Copyright
©The Author(s) 2021.
World J Hepatol. Oct 27, 2021; 13(10): 1378-1393
Published online Oct 27, 2021. doi: 10.4254/wjh.v13.i10.1378
Published online Oct 27, 2021. doi: 10.4254/wjh.v13.i10.1378
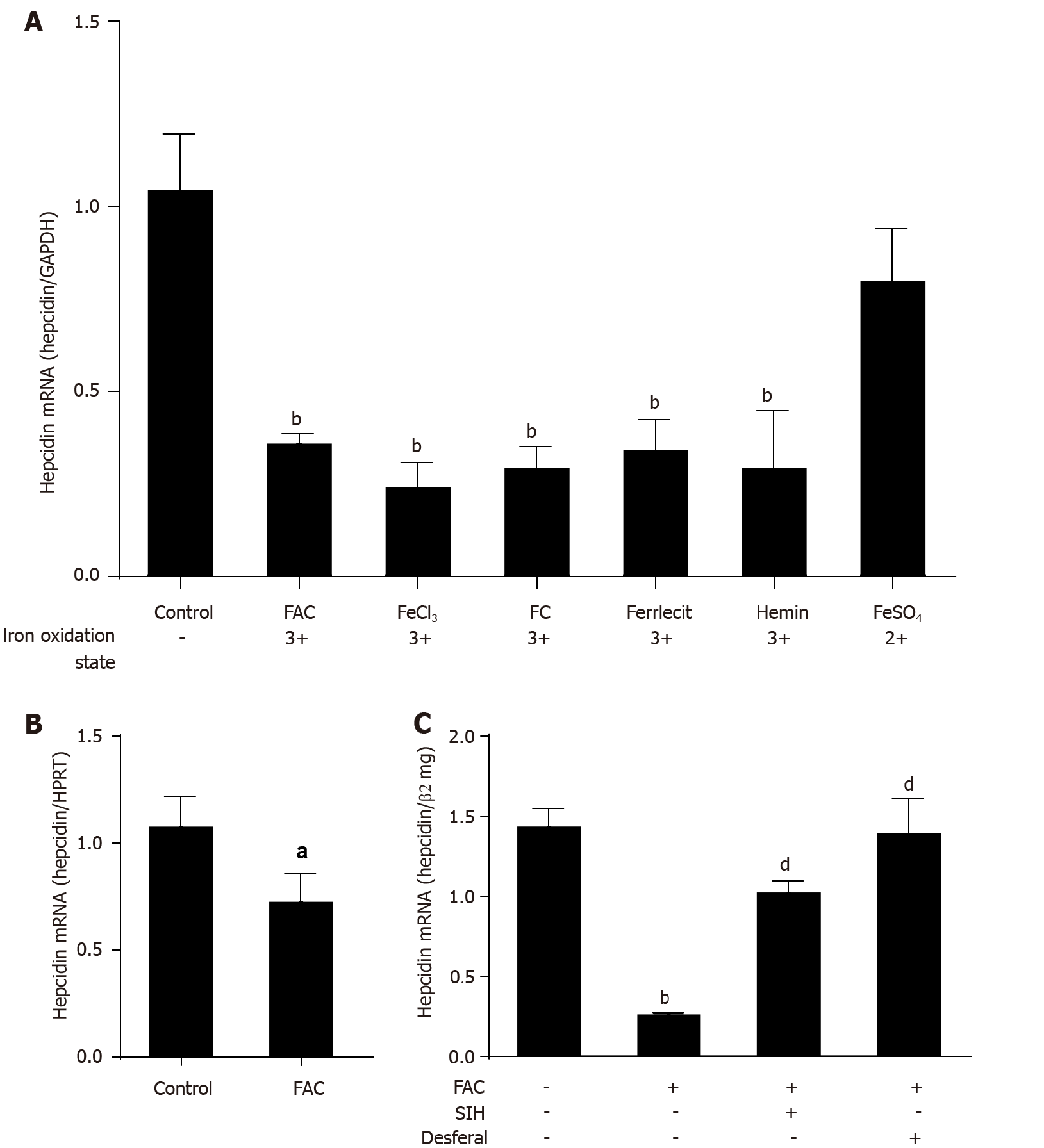
Figure 1 Efficient suppression of hepcidin by higher iron levels.
A: Huh7 cells were treated with 50 μmol/L of FAC, FeCl3, FC, ferrlecit, hemin or FeSO4 for 24 h; B: Murine primary hepatocytes were treated with FAC (50 μmol/L) for 24 h; C: Huh7 cells were treated with FAC (50 μmol/L) in the presence or absence of SIH (100 μmol/L) or Desferal (50 μmol/L) for 24 h. Total RNA was extracted from Huh7 cells or murine primary hepatocytes. Hepcidin mRNA levels were determined by quantitative real-time PCR, normalized to glyceraldehyde 3-phosphate dehydrogenase or hypoxanthine phosphoribosyltransferase or β2mg. Data are presented as mean ± SD. aP < 0.05, bP < 0.001 vs control; dP < 0.001 vs FAC group. FAC: Ferric ammonium citrate; FeCl3: Ferric chloride; FC; Ferric citrate; FeSO4: Ferrous sulfate; SIH: Salicylaldehyde isonicotinoyl hydrazine; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase; β2mg, β2-microglobulin; HPRT: Hypoxanthine phosphoribosyltransferase.
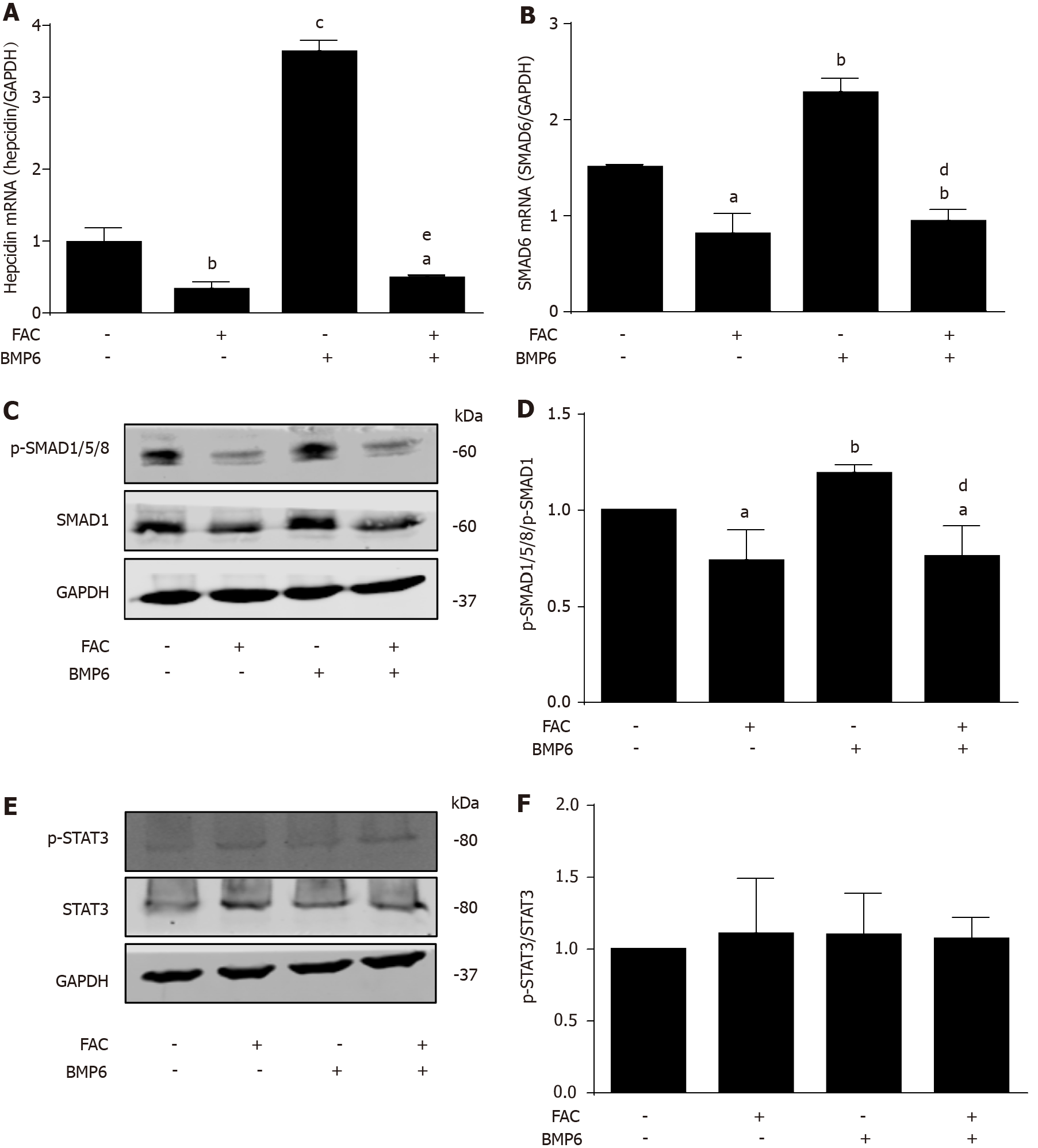
Figure 2 Ferric ammonium citrate profoundly blocks bone morphogenetic protein 6-mediated hepcidin signaling.
Huh7 cells were treated with or without bone morphogenetic protein 6 (BMP6) (40 ng/mL) in the presence or absence of ferric ammonium citrate (FAC) (50 μmol/L) for 24 h. Total RNA and protein were extracted from Huh7 cells. A: FAC decreased the hepcidin mRNA expression in the presence or absence of BMP6; B: FAC decreased small mothers against decapentaplegic 6 (SMAD6) mRNA expression in the presence or absence of BMP6; C, D: FAC decreased p-SMAD1/5/8 protein expression in the presence or absence of BMP6; E, F: Both BMP6 and FAC have no significant effect on phosphorylated signal transducer and activator of transcription 3 (p-STAT3) protein expression. SMAD1, p-SMAD1/5/8, STAT3, p-STAT3 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) protein levels were determined by Western blotting. Hepcidin and SMAD6 mRNA levels were determined by qRT-PCR, normalized to GAPDH. Western Blots are representatives of three independent experiments. Data are presented as mean ± SD. aP < 0.05, bP < 0.01, cP < 0.001 vs control; dP < 0.01, eP < 0.001 vs BMP6 group. FAC: Ferric ammonium citrate; BMP6. Bone morphogenetic protein 6; p-: Phospho-; SMAD: Small mothers against decapentaplegic; STAT3: Signal transducer and activator of transcription 3; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase.
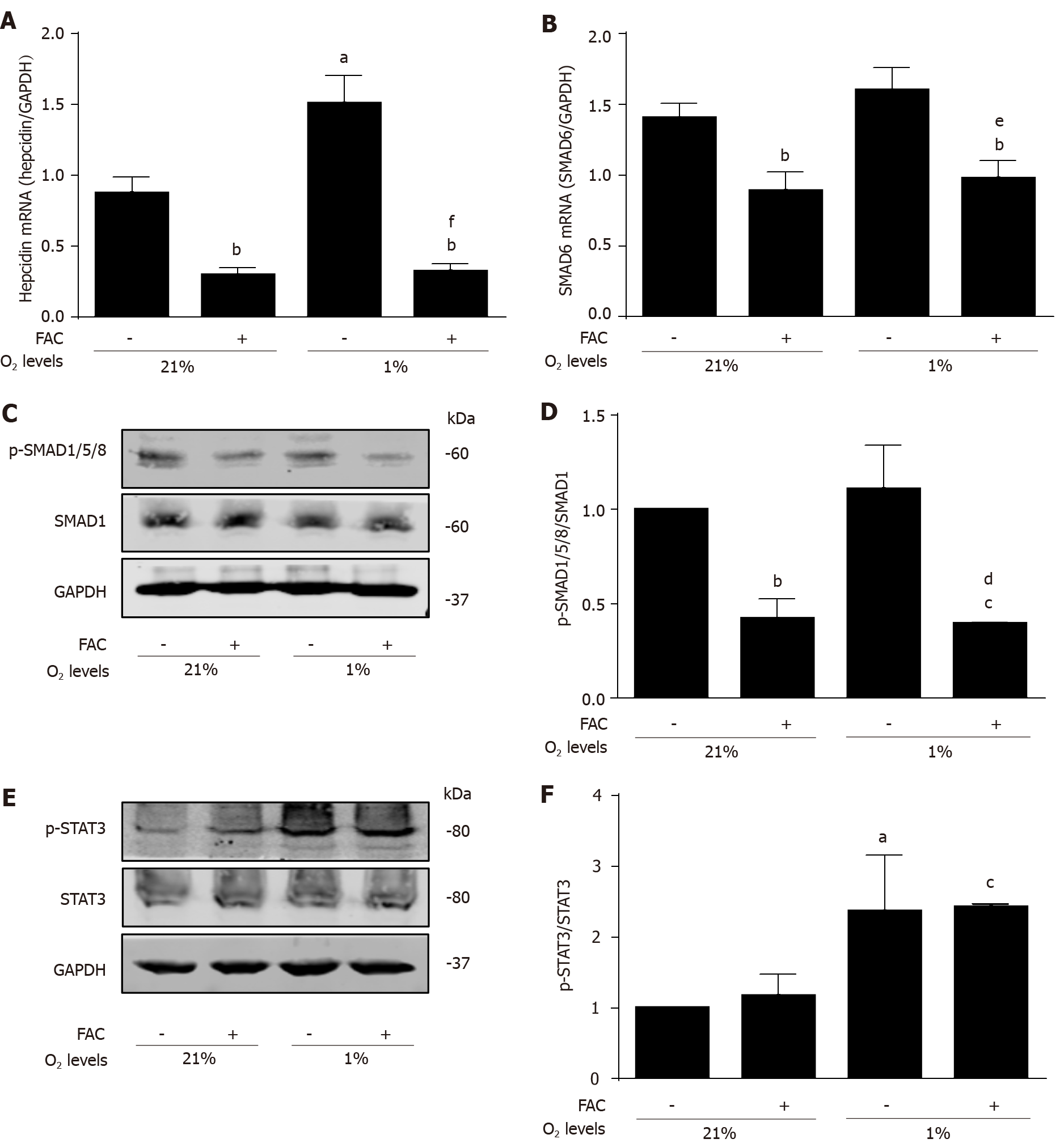
Figure 3 Ferric ammonium citrate efficiently inhibits hypoxia-mediated hepcidin response independent of signal transducer and activator of transcription 3.
Huh7 cells were exposed to normoxia (210 mL/L O2, 21% O2) or hypoxia (10 mL/L O2, 1% O2) in the presence or absence of ferric ammonium citrate (FAC) (50 μmol/L) for 24 h. Total RNA and protein were extracted from Huh7 cells. A: FAC decreased the basal and hypoxia-induced hepcidin mRNA expression; B: Hypoxia has no obvious effect on small mothers against decapentaplegic 6 (SMAD6) mRNA expression, but FAC decreased SMAD6 mRNA expression in the presence or absence of hypoxia; C, D: Hypoxia has no significant effect on p-SMAD1/5/8 protein expression, while FAC decreased p-SMAD1/5/8 protein expression in the presence or absence of hypoxia; E, F: Hypoxia increased phosphorylated signal transducer and activator of transcription 3 (p-STAT3) protein expression, while FAC has no significant effect on p-STAT3 protein expression in the presence or absence of hypoxia. SMAD1, p-SMAD1/5/8, STAT3, p-STAT3 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) protein levels were determined by Western blotting. Hepcidin and SMAD6 mRNA levels were determined by qRT-PCR, normalized to GAPDH. Western Blots are representatives of three independent experiments. Data are presented as mean ± SD. aP < 0.05, bP < 0.01, cP < 0.001 vs control (21% O2); dP < 0.05, eP < 0.01, fP < 0.001 vs control (1% O2). FAC: Ferric ammonium citrate; O2: oxygen; p-: Phospho-; SMAD: Small mothers against decapentaplegic; STAT3: Signal transducer and activator of transcription 3; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase.
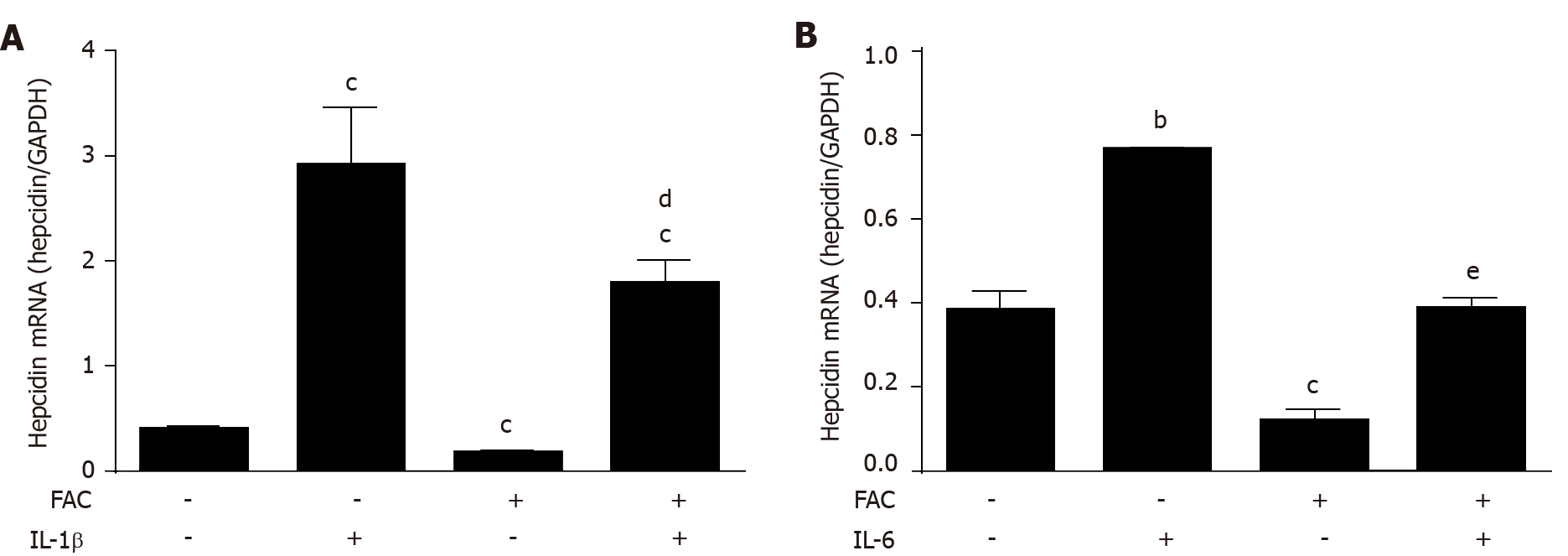
Figure 4 Ferric ammonium citrate efficiently blocks cytokine-mediated hepcidin expression.
Huh7 cells were treated with or without IL-1β (10 ng/mL) or IL-6 (10 ng/mL) in the presence or absence of ferric ammonium citrate (FAC) (50 μmol/L) for 24 h. Total RNA was extracted from Huh7 cells. A: FAC significantly decreased IL-1β-induced hepcidin mRNA expression; B: FAC efficiently blocks IL-6-induced hepcidin mRNA expression. Hepcidin mRNA levels were determined by qRT-PCR, normalized to glyceraldehyde 3-phosphate dehydrogenase. Data are presented as mean ± SD. bP < 0.01, cP < 0.001 vs control; dP < 0.05, eP < 0.001 vs IL-6 group. IL-1β: Interleukin 1β; IL-6: Interleukin 6; FAC: Ferric ammonium citrate; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase.
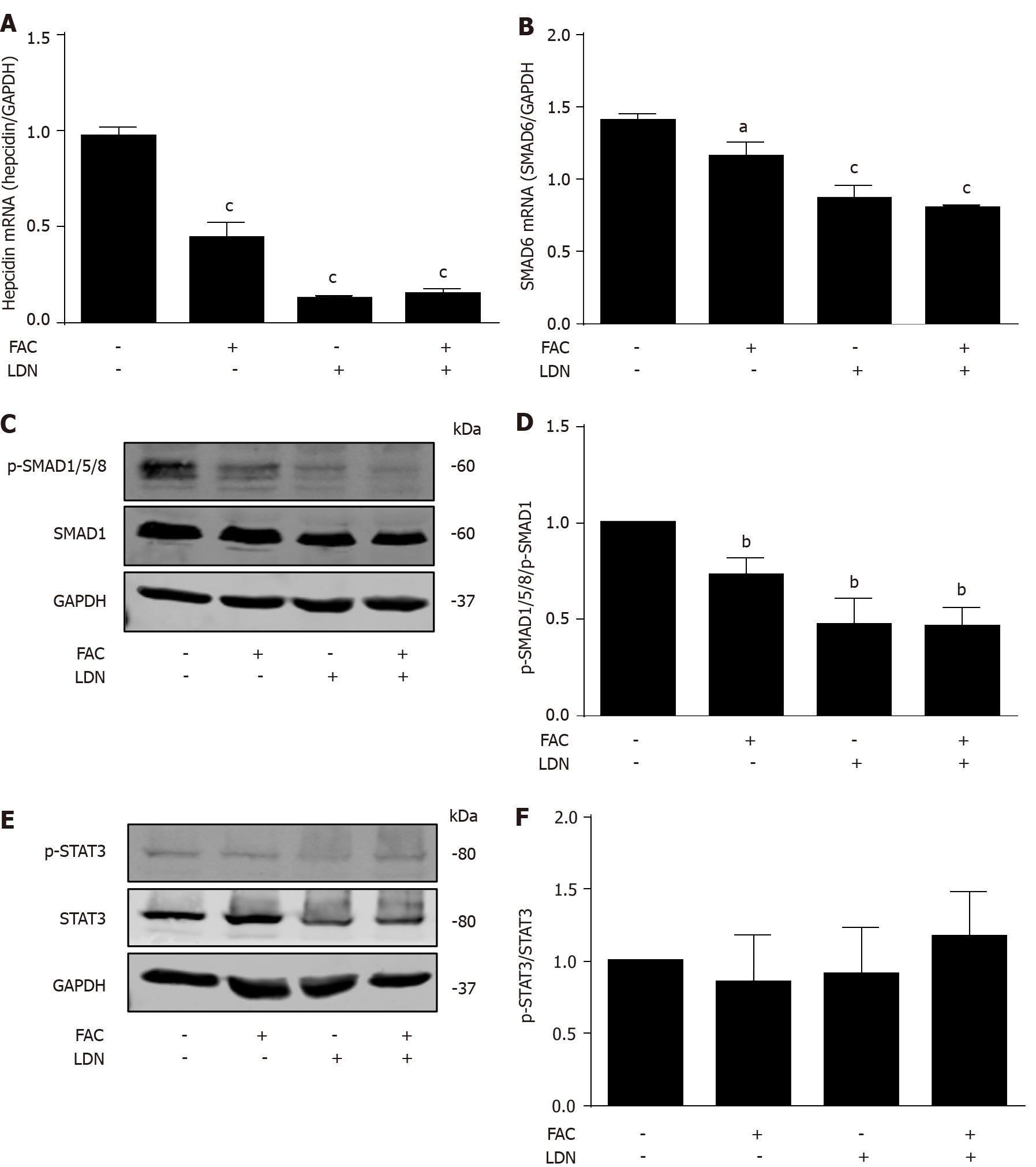
Figure 5 Inhibition of hepatocellular hepcidin by ferric ammonium citrate requires bone morphogenetic protein/small mothers against decapentaplegic signaling.
Huh7 cells were treated with or without ferric ammonium citrate (FAC) (50 μmol/L) in the presence or absence of LDN193189 Hydrochloride (LDN) (20 nmol/L) for 24 h. Total RNA and protein were extracted from Huh7 cells. A: FAC or LDN decreased the basal hepcidin mRNA expression, but FAC in combination with LDN did not further suppress hepcidin mRNA expression compared with LDN alone; B-D: FAC or LDN decreased the basal small mothers against decapentaplegic (SMAD)6 mRNA and p-SMAD1/5/8 protein expression, but FAC in combination with LDN did not further suppress SMAD6 and p-SMAD1/5/8 expression compared with LDN alone; E, F: Both FAC and LDN had no significant effect on phosphorylated signal transducer and activator of transcription 3 (p-STAT3) protein expression. SMAD1, p-SMAD1/5/8, STAT3, p-STAT3 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) protein levels were determined by Western blotting. Hepcidin and SMAD6 mRNA levels were determined by qRT-PCR, normalized to GAPDH. Western Blots are representatives of three independent experiments. Data are presented as mean ± SD. aP < 0.05, bP < 0.01, cP < 0.001 vs control. FAC: Ferric ammonium citrate; LDN: LDN193189 Hydrochloride; p-: Phospho-; SMAD: Small mothers against decapentaplegic; STAT3: Signal transducer and activator of transcription 3; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase.
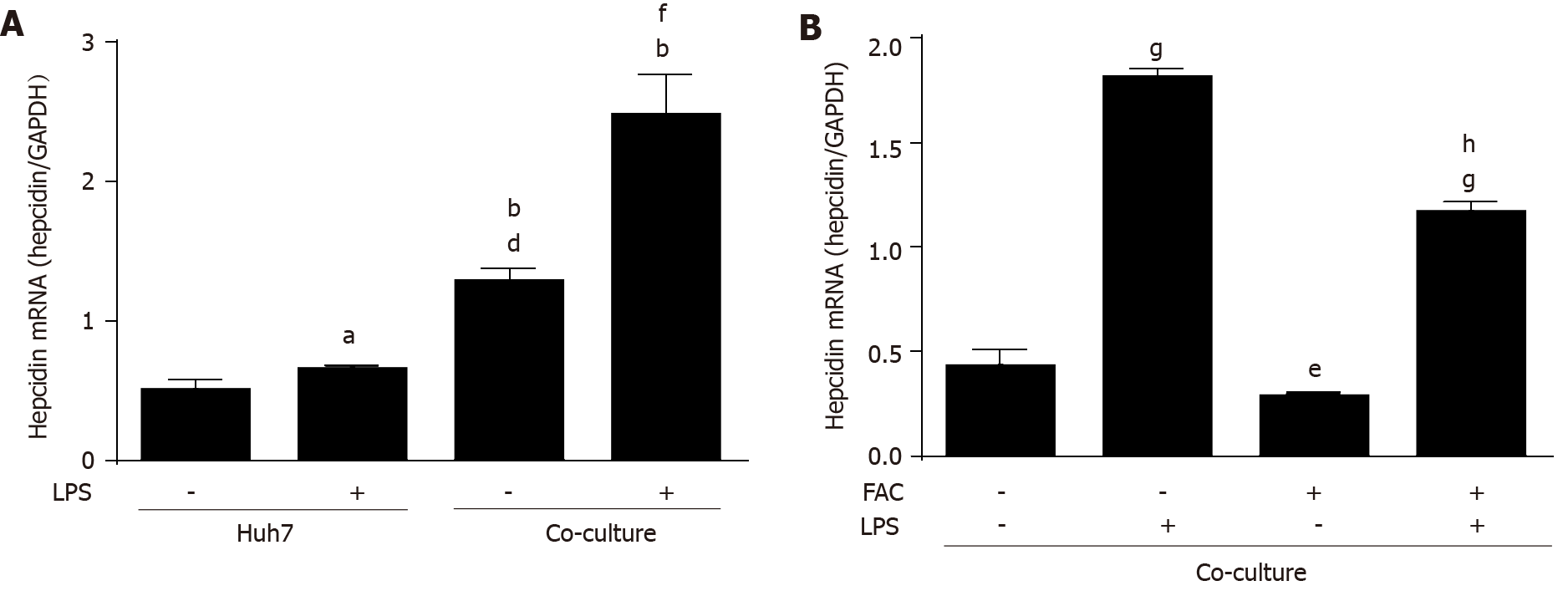
Figure 6 Ferric ammonium citrate decreases hepatic hepcidin expression induced by lipopolysaccharide in a macrophage-hepatocyte co-culture model.
Huh7 cells were treated with or without lipopolysaccharide (LPS) (500 ng/mL) for 24 h. Huh7 cells were directly co-cultured with THP-1 macrophages according to pathophysiological macrophage/hepatocyte cell ratio (1:4) and then treated with or without LPS (500 ng/mL) for 24 h in the presence or absence of ferric ammonium citrate (FAC) (50 μmol/L). Total RNA was extracted from Huh7 cells or Huh7 cells and THP-1 macrophages. A: Hepcidin mRNA levels were slightly increased by LPS in monoculture of Huh7 cells, and macrophages increased hepcidin mRNA levels compared with monoculture control and the presence of LPS further markedly increased hepcidin mRNA levels; B: FAC decreased the basal and LPS-induced hepcidin mRNA levels in the co-culture model. Hepcidin mRNA levels were determined by qRT-PCR, normalized to glyceraldehyde 3-phosphate dehydrogenase. Data are presented as mean ± SD. aP < 0.05, bP < 0.001 vs Huh7 control; dP < 0.001 vs Huh7 LPS group; eP < 0.05, fP < 0.01, gP < 0.001 vs co-culture control; hP < 0.001 vs co-culture LPS group. LPS: Lipopolysaccharide; FAC: Ferric ammonium citrate.
Figure 7 Scheme of iron-mediated blockage of hepcidin transcription via bone morphogenetic protein/small mothers against decapenta
- Citation: Yu LN, Wang SJ, Chen C, Rausch V, Elshaarawy O, Mueller S. Direct modulation of hepatocyte hepcidin signaling by iron. World J Hepatol 2021; 13(10): 1378-1393
- URL: https://www.wjgnet.com/1948-5182/full/v13/i10/1378.htm
- DOI: https://dx.doi.org/10.4254/wjh.v13.i10.1378