Copyright
©2014 Baishideng Publishing Group Co.
World J Hepatol. Apr 27, 2014; 6(4): 230-242
Published online Apr 27, 2014. doi: 10.4254/wjh.v6.i4.230
Published online Apr 27, 2014. doi: 10.4254/wjh.v6.i4.230
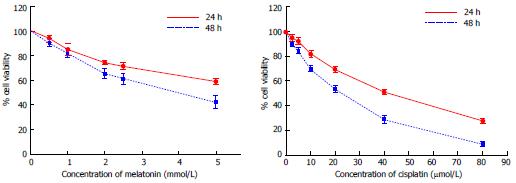
Figure 1 Effect of melatonin and cisplatin on cell viability analyzed using an MTT assay.
Hepatocellular carcinoma (HepG2) cells were treated with various concentrations of melatonin and cisplatin for 24 h and 48 h. Both melatonin and cisplatin reduced the percent viability of HepG2 cells in a time and concentration-dependent manner (P < 0.001). The experiments were performed in triplicate, and the results are presented as the means ± SE.
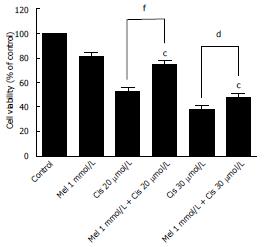
Figure 2 Effect of the combined treatment of melatonin and cisplatin on cell viability.
Hepatocellular carcinoma cells were treated with melatonin (1 mmol/L) and/or cisplatin (20 and 30 μmol/L) for 48 h and then analyzed by an MTT assay. Melatonin reduced the cisplatin cytotoxic effect at 20 μmol/L cisplatin. The results are presented as the mean ± SE. (cP < 0.001 compared with the control group, dP < 0.05, fP < 0.001 compared with cisplatin-treated group).
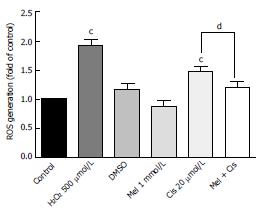
Figure 3 Melatonin reduced cisplatin-induced intracellular reactive oxygen species levels.
Hepatocellular carcinoma cells were treated with melatonin (1 mmol/L) and/or cisplatin (20 μmol/L) for 24 h. The results are presented as the means ± SE. (cP < 0.001 compared with the control group, dP < 0.05 compared with the cisplatin-treated group).
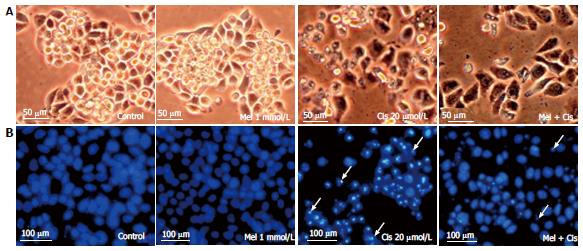
Figure 4 Histological changes.
Hepatocellular carcinoma cells were treated with melatonin (1 mmol/L) and/or cisplatin (20 μmol/L) for 48 h. The cells were stained with DAPI for fragmented DNA (apoptotic bodies) and observed under an inverted microscope. A: Morphological changes showing vacuoles in the cytoplasm of cisplatin-treated cells (scale bar = 50 μm); B: DAPI staining showing nuclear condensation and apoptotic bodies (white arrows) in cisplatin-treated cells. The combined treatment showed less apoptotic effects (scale bar = 100 μm).
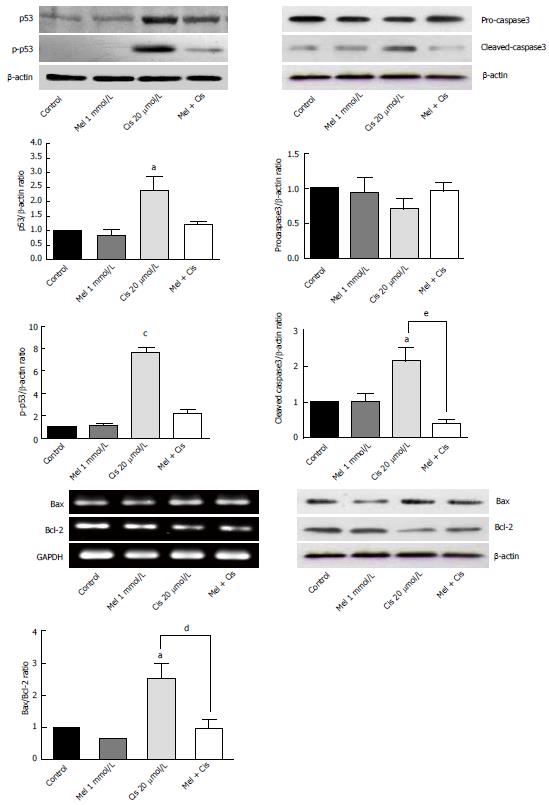
Figure 5 Reverse transcription-polymerase chain reaction and Western blot analyses of apoptosis regulators in treated hepatocellular carcinoma cells.
Melatonin had minor effects on all apoptosis markers, whereas cisplatin significantly increased apoptosis via the activation of p53 and caspase3 and the Bax/Bcl-2 ratio. Melatonin reduced cisplatin effects in the combined treatment. (aP < 0.05, cP < 0.001 compared with the control group, dP < 0.05, eP < 0.01 compared with the cisplatin-treated group).
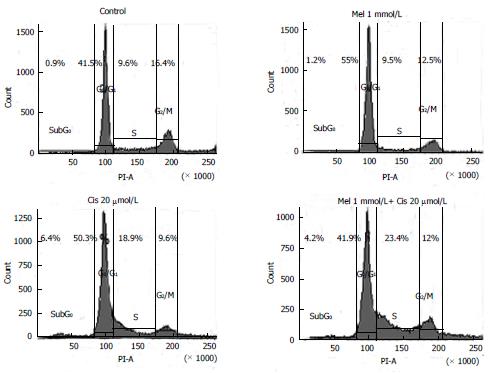
Figure 6 Effects of melatonin and cisplatin on the cell cycle, as evaluated using flow cytometric analysis.
Hepatocellular carcinoma (HepG2) cells were treated with melatonin (1 mmol/L) and/or cisplatin (20 μmol/L) for 24 h. Melatonin induced DNA accumulation in the HepG2 cells at the G0/G1 phase, whereas cisplatin affected cell cycle arrest at the sub-G0, G0/G1, and S phases. In the combined treatment, melatonin decreased the cisplatin effect on cell cycle arrest at the sub-G0 through G0/G1 phases but significantly increased S phase cell cycle arrest.
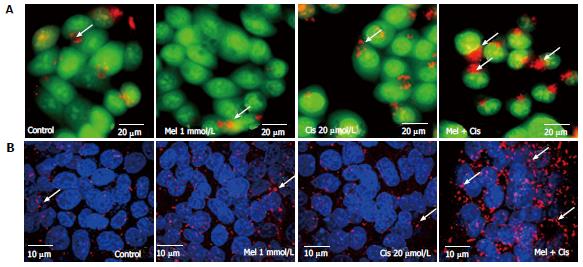
Figure 7 Autophagic detection by acridine orange staining and immunofluorescence.
Hepatocellular carcinoma (HepG2) cells were treated with 1 mmol/L melatonin and/or 20 µmol/L cisplatin for 24 h and evaluated for autophagy formation. The experiments were performed in triplicate, and representative micrographs are shown. A: Acridine orange staining showed lysosomal (red or orange) staining in the cells of all treatments. The increased acidic lysosomes in the combination treatment suggests potential lysosomal activation (scale bar = 20 μm); B: Confocal immunofluorescence micrographs of representative treated-HepG2 cells immunolabeled with the anti-LC3 antibody (scale bar = 10 μm). Melatonin and cisplatin treatment alone induced some fluorescent immunoreactivity in the cytoplasm, but the combined treatment induced intense immunoreactivity.
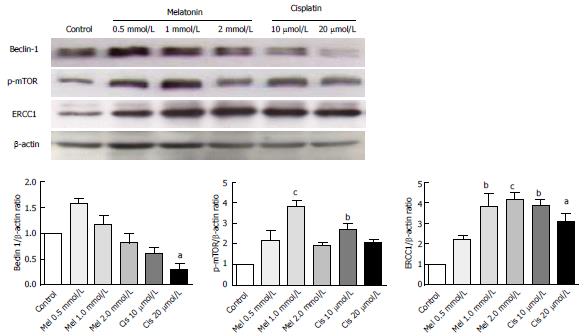
Figure 8 Analysis of autophagy and DNA repair-related proteins.
Hepatocellular carcinoma cells were treated with various concentrations of melatonin and cisplatin. At the selected concentrations, 1 mmol/L melatonin and 20 μmol/L cisplatin have a similar effect by significantly increasing p-mTOR and ERCC 1 from the basal levels. However, the effect on Beclin-1 was the opposite, with melatonin increasing Beclin-1 and cisplatin decreasing Beclin-1 from the basal level. The results are presented as the mean ± SE. (aP < 0.05, bP < 0.01, cP < 0.001 compared with the control group). ERCC1: Excision repair cross complementary-1.
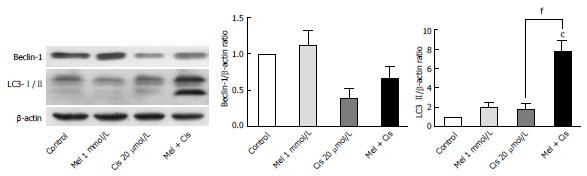
Figure 9 Alteration of autophagy-forming proteins.
Hepatocellular carcinoma cells were treated with melatonin and/or cisplatin for 24 h. Melatonin slightly increased Beclin-1 and LC3-II, whereas cisplatin significantly decreased Beclin-1 and slightly increased LC3-II. Melatonin inhibited the cisplatin effect on Beclin-1 but increased LC3-II in the combined treatment. (cP < 0.001 compared with the control group, fP < 0.001 compared with the cisplatin-treated group).
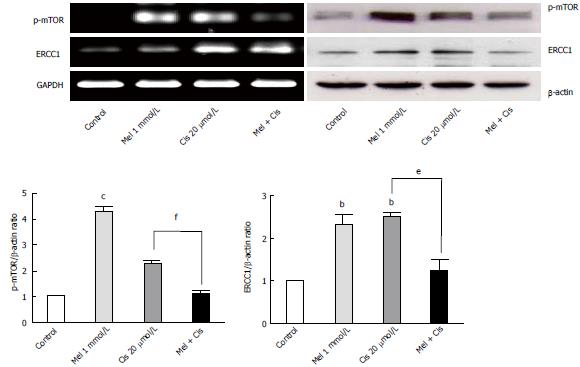
Figure 10 RT-PCR and Western blot analyses of autophagy regulators.
Both melatonin and cisplatin increased p-mTOR and ERCC 1 levels in the HepG2 cells, but the combined treatment resulted in the suppression of both proteins. (bP < 0.01, cP < 0.001 compared with the control group, eP < 0.01, fP < 0.001 compared with the cisplatin-treated group). ERCC1: Excision repair cross complementary-1.
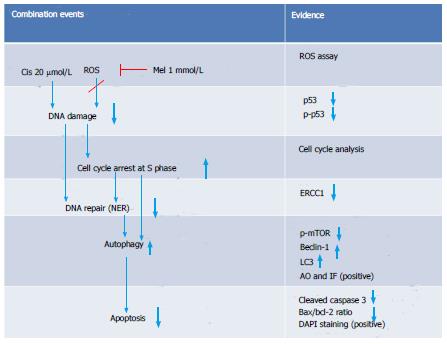
Figure 11 Schematic overview of the major findings in the combination group.
-
Citation: Bennukul K, Numkliang S, Leardkamolkarn V. Melatonin attenuates cisplatin-induced HepG2 cell death
via the regulation of mTOR and ERCC1 expressions. World J Hepatol 2014; 6(4): 230-242 - URL: https://www.wjgnet.com/1948-5182/full/v6/i4/230.htm
- DOI: https://dx.doi.org/10.4254/wjh.v6.i4.230