vessel invasion | Positive | 7 | 66.0 | 52.9 | 0.10 |
| Negative | 305 | 66.0 | 52.9 | ||
When 44 patients who underwent a second liver resection for liver recurrence were divided into those with conventional resection (n = 37) and those with combined major-vessel resection to liver resection (n = 7), maximum tumor diameter was greater in the combined resection group than in the conventional resection group (P = 0.05, Table 5). Overall survival tended to be poorer in the combined resection group than in the conventional resection group, although significance was not reached (P = 0.09, Figure 4). Univariate analysis identified patient age (P = 0.03), extent of hepatectomy (P = 0.02), adjuvant chemotherapy (P = 0.03), and histologic major-vessel invasion (P < 0.01) as significant prognostic determinants (Table 7). Multivariate analysis identified factors independently affecting survival as extent of hepatectomy (major; RR, 0.264; CI, 0.072 to 0.970; P = 0.0449), use of adjuvant chemotherapy (RR, 0.119; CI, 0.019 to 0.751; P = 0.0235), and lack of histologic major-vessel invasion (0.057; CI, 0.011 to 0.286; P = 0.0005).
| Survival (%) | |||||
| Variables | n | 3 years | 5 years | P value | |
| Patient-related | |||||
| Age (yr) | ≤ 63 | 23 | 95.0 | 62.9 | 0.03 |
| ≥ 64 | 21 | 67.9 | 34.5 | ||
| Gender | Male | 24 | 85.6 | 56.5 | 0.21 |
| Female | 20 | 77.8 | 43.5 | ||
| Primary-related | |||||
| Site | Colon | 33 | 82.9 | 49.1 | 0.80 |
| Rectum | 11 | 80.0 | 54.9 | ||
| Histology | Moderate | 31 | 85.9 | 52.3 | 0.40 |
| Others | 13 | 72.7 | 45.5 | ||
| Dukes stage | A/B | 14 | 71.4 | 38.1 | 0.15 |
| C | 30 | 87.9 | 57.7 | ||
| Liver-related | |||||
| Distribution | Unilobar | 32 | 79.1 | 46.5 | 0.63 |
| Bilobar | 12 | 90.0 | 60.0 | ||
| Number | 1 | 24 | 76.1 | 40.0 | 0.61 |
| ≥ 2 | 20 | 88.9 | 62.3 | ||
| Maximum tumor size (mm) | ≤ 30 | 22 | 73.3 | 47.5 | 0.41 |
| > 30 | 22 | 90.2 | 52.4 | ||
| Prehepatectomy CEA (ng/mL) | |||||
| < 9 | 18 | 76.0 | 53.2 | 0.56 | |
| ≥ 9 | 18 | 93.8 | 67.7 | ||
| Extrahepatic metastases | Present | 9 | 77.8 | 62.2 | 0.99 |
| Absent | 35 | 83.2 | 47.2 | ||
| Treatment-related | |||||
| Extent of hepatectomy | Major | 10 | 90.0 | 64.3 | 0.02 |
| Minor | 34 | 79.7 | 46.1 | ||
| Tumor-free margin | Not exposed | 37 | 81.2 | 53.9 | 0.39 |
| Exposed | 7 | 85.7 | 34.3 | ||
| Hepatectomy with ablation | Performed | 3 | 66.7 | 33.3 | 0.98 |
| Not performed | 41 | 83.3 | 52.2 | ||
| Prehepatectomy chemotherapy | |||||
| Performed | 6 | 50.0 | 50.0 | 0.73 | |
| Not performed | 38 | 80.0 | 50.0 | ||
| Adjuvant chemotherapy | Performed | 37 | 86.1 | 52.9 | 0.03 |
| Not performed | 7 | 0.0 | 0.0 | ||
| Combined resection | Performed | 7 | 66.7 | 33.3 | 0.09 |
| Not performed | 37 | 84.6 | 53.8 | ||
| Major vessel invasion | Positive | 4 | 33.3 | 0.0 | < 0.01 |
| Negative | 40 | 85.9 | 54.8 | ||
In the present study, hepatic resection combined with major blood vessel resection/reconstruction for colorectal liver metastases could be performed with acceptable morbidity and no mortality, although the procedure was associated with greater blood loss and required blood transfusion more frequently than conventional liver resections. For vascular control during combined resections including the IVC, total hepatic vascular exclusion[10] and/or hypothermic isolated hepatic perfusion[11] have been used previously. However, most patients in this study did not require venovenous bypass or hypothermic isolated hepatic perfusion, which can involve a clinically significant hemodynamic instability. Such measures could be avoided probably because most of our patients had the IVC reconstructed by primary closure during clamping of a single side of the IVC or total hepatic IVC (clamping below the hepatic venous confluence). The resected IVC can be repaired primarily if the resected segment is small[5,12]. Importantly, however, persistent leg edema has been reported when the IVC was narrowed by 50%, despite maintenance of IVC patency[13]. Therefore, the partially resected IVC was often reconstructed using a patch graft. Grafts for patch repair have reportedly included saphenous vein[14], superficial femoral vein[15], and left renal vein[5]. We used a pericardial patch graft for the IVC defect. This graft can be obtained easily even in repeat resections where severe intra-abdominal adhesions may be encountered. This also avoids additional skin incisions and risk of compromising renal function.
Repeat liver resection, large tumors, and prehepatectomy chemotherapy were selected factors predicting resection and reconstruction of major vessels during hepatectomy. A trend associating increased frequency of tumor invasion of major vessels with increased size of metastases readily can be expected. Prehepatectomy chemotherapy was given to patients initially deemed to have unresectable liver involvement or marginally resectable metastases and so one also might expect their tumors to invade major vessels frequently. Distortion and anatomic disorientation caused by rotation of the liver remnant often accompanies regeneration after repeat resections. Repeat resections often induce adhesions of unencapsulated liver surfaces to surrounding organs. Such alteration of anatomy was probably the main reason for repeat resection as a risk factor for major-vessel invasion.
Resection of colorectal liver metastases infiltrating major vessels is technically feasible although its long-term outcome has yet to be fully described. Miyazaki et al[5] reported 5-year and median survivals of 22% and 19.2 mo following colorectal metastasis resection combined with IVC resection. Aoki et al[16] reported a median survival time for patients with resection/reconstruction of the IVC or hepatic venous confluence of 25.8 mo. Similar results were obtained in the present study; 5-year and median survival of the 25 patients with resection of major vessels were 24.9% and 39 mo after hepatectomy. When patients were divided into conventional resection vs combined major vessel resection both at initial and second hepatectomy, overall survival and the disease-free rate in the combined resection group were significantly poorer than in the conventional resection group at initial hepatectomy, although preoperative tumor-related factors (tumor size and CEA) differed significantly between the groups.
Combined major-vessel resection/reconstruction was also identified as a prognosticator at initial hepatectomy for liver metastases by multivariate analysis. Even at a second hepatectomy performed in a limited number of patients with liver recurrence, overall survival tended to be poorer in the combined resection group than in the conventional resection group. As for prognostic factors in the second resection, combined major-vessel resection/reconstruction tended to be a negative prognosticator but fell short of significance by univariate analysis.
The presence of histologic major-vessel invasion was identified as a factor adversely affecting survival. Most reported surgical experience with combined major-vessel resection/reconstruction for colorectal liver metastases has involved small numbers of patients, precluding definite conclusions about long-term survival. In previous reported series, however, the prognosis for patients with advanced tumors invading the IVC or major hepatic venous confluence seemed unsatisfactory compared to the prognosis for patients without major vessel invasion[5,16]. Impact of combined major-vessel resection/reconstruction on survival may be clearly demonstrated when comparison is made between patients who did not get the surgery and those that did. However, reasons for not performing such surgery were heterogeneous, (intrahepatic and extrahepatic disease status and patients’ status), and so it was difficult to obtain similar background characteristics between these patients. Comparison of nutritional or functional assessment was also difficult for the same reasons.
Even in reports including several kinds of liver cancers, 5-year survival was unsatisfactory in cases with vascular invasion, approximately 30%[13,17]. Early tumor recurrence in patients with extensive local tumor spread also has been reported after ex situ liver surgery[18]. In treating hepatocellular carcinoma, Yang et al[19] reported that portal vein invasion predominated in patients whose first recurrence was in the liver, while hepatic vein invasion was predominant in patients who had only extrahepatic metastases without intrahepatic metastases. When colorectal liver metastases invade the IVC or major hepatic vein, dissemination of tumor cells through these veins may lead to extrahepatic recurrences, as occurs with hepatocellular carcinoma. However, the site of initial recurrence did not differ significantly between our combined and conventional groups after initial liver resection (extrahepatic recurrence, 64% vs 64%; P = 0.82) or second resection (extrahepatic recurrence, 80% vs 54%; P = 0.07; data not shown).
Current chemotherapy regimens can achieve either stabilization or decrease in tumor in more than 80% of patients[20,21]. Chemotherapy prior to hepatectomy allows us to extend indications for surgery in the presence of multiple metastases, permitting long-term survival, especially in chemotherapy responders[22-24]. Ng et al[25] reported that in response to chemotherapy, death of viable cells is randomly distributed. Necrotic elements in the center of the tumor are replaced by fibrosis, which draws remaining viable cells toward the center, reducing tumor volume. Furthermore, chemotherapy-associated decreases in micrometastases surrounding liver tumors are related to clinical responses and a favorable outcome[22], allowing complete removal of liver tumors to be achieved by a less extensive resection. Therefore, aggressive surgical approaches for liver metastases involving major vessels are best limited to patients showing a response or at least stability during effective prehepatectomy chemotherapy.
Hepatic resection combined with major-vessel resection/reconstruction for colorectal liver metastases can be performed with acceptable operative risk. Although no definite conclusion on long-term survival can be drawn from our study because of a limited number of patients, their overall survival was unsatisfactory. Ongoing advances in perioperative chemotherapy will be necessary to achieve better survival.
No definite consensus on long-term survival benefit of combined resection of liver with major vessels in treating colorectal liver metastases has yet been reached.
Only a little information exists about the impact of combined resection of liver with major vessels on the long-term outcome in patients following liver resection for colorectal metastases.
Hepatic resection combined with major-vessel resection/reconstruction for colorectal liver metastases can be performed with acceptable operative risk.
Surgical approaches for liver metastases involving major vessels are best limited to patients showing a response during prehepatectomy chemotherapy.
This study is fair, which can be accepted after answering some questions and revising. In fact, the greatest weakness of this retrospective study is that comparing 25 pts with vascular resection (to create a clear margin without adenoma) to 312 pts without need of vascular resection (to create a clear margin without adenoca) is like the trite old saying “comparing apples to oranges”.
Peer reviewer: Robert A Fisher, MD, HM Lee Professor of Surgery (tenured), Department of Surgery, Division of Transplant Surgery, Medical College of Virginia Hospitals, Virginia Commonwealth University, MCV Campus, VA 23298-0057, United States
S- Editor Zhang HN L- Editor Hughes D E- Editor Ma WH
| 1. | Wicherts DA, Miller R, de Haas RJ, Bitsakou G, Vibert E, Veilhan LA, Azoulay D, Bismuth H, Castaing D, Adam R. Long-term results of two-stage hepatectomy for irresectable colorectal cancer liver metastases. Ann Surg. 2008;248:994-1005. |
| 2. | Kianmanesh R, Farges O, Abdalla EK, Sauvanet A, Ruszniewski P, Belghiti J. Right portal vein ligation: a new planned two-step all-surgical approach for complete resection of primary gastrointestinal tumors with multiple bilateral liver metastases. J Am Coll Surg. 2003;197:164-170. |
| 3. | Jaeck D, Oussoultzoglou E, Rosso E, Greget M, Weber JC, Bachellier P. A two-stage hepatectomy procedure combined with portal vein embolization to achieve curative resection for initially unresectable multiple and bilobar colorectal liver metastases. Ann Surg. 2004;240:1037-1049; discussion 1049-1051. |
| 4. | Tanaka K, Shimada H, Nagano Y, Endo I, Sekido H, Togo S. Outcome after hepatic resection versus combined resection and microwave ablation for multiple bilobar colorectal metastases to the liver. Surgery. 2006;139:263-273. |
| 5. | Miyazaki M, Ito H, Nakagawa K, Ambiru S, Shimizu H, Okuno A, Nukui Y, Yoshitomi H, Kusashio K, Furuya S. Aggressive surgical resection for hepatic metastases involving the inferior vena cava. Am J Surg. 1999;177:294-298. |
| 6. | Yamanaka N, Okamoto E, Oriyama T, Fujimoto J, Furukawa K, Kawamura E, Tanaka T, Tomoda F. A prediction scoring system to select the surgical treatment of liver cancer. Further refinement based on 10 years of use. Ann Surg. 1994;219:342-346. |
| 7. | Kumada K, Shimahara Y, Fukui K, Itoh K, Morikawa S, Ozawa K. Extended right hepatic lobectomy: combined resection of inferior vena cava and its reconstruction by EPTFE graft (Gore-Tex). Case report. Acta Chir Scand. 1988;154:481-483. |
| 8. | Togo S, Tanaka K, Endo I, Kurosawa H, Morioka D, Miura Y, Nagano Y, Masui H, Sekido H, Shimada H. Reconstruction of the hepatic vein using a patch graft from the autologous pericardium. Int Surg. 2002;87:233-235. |
| 9. | The Brisbane 2000 Terminology of Liver Anatomy and Resection. Terminology Committee of the International Hepato-Pancreato-Biliary Association. HPB. 2000;2:333-339. |
| 10. | Huguet C, Nordlinger B, Galopin JJ, Bloch P, Gallot D. Normothermic hepatic vascular exclusion for extensive hepatectomy. Surg Gynecol Obstet. 1978;147:689-693. |
| 11. | Hannoun L, Delriviere L, Gibbs P, Borie D, Vaillant JC, Delva E. Major extended hepatic resections in diseased livers using hypothermic protection: preliminary results from the first 12 patients treated with this new technique. J Am Coll Surg. 1996;183:597-605. |
| 12. | Lodge JP, Ammori BJ, Prasad KR, Bellamy MC. Ex vivo and in situ resection of inferior vena cava with hepatectomy for colorectal metastases. Ann Surg. 2000;231:471-479. |
| 13. | Hemming AW, Reed AI, Langham MR Jr, Fujita S, Howard RJ. Combined resection of the liver and inferior vena cava for hepatic malignancy. Ann Surg. 2004;239:712-719; discussion 719-721. |
| 14. | Moriura S, Nimura Y, Hayakawa N, Maeda S, Kamiya J, Kondo S, Shionoya S, Nagino M. Combined resection of the inferior vena cava for hepato-biliary and pancreatic malignancies. Hepatogastroenterology. 1990;37:253-255. |
| 15. | Emre S, Schwartz ME, Katz E, Miller CM. Liver resection under total vascular isolation. Variations on a theme. Ann Surg. 1993;217:15-19. |
| 16. | Aoki T, Sugawara Y, Imamura H, Seyama Y, Minagawa M, Hasegawa K, Kokudo N, Makuuchi M. Hepatic resection with reconstruction of the inferior vena cava or hepatic venous confluence for metastatic liver tumor from colorectal cancer. J Am Coll Surg. 2004;198:366-372. |
| 17. | Azoulay D, Andreani P, Maggi U, Salloum C, Perdigao F, Sebagh M, Lemoine A, Adam R, Castaing D. Combined liver resection and reconstruction of the supra-renal vena cava: the Paul Brousse experience. Ann Surg. 2006;244:80-88. |
| 18. | Oldhafer KJ, Lang H, Schlitt HJ, Hauss J, Raab R, Klempnauer J, Pichlmayr R. Long-term experience after ex situ liver surgery. Surgery. 2000;127:520-527. |
| 19. | Yang Y, Nagano H, Ota H, Morimoto O, Nakamura M, Wada H, Noda T, Damdinsuren B, Marubashi S, Miyamoto A. Patterns and clinicopathologic features of extrahepatic recurrence of hepatocellular carcinoma after curative resection. Surgery. 2007;141:196-202. |
| 20. | Giacchetti S, Perpoint B, Zidani R, Le Bail N, Faggiuolo R, Focan C, Chollet P, Llory JF, Letourneau Y, Coudert B. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2000;18:136-147. |
| 21. | Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041-1047. |
| 22. | Tanaka K, Shimada H, Kubota K, Ueda M, Endo I, Sekido H, Togo S. Effectiveness of prehepatectomy intra-arterial chemotherapy for multiple bilobar colorectal cancer metastases to the liver: a clinicopathologic study of peritumoral vasculobiliary invasion. Surgery. 2005;137:156-164. |
| 23. | Bismuth H, Adam R, Levi F, Farabos C, Waechter F, Castaing D, Majno P, Engerran L. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg. 1996;224:509-520; discussion 520-522. |
| 24. | Adam R, Avisar E, Ariche A, Giachetti S, Azoulay D, Castaing D, Kunstlinger F, Levi F, Bismuth F. Five-year survival following hepatic resection after neoadjuvant therapy for nonresectable colorectal. Ann Surg Oncol. 2001;8:347-353. |