Copyright
©The Author(s) 2021.
World J Hepatol. Mar 27, 2021; 13(3): 343-361
Published online Mar 27, 2021. doi: 10.4254/wjh.v13.i3.343
Published online Mar 27, 2021. doi: 10.4254/wjh.v13.i3.343
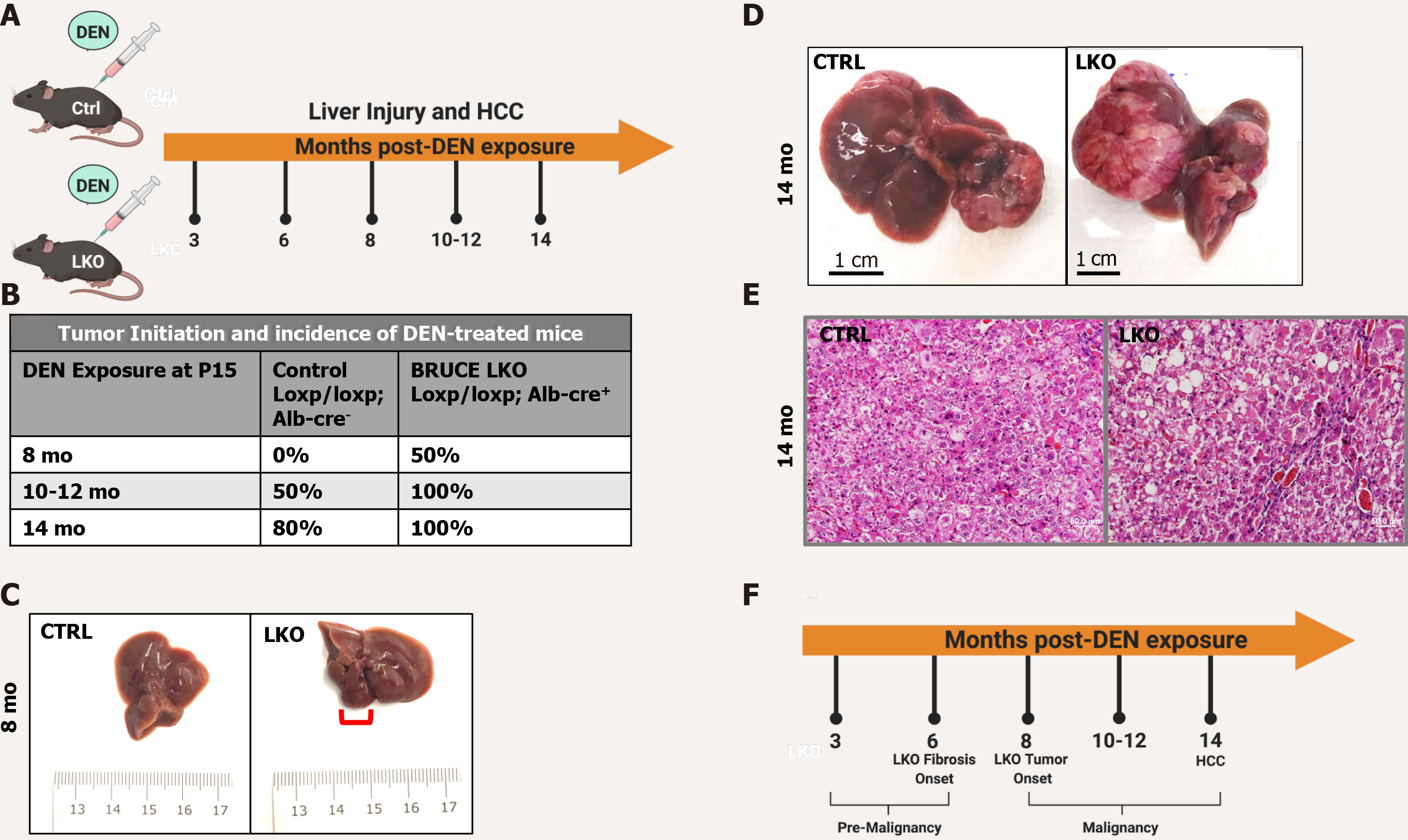
Figure 1 Diethylnitrosamine-induced hepatic malignancy leads to earlier tumor initiation and an exacerbated patient hepatocellular carcinoma-like phenotype in BRUCE LKO mice.
A: Diethylnitrosamine (DEN) model. Control and BRUCE LKO mice were treated with DEN over a time course schedule as indicated; B: Tumor onset in LKO mice happened after 8 mo post-DEN exposure, while tumor onset did not begin in control mice until 10-12 mo; C: Tumor in LKO mouse traced in red; D: After 14 mo of DEN exposure, control and LKO mice develop hepatocellular carcinoma; however, the LKO mice have a more exacerbated phenotype; E: Hematoxylin and Eosin staining reveals a trabecular histologic feature in LKO but not control; F: Timeline of key events of DEN-induced hepatic malignancy model. DEN: Diethylnitrosamine; LKO: Liver-specific knockout; HCC: Hepatocellular carcinoma; CTRL: Control; BRUCE: BIR repeat-containing ubiquitin conjugating enzyme.
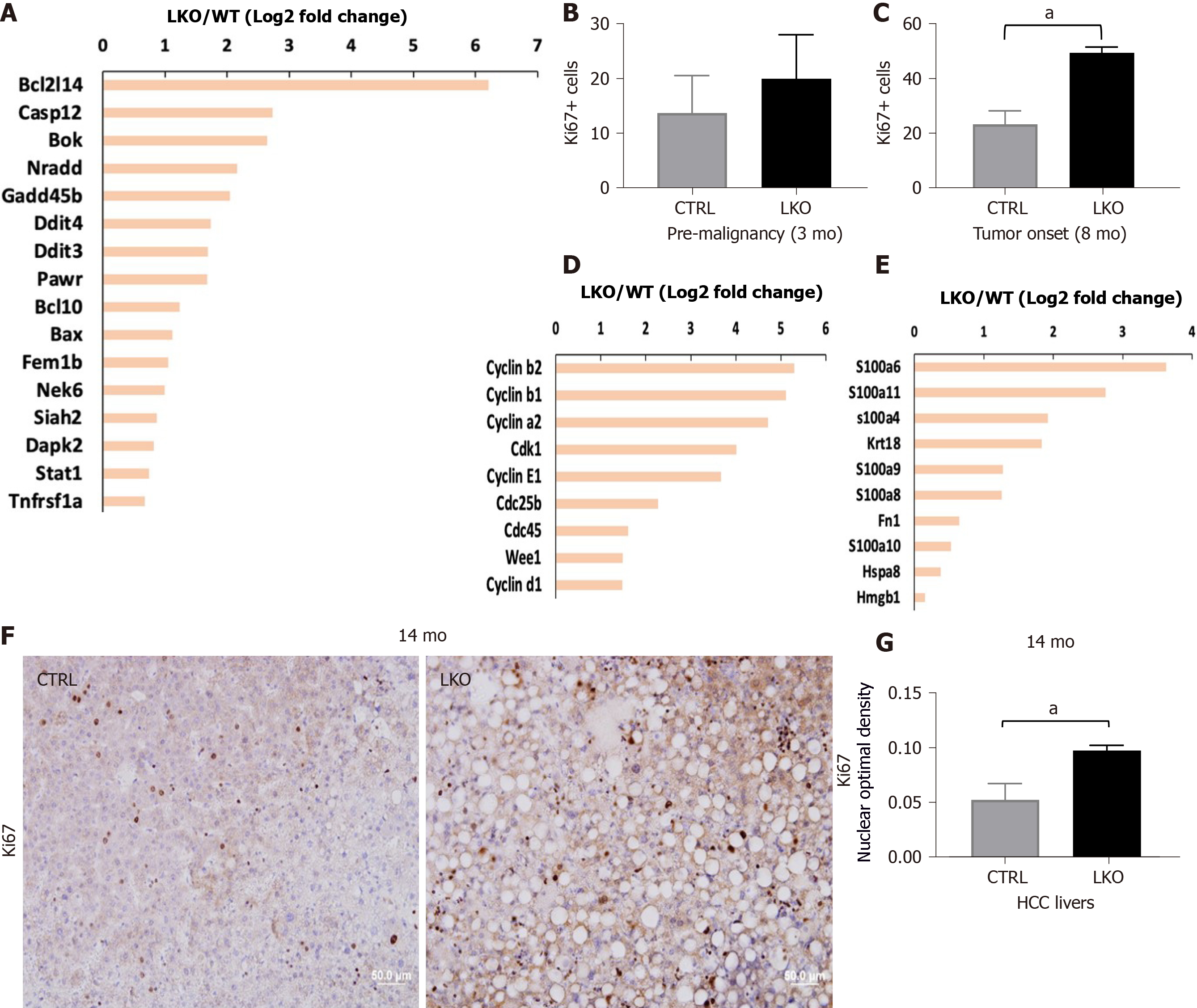
Figure 2 BRUCE deficiency increases diethylnitrosamine-induced liver injury and hepatic proliferation.
A: Apoptotic gene expression is increased in liver-specific BRUCE KO livers at the time of tumor onset; B: Hepatic proliferation was measured by immunohistochemistry staining of the livers against Ki67. Livers exposed to diethylnitrosamine for 3 mo have an increase of Ki67+ cells; C: At the time of tumor onset in LKO livers there is an increase of Ki67+ positive cells; D: At the time of tumor onset, RNA-seq analysis reveals an increase of known cell cycle markers; E: Damage associated molecular patterns in the LKO livers; F and G: Ki67 staining by immunohistochemistry in 14 mo hepatocellular carcinoma livers, as well as quantification, show an increase of proliferation in LKO livers. aP< 0.05. HCC: Hepatocellular carcinoma; LKO: Liver-specific knockout; CTRL: Control.
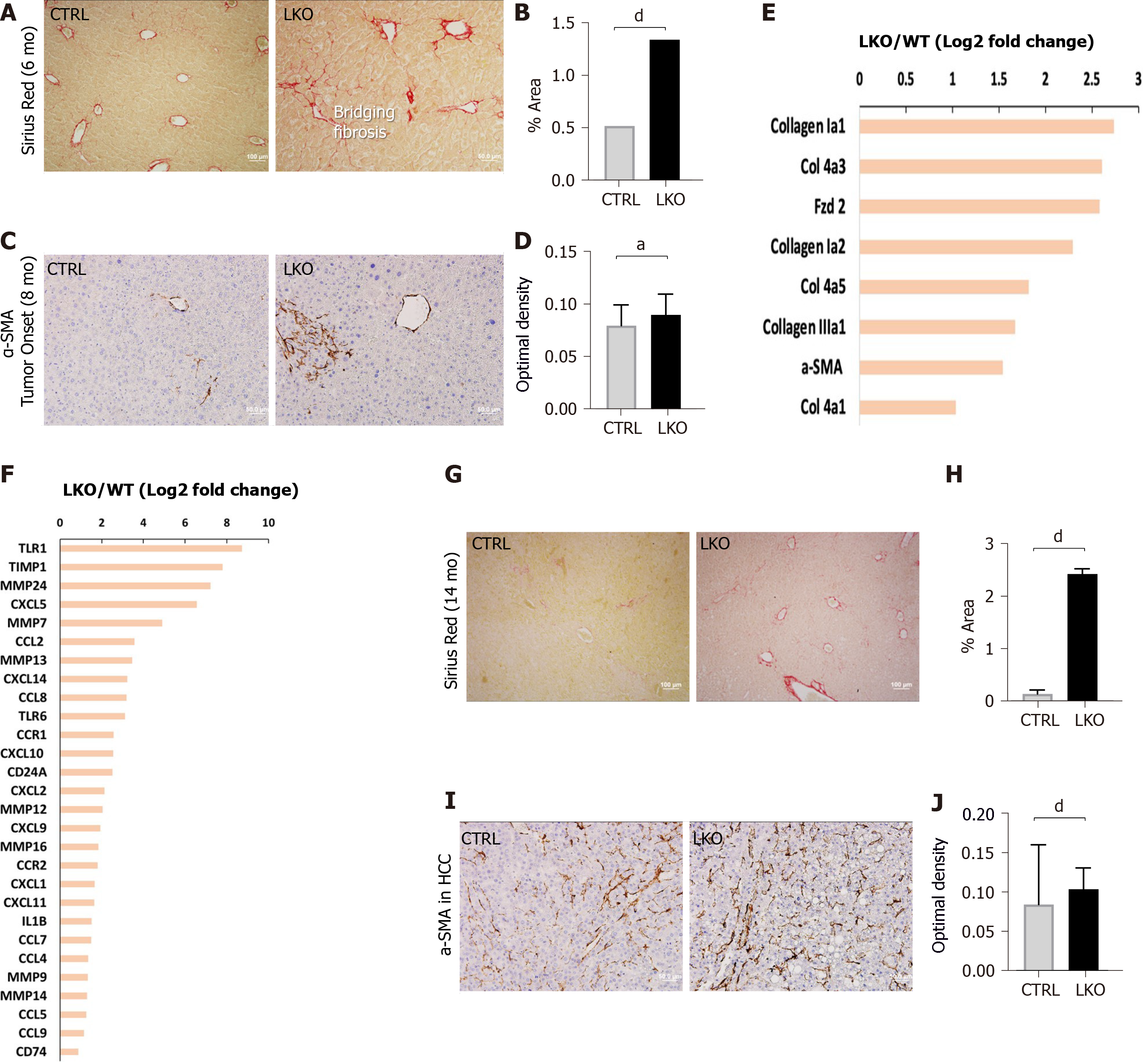
Figure 3 BRUCE deficiency accelerates and increases diethylnitrosamine-induced fibrosis.
A and B: Sirius red staining of control and liver-specific BRUCE KO livers 6 mo post-diethylnitrosamine (DEN) exposure show an increase of Sirius red staining in the LKO livers, verified by quantification to the right; C and D: α-smooth muscle actin (SMA) immunohistochemistry at LKO tumor onset show an increase in α-SMA in LKO livers which is quantified in; E and F: RNA-seq analysis at the time of LKO tumor onset reveal that LKO livers demonstrate key patterns of human hepatic fibrosis, such as increased collagens and increased α-SMA as well as increased inflammation-related markers, such as CCL2; G and H: Sirius red staining of 14 mo post-DEN exposed HCC livers reveal an increase of collagen deposition, which was quantified; I and J: α-SMA immunohistochemistry of HCC livers, including quantification demonstrate an increase of activated hepatic stellate cells in 14 mo DEN-exposed LKO livers. aP < 0.05; dP < 0.001. HCC: Hepatocellular carcinoma; LKO: Liver-specific knockout; α-SMA: α-smooth muscle actin; CTRL: Control.
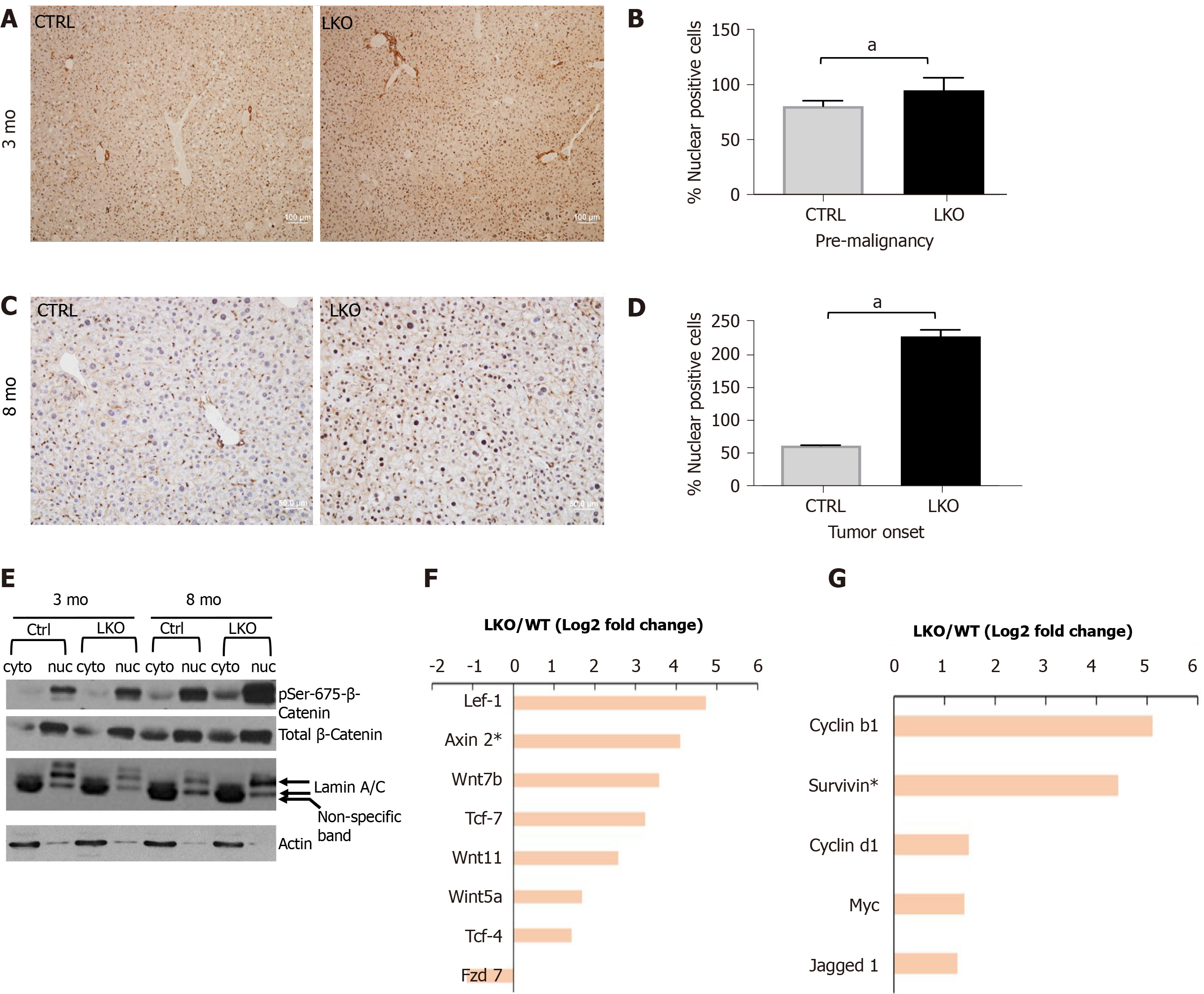
Figure 4 BRUCE deficiency promotes β-catenin activation in mice.
A and B: After three months post-diethylnitrosamine (DEN) exposure, there is an increase in nuclear β-catenin staining by immunohistochemistry in the BRUCE KO livers. See quantification to the right; C and D: At tumor onset in LKO livers, nuclear β-catenin, shown by immunohistochemical staining, is increased in the LKO livers and quantified to the right; E: Phosphorylation of β-catenin at Ser-675 is increased in both the cytoplasmic and nuclear fractions of the LKO livers both pre-malignancy (3 mo post-DEN) and at tumor onset (8 mo post-DEN); F and G: At the time of tumor onset in LKO livers (8 mo post-DEN exposure), RNA sequencing analysis determined an increase in several canonical Wnt/β-catenin pathway members and target genes. aP< 0.05. LKO: Liver-specific knockout; CTRL: Control.
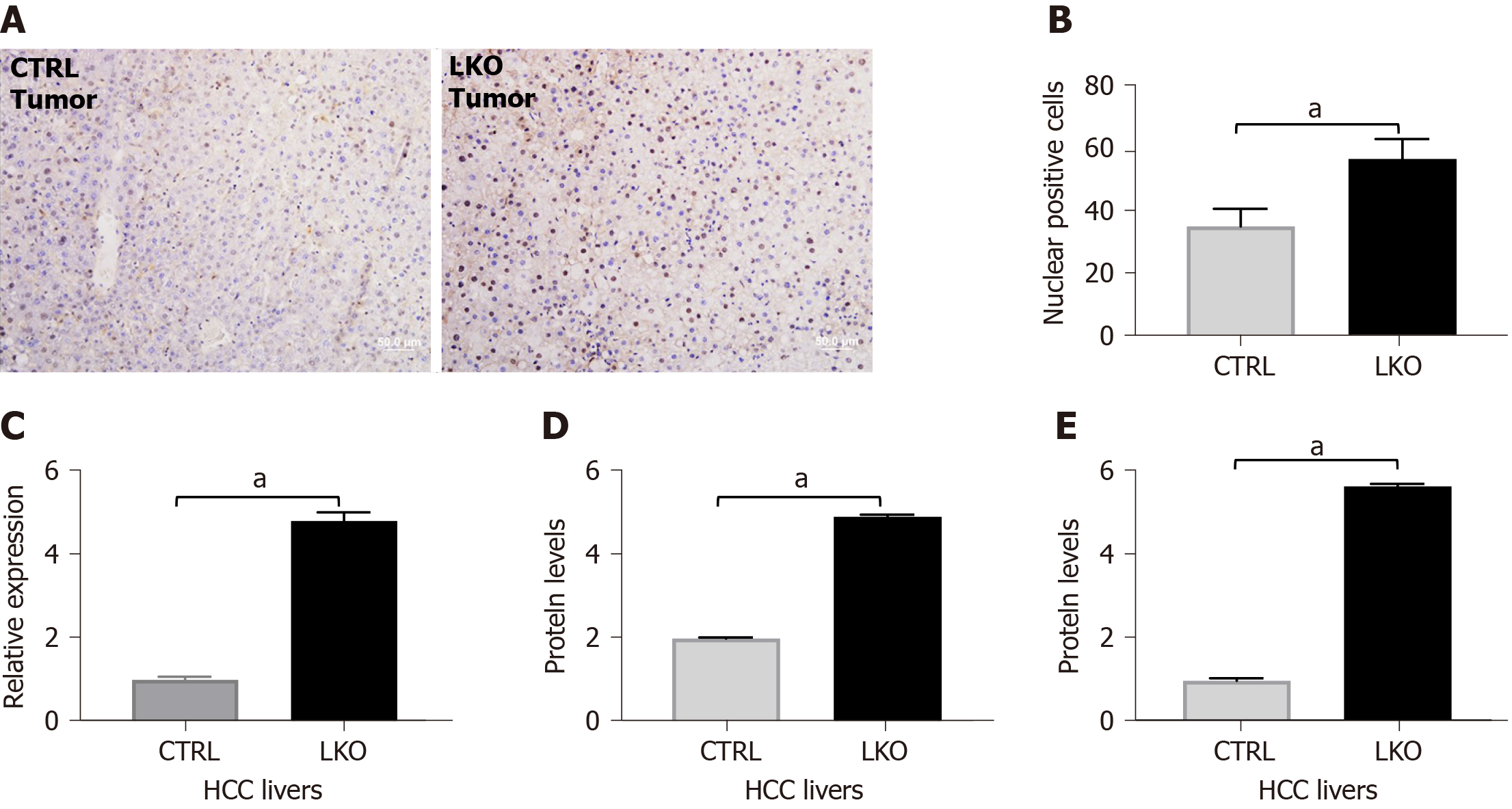
Figure 5 BRUCE deficiency increases nuclear β-catenin and activity in hepatocellular carcinoma livers.
A and B: Immunohistochemistry of β-catenin in liver tumors after 14 mo of DEN-exposure which is quantified in; C: RT-PCR analysis of β-catenin in liver tumors after 14 mo post-DEN revealed an increase in mRNA levels in BRUCE knockout livers compared to control; D and E: Graphical representation of western blot analysis of β-catenin in liver tumors after 14 mo of DEN-exposure and cyclin D1, a downstream target of β-catenin. aP < 0.05. HCC: Hepatocellular carcinoma; LKO: Liver-specific knockout; CTRL: Control.
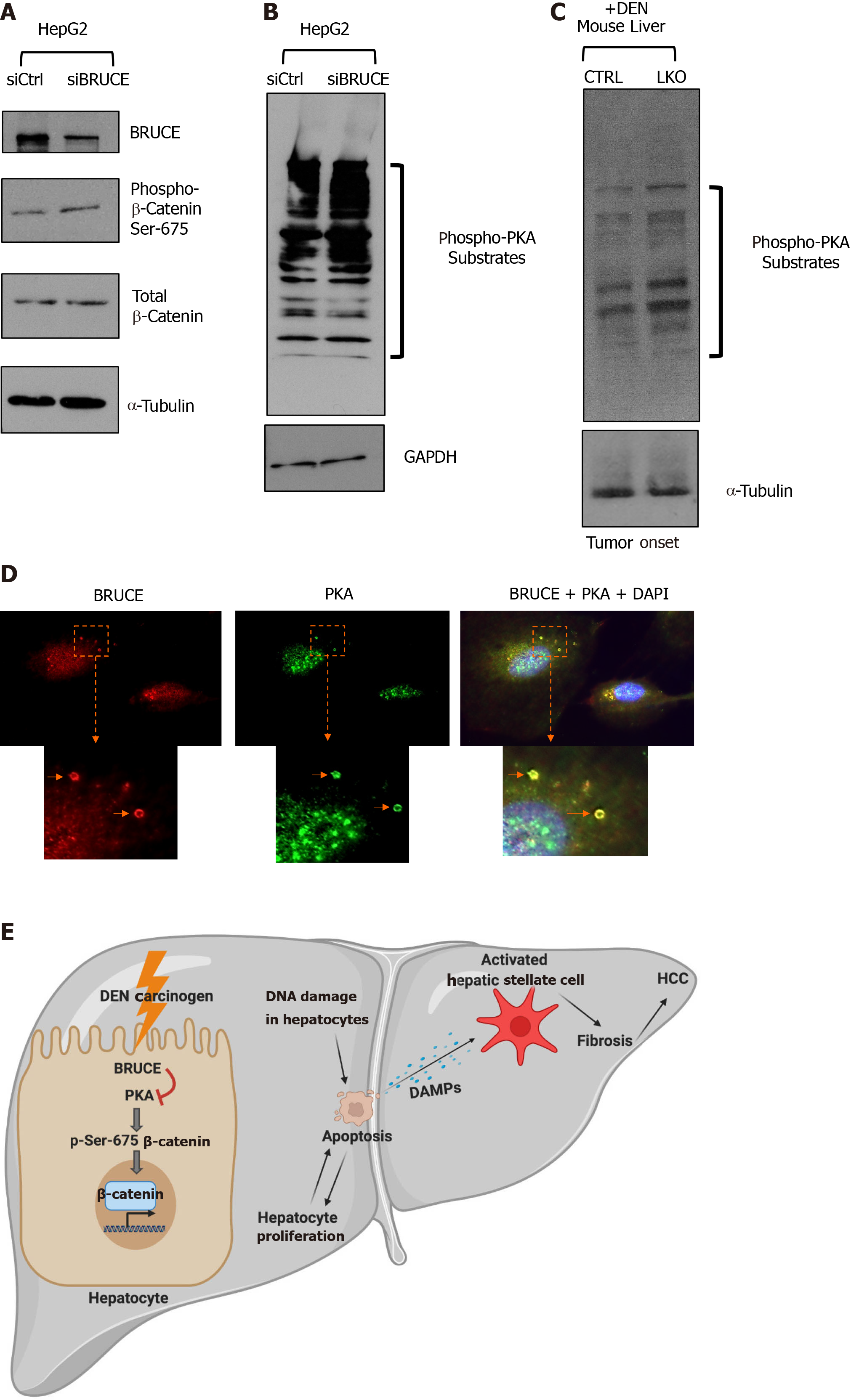
Figure 6 BRUCE-dependent regulation of β-catenin links to protein kinase activity.
A: Whole cell lysates of HepG2 cells transfected with either an siCtrl or siBRUCE were blotted for BRUCE, phospho- and total-β-catenin, as well as a tubulin control; B: Lysates described in (A) were blotted for phospho-protein kinase A (PKA) substrates to measure PKA activity, as well as a glyceraldehyde-3-phosphate dehydrogenase control; C: Western blot analysis of mouse liver tissue lysates from control and liver-specific BRUCE knockout (LKO) exposed to diethylnitrosamine (DEN) for phospho-PKA substrates showing an increase in PKA activity in LKO livers at the time of tumor onset (8 mo); D: Immunofluorescence staining showing colocalization of BRUCE (red) and PKA (green) in endosomes (arrows) in normal human THLE2 hepatocyte line with cell nucleus counterstained with DAPI. The cellular areas outlined in dashed squares are enlarged and shown below; scale bar 20 μm; E: A working model showing a new BRUCE-PKA-β-catenin signaling axis involved in the regulation of fibrosis and HCC. BRUCE regulates β-catenin activation by inhibiting PKA-dependent phosphorylation-activation of β-catenin for hepatic proliferation and carcinogenesis. Mechanistically, BRUCE interacts with PKA in the hepatocyte cytoplasm to restrain PKA activity. When this interaction is disrupted by KO of BRUCE in the mouse liver, or by KD of BRUCE expression in liver cancer cell line, the repression of PKA is derepressed and PKA-dependent phosphorylation-activation of β-catenin at Ser-675 occurs which results in hepatic proliferation. Meanwhile hepatocytes undergo apoptosis induced by DEN-DNA damage and these apoptotic hepatocytes release damage associated molecular patterns to activate hepatic stellate cells. The BRUCE-PKA-β-catenin signaling axis, together with DEN induced DNA damage, hepatic cell death, and oxidative stress, result in an early onset of fibrosis and accelerated HCC. DEN: Diethylnitrosamine; LKO: Liver-specific knockout; BRUCE: BIR repeat-containing ubiquitin conjugating enzyme; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; PKA: Protein kinase A; DAPI: 4',6-diamidino-2-phenylindole; HCC: Hepatocellular carcinoma; DAMPs: Damage associated molecular patterns; CTRL: Control.
- Citation: Vilfranc CL, Che LX, Patra KC, Niu L, Olowokure O, Wang J, Shah SA, Du CY. BIR repeat-containing ubiquitin conjugating enzyme (BRUCE) regulation of β-catenin signaling in the progression of drug-induced hepatic fibrosis and carcinogenesis. World J Hepatol 2021; 13(3): 343-361
- URL: https://www.wjgnet.com/1948-5182/full/v13/i3/343.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i3.343