Copyright
©The Author(s) 2022.
World J Hepatol. Jun 27, 2022; 14(6): 1248-1257
Published online Jun 27, 2022. doi: 10.4254/wjh.v14.i6.1248
Published online Jun 27, 2022. doi: 10.4254/wjh.v14.i6.1248
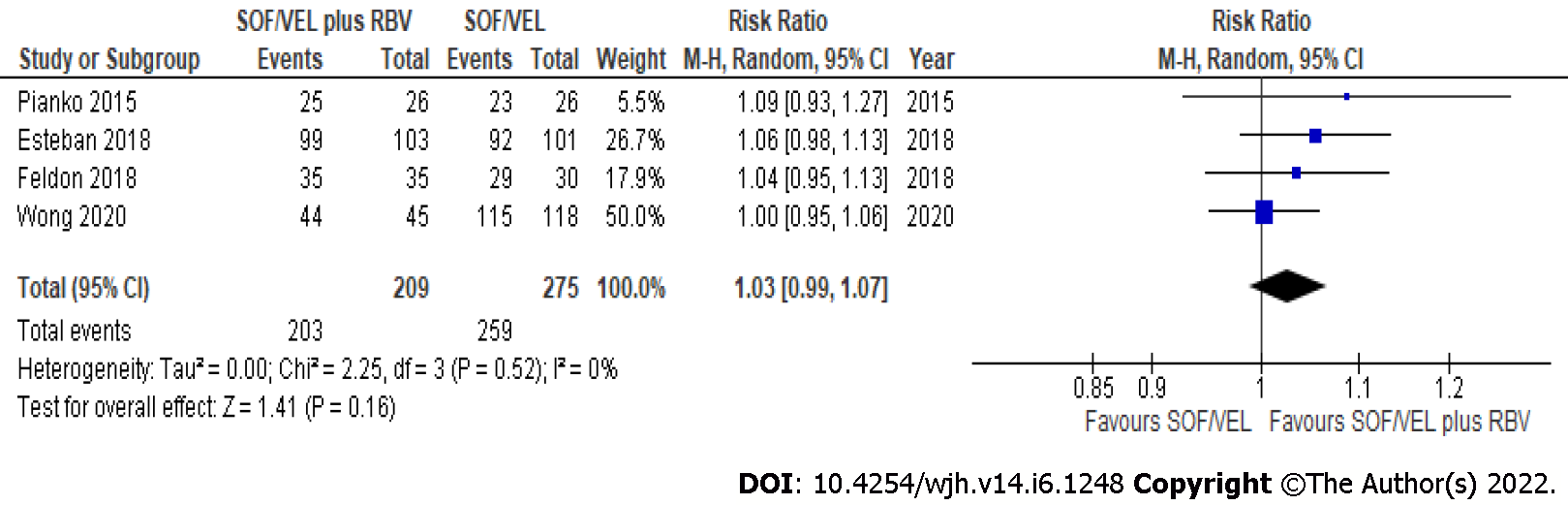
Figure 1 Sustained virological response by intention-to-treat analysis from sofosbuvir/velpatasvir with or without ribavirin.
SOF/VEL: Sofosbuvir/velpatasvir; RBV: Ribavirin.
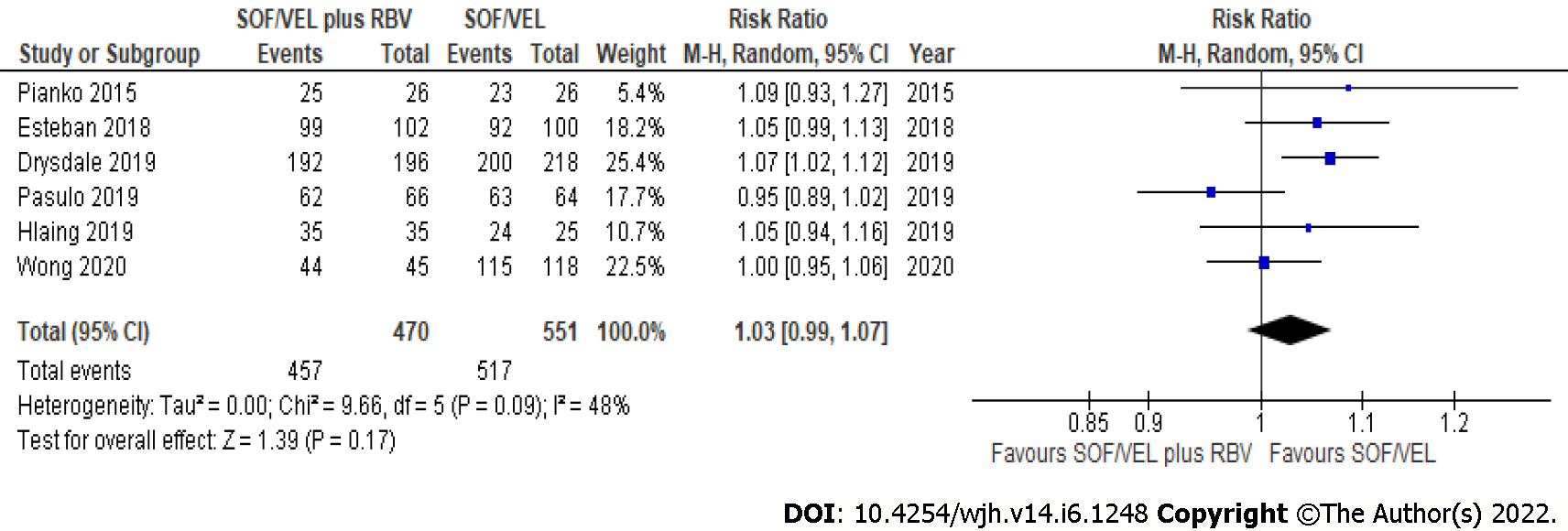
Figure 2 Sustained virological response by per-protocol analysis from sofosbuvir/velpatasvir with or without ribavirin.
SOF/VEL: Sofosbuvir/velpatasvir; RBV: Ribavirin.
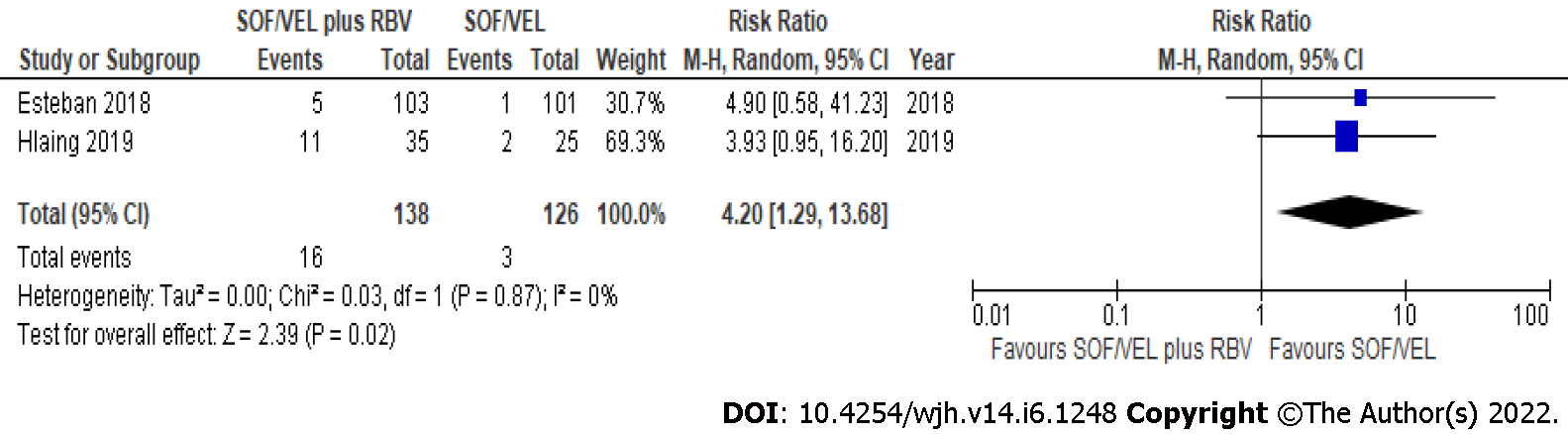
Figure 3 Severe adverse events from sofosbuvir/velpatasvir with or without ribavirin.
SOF/VEL: Sofosbuvir/velpatasvir; RBV: Ribavirin.
- Citation: Loo JH, Xu WXF, Low JT, Tay WX, Ang LS, Tam YC, Thurairajah PH, Kumar R, Wong YJ. Efficacy and safety of sofosbuvir/velpatasvir with or without ribavirin in hepatitis C genotype 3 compensated cirrhosis: A meta-analysis. World J Hepatol 2022; 14(6): 1248-1257
- URL: https://www.wjgnet.com/1948-5182/full/v14/i6/1248.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i6.1248