Copyright
©The Author(s) 2015.
World J Hepatol. Oct 18, 2015; 7(23): 2459-2469
Published online Oct 18, 2015. doi: 10.4254/wjh.v7.i23.2459
Published online Oct 18, 2015. doi: 10.4254/wjh.v7.i23.2459
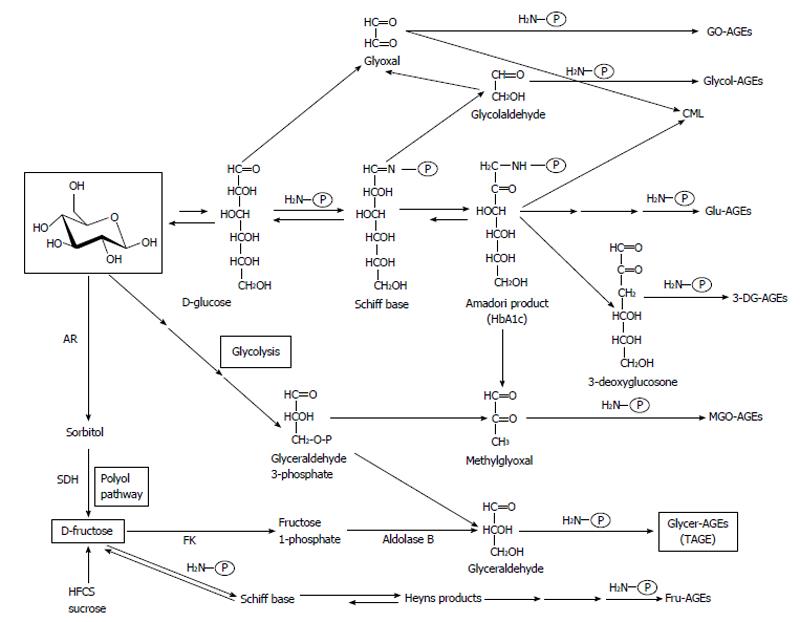
Figure 1 Alternative routes for the formation of advanced glycation end-products in vivo.
Reducing sugars, such as glucose, fructose, and glyceraldehyde are known to react non-enzymatically with the amino groups of proteins to form reversible Schiff bases and Amadori product/Heyns products. These early glycation products undergo further complex reactions such as rearrangement, dehydration, and condensation to become irreversibly cross-linked, heterogeneous fluorescent derivatives, termed advanced glycation end-products (AGEs). Glu-AGEs: Glucose-derived AGEs; Fru-AGEs: Fructose-derived AGEs; Glycer-AGEs: Glyceraldehyde-derived AGEs; Glycol-AGEs: Glycolaldehyde-derived AGEs; MGO-AGEs: Methylglyoxal-derived AGEs; GO-AGEs: Glyoxal-derived AGEs; 3-DG-AGEs: 3-deoxyglucosone-derived AGEs; CML: Nε-(carboxymethyl)lysine; P-NH2: Free amino residue of a protein; AR: Aldose reductase; SDH: Sorbitol dehydrogenase; FK: Fructokinase; HFCS: High-fructose corn syrup; HbA1c: Hemoglobin A1c; TAGE: Toxic advanced glycation end-products.
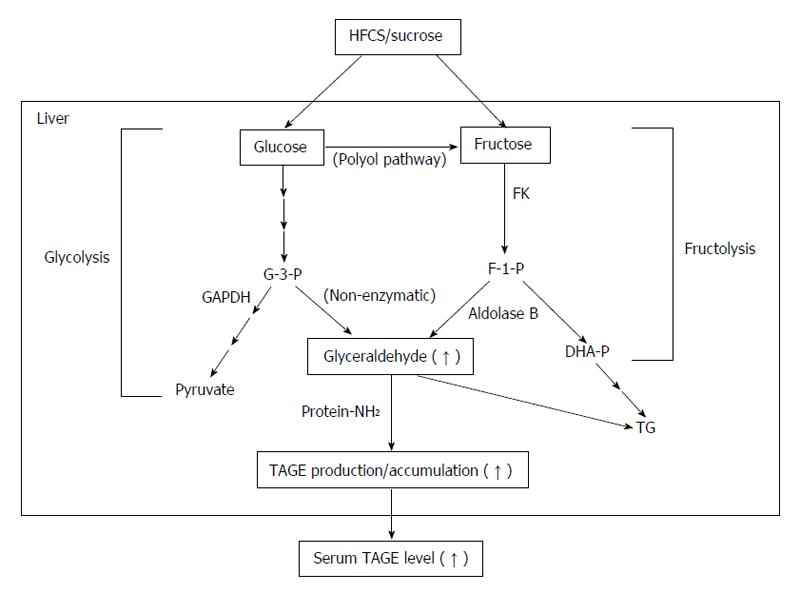
Figure 2 In vivo production routes of glycer-advanced glycation end-products (toxic advanced glycation end-products).
The chronic and excessive ingestion of sugar-sweetened beverages (HFCS/sucrose) increases the levels of the sugar metabolite, glyceraldehyde in the liver. The glycolytic intermediate glyceraldehyde-3-phosphate (G-3-P) is normally catabolized (glycolysis) by the enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH). G-3-P accumulates intracellularly with a decline in GAPDH activity. The metabolism of G-3-P then shifts to another route, resulting in an increase in the amount of glyceraldehyde, which promotes the formation of glycer-advanced glycation end-products (AGEs) (TAGE). Fructose from the daily diet and polyol pathway is phosphorylated to fructose-1-phosphate (F-1-P) by fructokinase and is then catabolized to glyceraldehyde and dihydroxyacetone phosphate by aldolase B (fructolysis). The newly synthesized glyceraldehyde is then transported or leaks passively across the plasma membrane. Glyceraldehyde promotes the formation of TAGE both intracellularly and extracellularly. DHA-P: Dihydroxyacetone-phosphate; FK: Fructokinase; HFCS: High-fructose corn syrup; TAGE: Toxic advanced glycation end-products; TG: Triglyceride; Protein-NH2: Free amino residue of a protein.
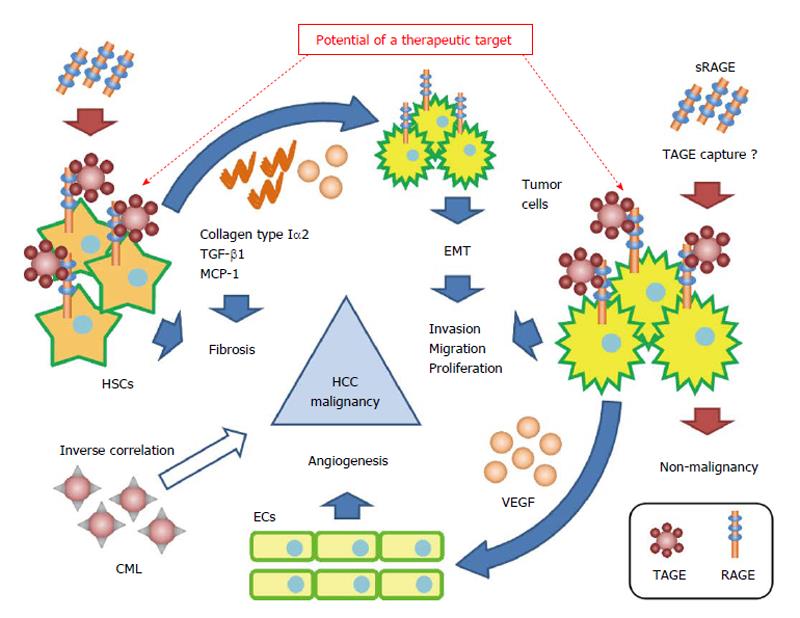
Figure 3 Proposed model for the contribution of the toxic advanced glycation end-products-receptor axis in nonalcoholic steatohepatitis-related hepatocellular carcinoma.
The interaction between TAGE and RAGE alters intracellular signaling in tumor cells and hepatic stellate cells, and induces angiogenesis, invasion, migration, proliferation, and fibrosis. This cooperation by the TAGE-RAGE axis may lead to the malignant progression of nonalcoholic steatohepatitis (NASH)-related hepatocellular carcinoma (HCC). CML and sRAGE inversely correlate with the risk of HCC, and sRAGE, which plays the role of a decoy receptor of RAGE, prevents the malignant progression of HCC. The TAGE-RAGE axis may become a treatment target in NASH and NASH-related HCC. CML: Nε-(carboxymethyl)lysine; ECs: Endothelial cells; EMT: Epithelial mesenchymal transition; HSCs: Hepatic stellate cells; MCP-1: Monocyte chemoattractant protein-1; RAGE: Receptor for advanced glycation end-products; sRAGE: Soluble receptor for advanced glycation end; TAGE: Toxic advanced glycation end-products; TGF-β1: Transforming growth factor-β1; VEGF: Vascular endothelial growth factor.
- Citation: Takino JI, Nagamine K, Hori T, Sakasai-Sakai A, Takeuchi M. Contribution of the toxic advanced glycation end-products-receptor axis in nonalcoholic steatohepatitis-related hepatocellular carcinoma. World J Hepatol 2015; 7(23): 2459-2469
- URL: https://www.wjgnet.com/1948-5182/full/v7/i23/2459.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i23.2459