Copyright
©The Author(s) 2020.
World J Hepatol. Jan 27, 2020; 12(1): 10-20
Published online Jan 27, 2020. doi: 10.4254/wjh.v12.i1.10
Published online Jan 27, 2020. doi: 10.4254/wjh.v12.i1.10
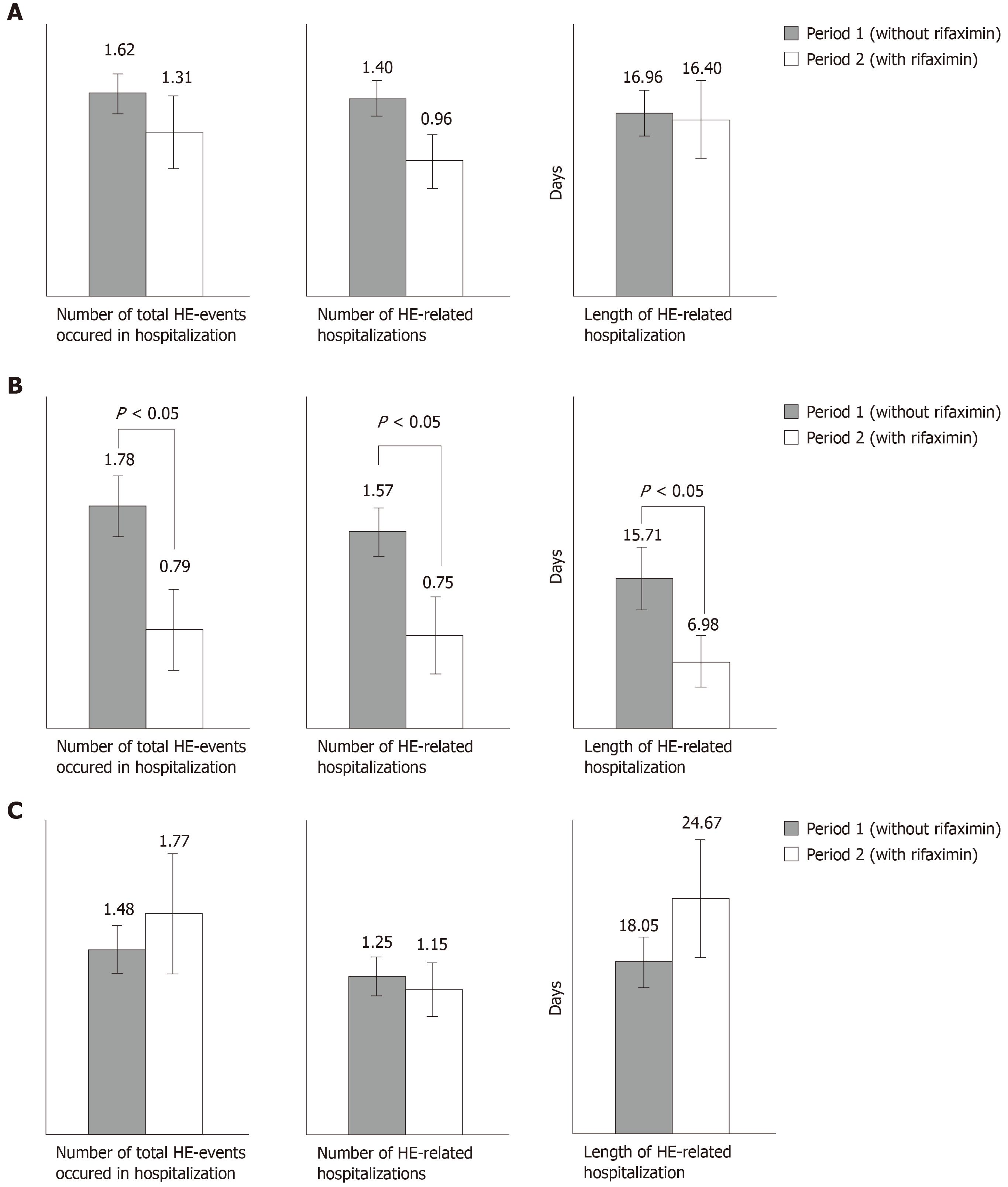
Figure 1 Comparison of hepatic encephalopathy events, hepatic encephalopathy-related hospitalizations and length of hepatic encephalopathy-related hospitalization with or without rifaximin for entire cohort and subgroups “prevention of recurrent hepatic encephalopathy” and “prevention of an acute episode on persistent hepatic encephalopathy”.
A: Entire cohort; B: Prevention of recurrent hepatic encephalopathy (HE) episodes; C: Prevention of recurrent acute exacerbations on persistent HE. The results are expressed for 100 d of follow-up. HE: Hepatic encephalopathy.
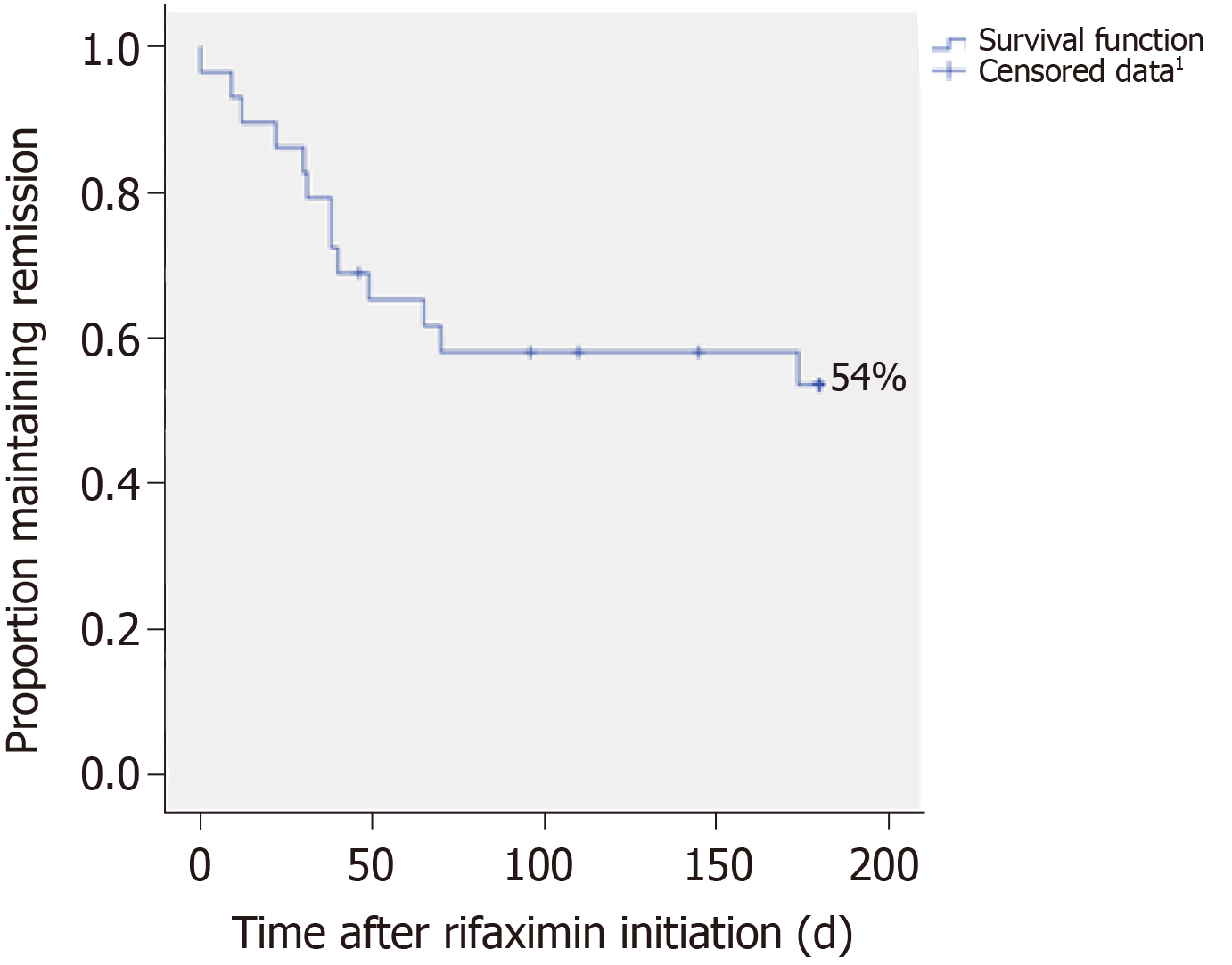
Figure 2 Probability of hepatic encephalopathy recurrence during rifaximin therapy in the subgroup “Prevention of recurrent hepatic encephalopathy”, n = 29; Kaplan-Meier method.
1Censored data correspond to discontinuation of treatment before the occurrence of hepatic encephalopathy (HE). At the end of follow-up, it corresponded to “HE-free” patients, after six months of treatment.
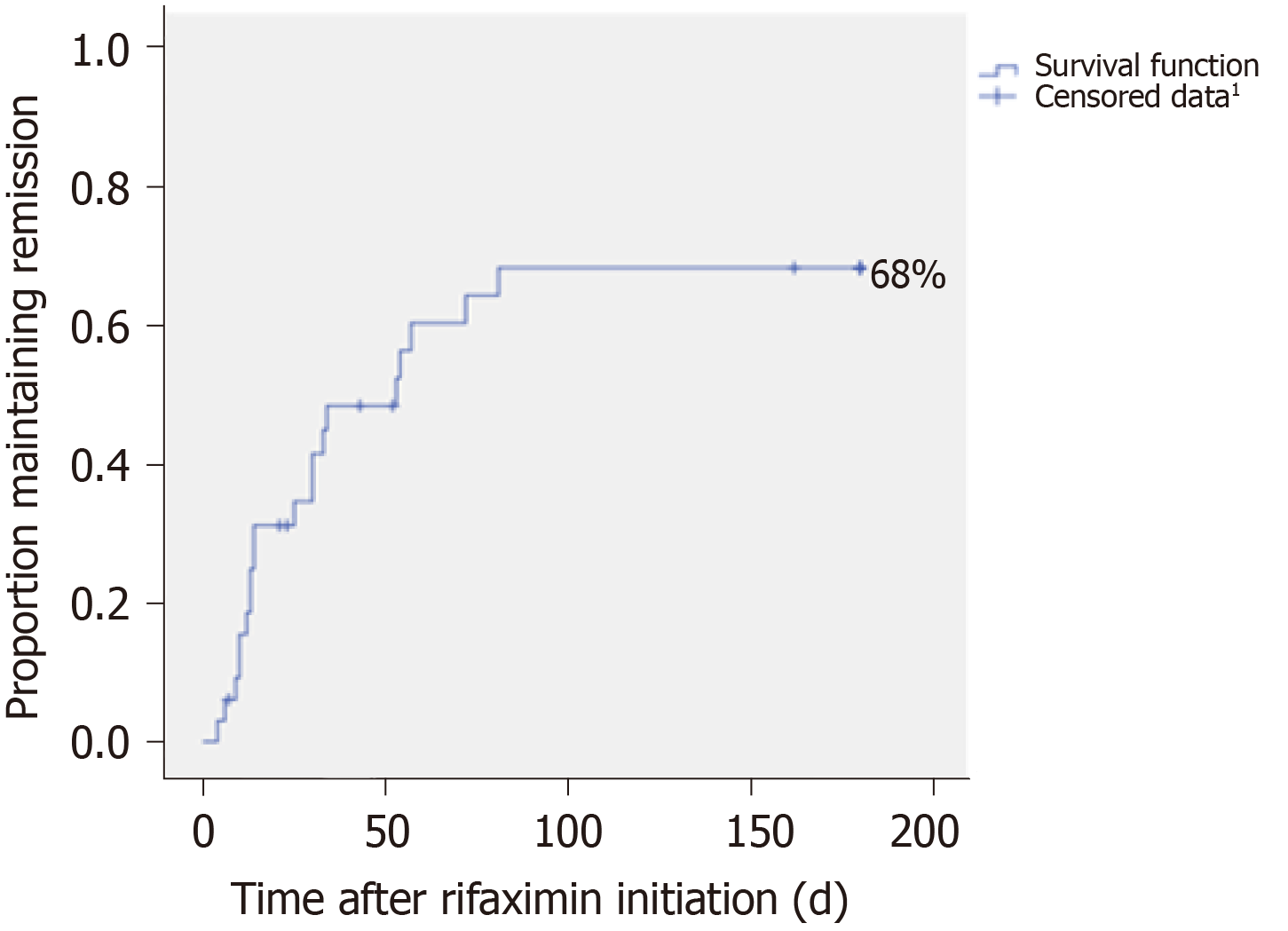
Figure 3 Cessation probability of persistent hepatic encephalopathy during rifaximin therapy in the subgroup “Prevention of an acute episode on persistent hepatic encephalopathy”, n = 33; Kaplan-Meier method.
1Censored data correspond to discontinuation of treatment before cessation of hepatic encephalopathy (HE). At the end of follow-up, it corresponded to patients with maintenance of persistent HE.
- Citation: Chautant F, Guillaume M, Robic MA, Cadranel JF, Peron JM, Lison H, Cool C, Bureau C, Duhalde V. Lessons from “real life experience” of rifaximin use in the management of recurrent hepatic encephalopathy. World J Hepatol 2020; 12(1): 10-20
- URL: https://www.wjgnet.com/1948-5182/full/v12/i1/10.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i1.10