Copyright
©The Author(s) 2016.
World J Hepatol. Nov 8, 2016; 8(31): 1318-1326
Published online Nov 8, 2016. doi: 10.4254/wjh.v8.i31.1318
Published online Nov 8, 2016. doi: 10.4254/wjh.v8.i31.1318
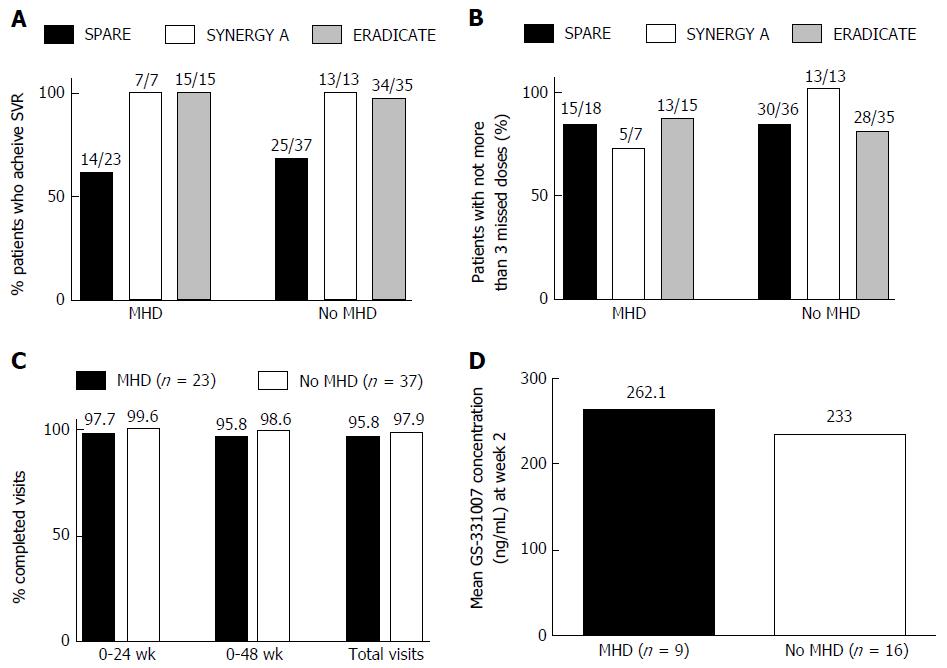
Figure 1 Sustained virologic response and measures of adherence among participants with and without mental health disease.
A: Sustained virologic response (SVR) achieved (ERADICATE, SYNERGY-A, SPARE). The comparisons of achieved SVR between groups within each study showed no significant differences for those with mental health disease (MHD) from those without MHD; B: Patients with not more than 3 missed doses (ERADICATE, SYNERGY-A, SPARE). The comparisons of adherence tracked by number of patients who had 3 or fewer total missed doses by pill count showed no significant differences for those with MHD from those without MHD in each study; C: Visit adherence (SPARE). Comparisons of total numbers of required visits completed showed no difference between those with MHD and those without during treatment (0-24 wk), through SVR24 (0-48 wk) and overall (P = 0.12); D: GS-331007 concentration at week 2 (SPARE). Comparisons of mean GS-331007 concentration showed no difference between those with MHD and those without at week 2 of treatment (P = 0.72).
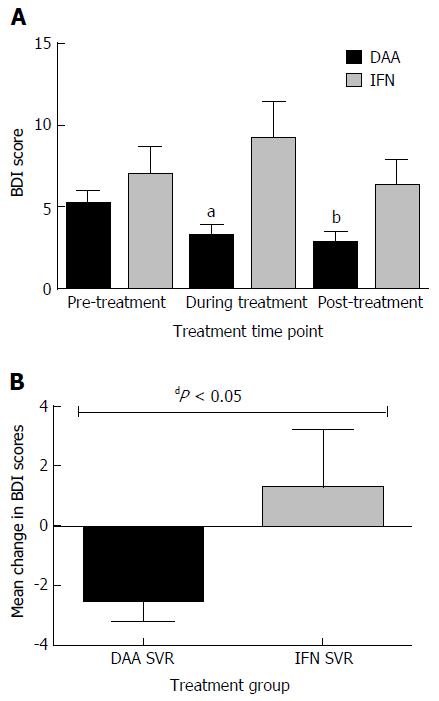
Figure 2 Beck’s Depression Inventory scores among participants with hepatitis C/human immunodeficiency virus coinfection and treated with ledipasvir-sofosbuvir and interferon-based therapy.
A: Mean BDI scores pre-, during, and post-treatment among all participants; Decrease in mean score from baseline to during treatment, aP = 0.0034, DAA vs IFN; Decrease in mean score from baseline to post-treatment, bP = 0.0012, DAA vs IFN; B: Change in mean BDI scores from baseline to post-treatment among participants who achieved SVR, dP = 0.0004, DAA vs IFN. SVR: Sustained virologic response; BDI: Beck’s Depression Inventory; DAA: Directly acting antiviral; IFN: Interferon.
- Citation: Tang LSY, Masur J, Sims Z, Nelson A, Osinusi A, Kohli A, Kattakuzhy S, Polis M, Kottilil S. Safe and effective sofosbuvir-based therapy in patients with mental health disease on hepatitis C virus treatment. World J Hepatol 2016; 8(31): 1318-1326
- URL: https://www.wjgnet.com/1948-5182/full/v8/i31/1318.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i31.1318