Copyright
©The Author(s) 2025.
World J Hepatol. Jul 27, 2025; 17(7): 106810
Published online Jul 27, 2025. doi: 10.4254/wjh.v17.i7.106810
Published online Jul 27, 2025. doi: 10.4254/wjh.v17.i7.106810
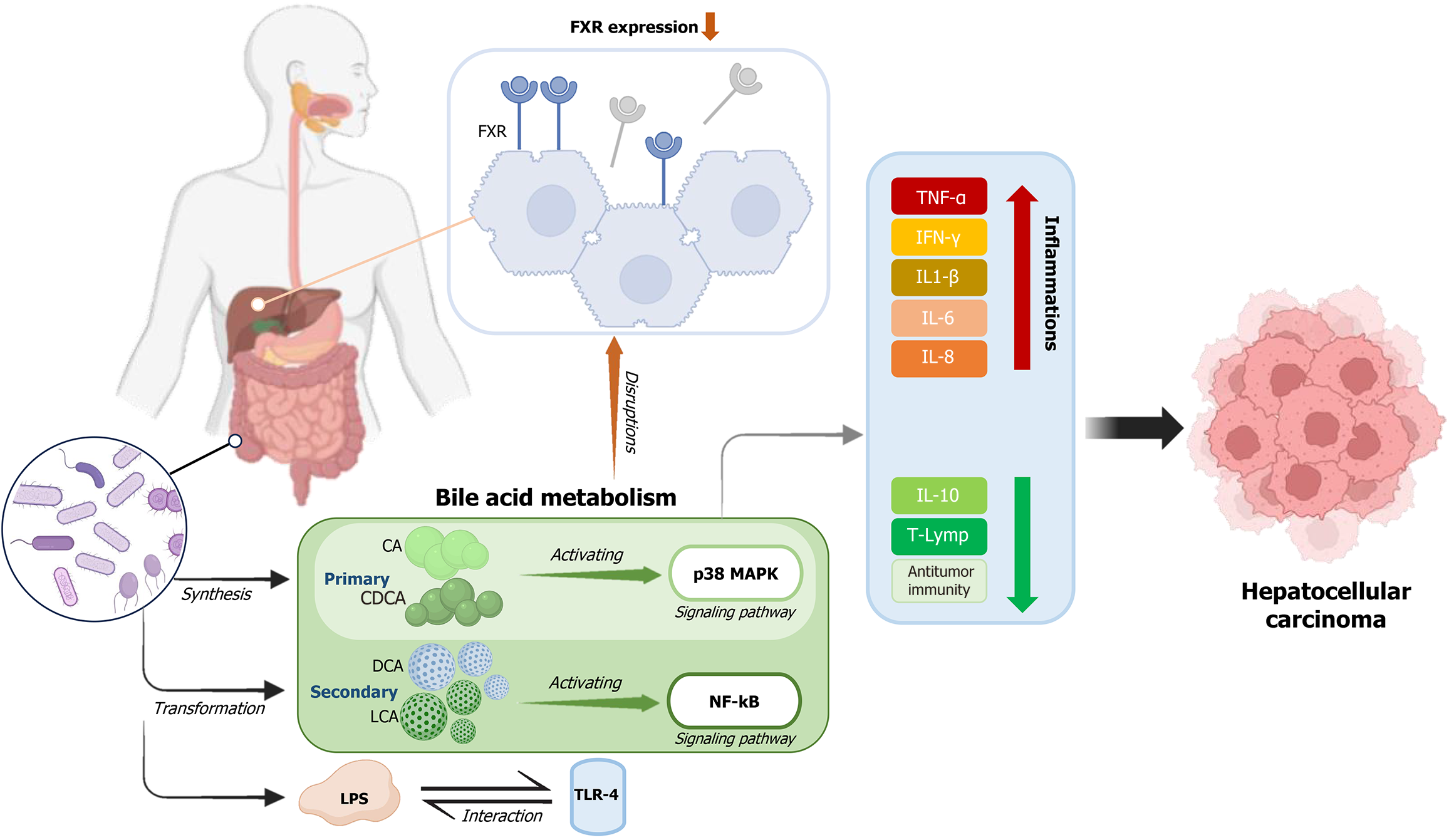
Figure 1 Gut-liver axis.
The gut microbiota participates in various stages of bile acid metabolism, producing primary bile acids like cholic acid (CA) and chenodeoxycholic acid (CDCA), which are further transformed into secondary bile acids such as deoxycholic acid (DCA) and lithocholic acid. The buildup of hydrophobic bile acids, particularly CA and CDCA, can trigger apoptosis in hepatocytes by activating the p38 mitogen-activated protein kinase signaling pathway, further contributing to chronic inflammation. The farnesoid X receptor (FXR) is essential in safeguarding against liver cancer development. Pathological conditions, such as cholestasis, can lower FXR expression, intensifying the inflammatory response. Additionally, less-immunogenic lipopolysaccharide, produced by gut microbes, interact with toll-like receptor 4 on hepatic stellate cells (HSCs). This interaction activates HSCs, promoting liver fibrosis and facilitating the advancement of hepatocellular carcinoma. DCA is known to cause DNA damage by activating the nuclear factor kappa B signaling pathway, leading to the release of inflammatory cytokines, including interleukin-1β, interleukin-6, tumor necrosis factor-alpha, interferon-gamma, interleukin-8, and reactive oxygen species. These processes exacerbate liver damage and contribute to the progression of Hepatocellular carcinoma. CA: Cholic acid; CDCA: Chenodeoxycholic acid; DCA: Deoxycholic acid; FXR: Farnesoid X receptor; TNF-α: Tumor necrosis factor-alpha; IL: Interleukin; IFN-γ: Interferon-gamma.
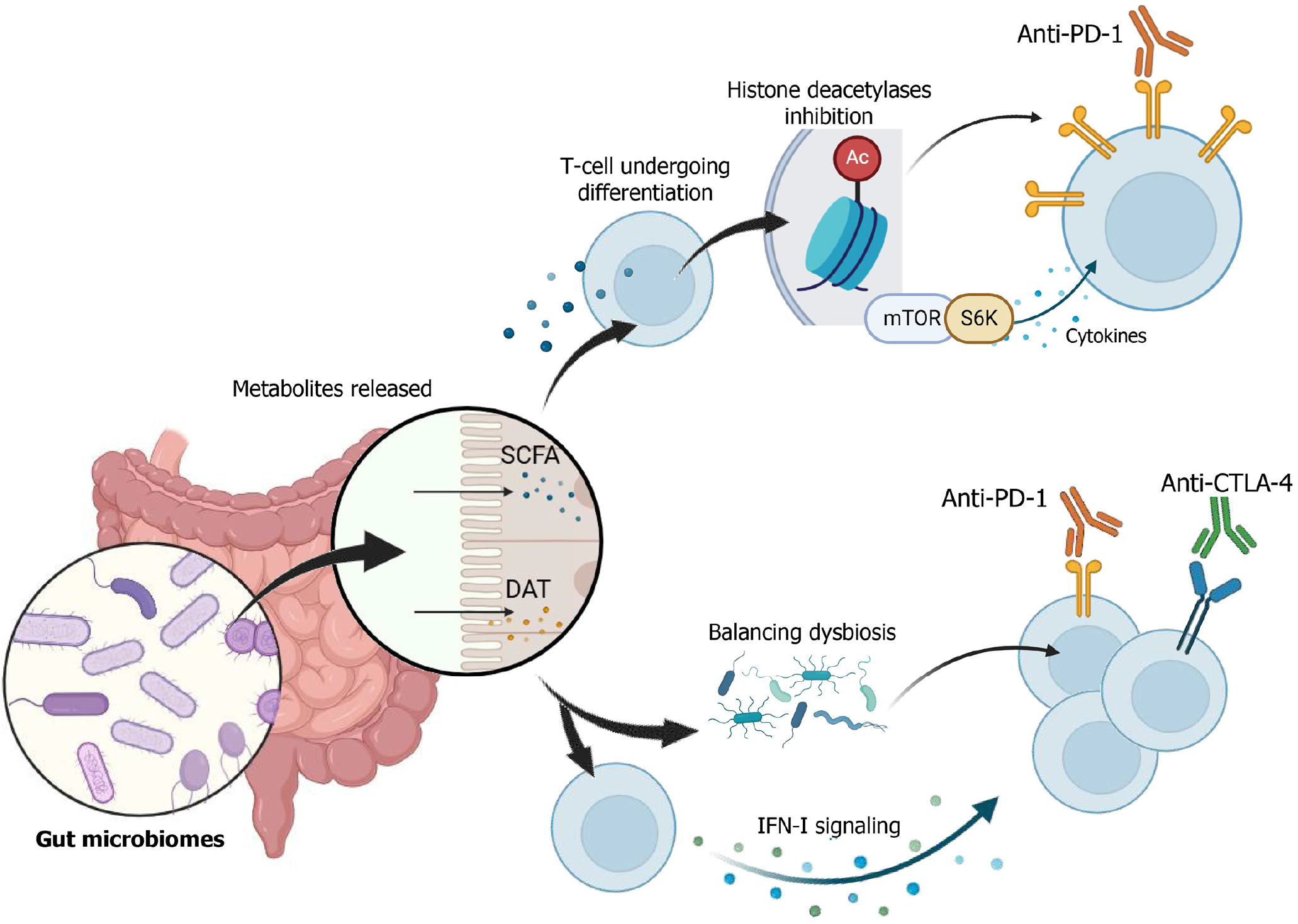
Figure 2 Mechanism of action of gut microbiomes and immune checkpoint inhibitors.
Gut microbiomes will release metabolites, such as short-chain fatty acids (SCFA) and desamino tyrosine (DAT). SCFA works by entering T cells and inhibiting histone deacetylases (HDAC). Inhibition of HDAC can affect the mechanistic target of rapamycin-ribosomal S6 kinase pathway, which will express cytokines. It leads to the upregulation of programmed cell death 1 ligands and will enhance immunotherapy response. Furthermore, DAT expression may alter gut microbiome composition and balance dysbiosis. DAT also promotes type I interferon signaling, which will promote cytotoxic T-cell and cancer apoptosis, which eventually enhance immune checkpoint inhibitor effectiveness. SCFA: Short-chain fatty acids; DAT: Desamino tyrosine; PD-1: Programmed cell death 1; CTLA-4: Cytotoxic T-lymphocyte-associated protein 4.
- Citation: Pamungkas KMN, Lesmana Dewi PIS, Alamsyah AZ, Dewi NLPY, Dewi NNGK, Mariadi IK, Sindhughosa DA. Microbiome dysbiosis and immune checkpoint inhibitors: Dual targets in Hepatocellular carcinoma management. World J Hepatol 2025; 17(7): 106810
- URL: https://www.wjgnet.com/1948-5182/full/v17/i7/106810.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i7.106810