Copyright
©The Author(s) 2016.
World J Hepatol. Feb 28, 2016; 8(6): 331-339
Published online Feb 28, 2016. doi: 10.4254/wjh.v8.i6.331
Published online Feb 28, 2016. doi: 10.4254/wjh.v8.i6.331
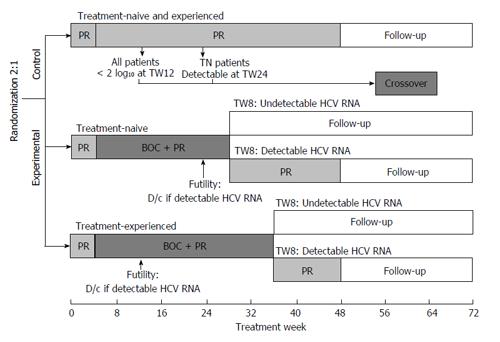
Figure 1 Study design.
BOC: Boceprevir; D/c: Discontinued; HCV: Hepatitis C virus; PR: Peginterferon/ribavirin; TW: Treatment week.
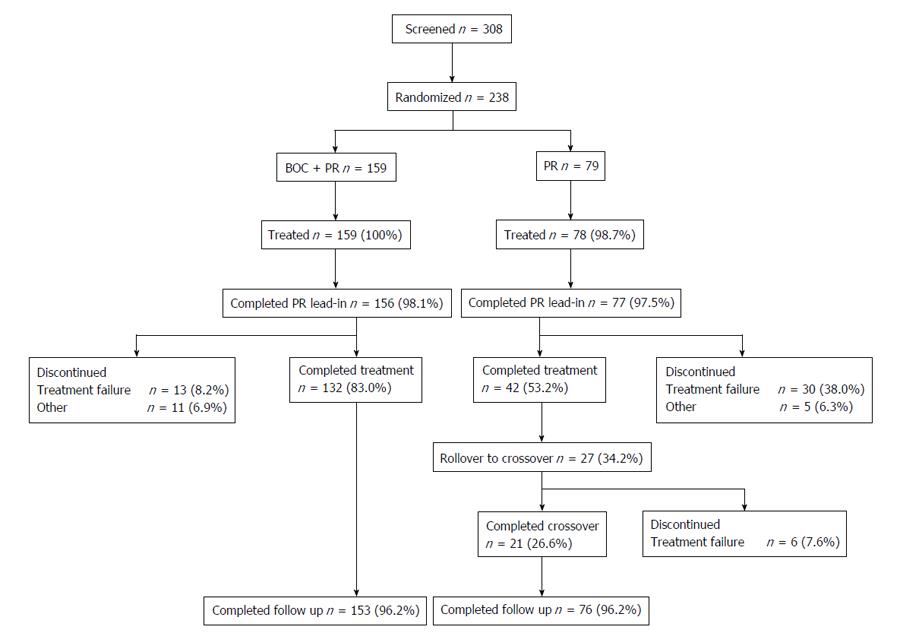
Figure 2 Patient disposition.
BOC: Boceprevir; PR: Peginterferon/ribavirin.
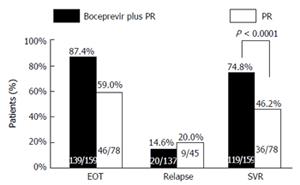
Figure 3 Analysis of sustained virologic response, end of treatment response, and relapse rate.
If a patient had missing data at and after the FW24 window and had undetectable HCV RNA at FW12, the patient was considered a sustained virologic responder. The P value was adjusted for stratification factors of IL28B genotype (CC vs non-CC) and previous treatment (TN vs TE), based on the Miettinen and Nurminen method. EOT was defined as the last dose date in treatment phase ± 14 d inclusive. The closest value to the last dose date was considered to be the EOT value. If there was no value within this window, the closest available value after this window was used. Relapse was defined as any patient who had detectable HCV RNA following end of all study therapy, after becoming undetectable and remaining so until end of treatment. EOT: End of treatment; HCV: Hepatitis C virus; PR: Peginterferon/ribavirin; SVR: Sustained virologic response; TE: Treatment experienced; TN: Treatment naive; IL28B: Interleukin-28B.
- Citation: Isakov V, Nikitin I, Chulanov V, Ogurtsov P, Lukyanova E, Long J, Wahl J, Helmond FA, The P08160 Trial Investigators. Boceprevir plus peginterferon/ribavirin for treatment of chronic hepatitis C in Russia. World J Hepatol 2016; 8(6): 331-339
- URL: https://www.wjgnet.com/1948-5182/full/v8/i6/331.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i6.331