Copyright
©The Author(s) 2016.
World J Hepatol. Dec 28, 2016; 8(36): 1623-1628
Published online Dec 28, 2016. doi: 10.4254/wjh.v8.i36.1623
Published online Dec 28, 2016. doi: 10.4254/wjh.v8.i36.1623
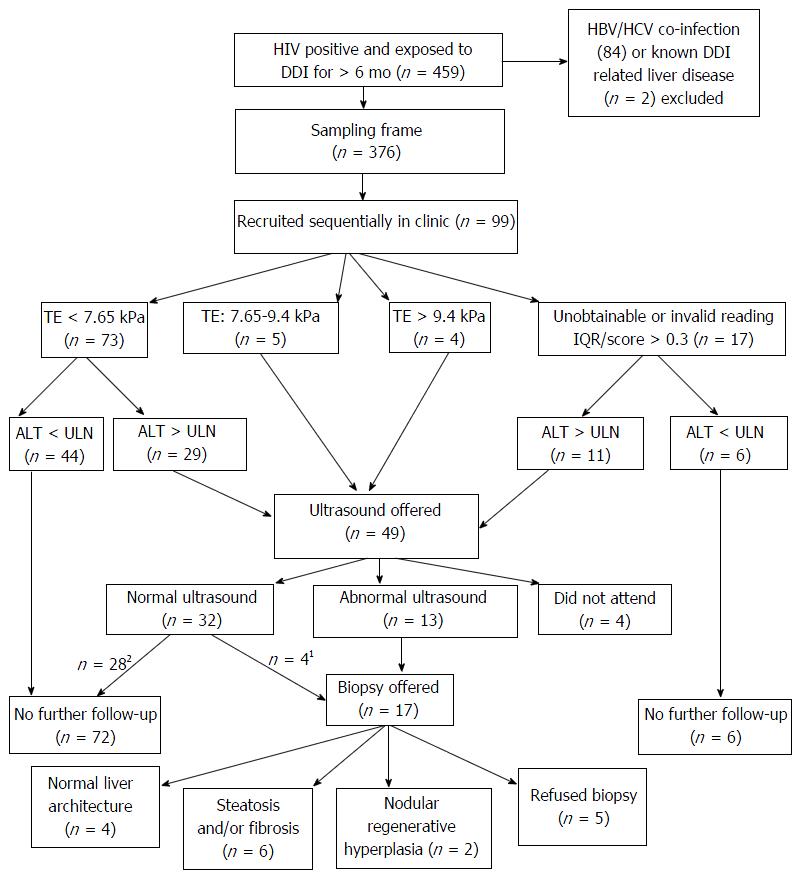
Figure 1 Screening algorithm to detect Didanosine related liver disease in human immunodeficiency virus positive patients previously exposed to Didanosine for > 6 mo.
1ALT > upper limit normal (ULN) or platelets < lower limit normal (LLN) or TE > 7.65 kPa; 2ALT < ULN and platelets > LLN and TE < 7.65 kPa. TE: Transient elastography; HIV: Human immunodeficiency virus; DDI: Didanosine; ALT: Alanine transaminase; HBV: Hepatitis B virus; HCV: Hepatitis C virus; IQR: Interquartile range.
- Citation: Logan S, Rodger A, Maynard-Smith L, O’Beirne J, Fernandez T, Ferro F, Smith C, Bhagani S. Prevalence of significant liver disease in human immunodeficiency virus-infected patients exposed to Didanosine: A cross sectional study. World J Hepatol 2016; 8(36): 1623-1628
- URL: https://www.wjgnet.com/1948-5182/full/v8/i36/1623.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i36.1623