Copyright
©The Author(s) 2018.
World J Hepatol. Sep 27, 2018; 10(9): 543-548
Published online Sep 27, 2018. doi: 10.4254/wjh.v10.i9.543
Published online Sep 27, 2018. doi: 10.4254/wjh.v10.i9.543
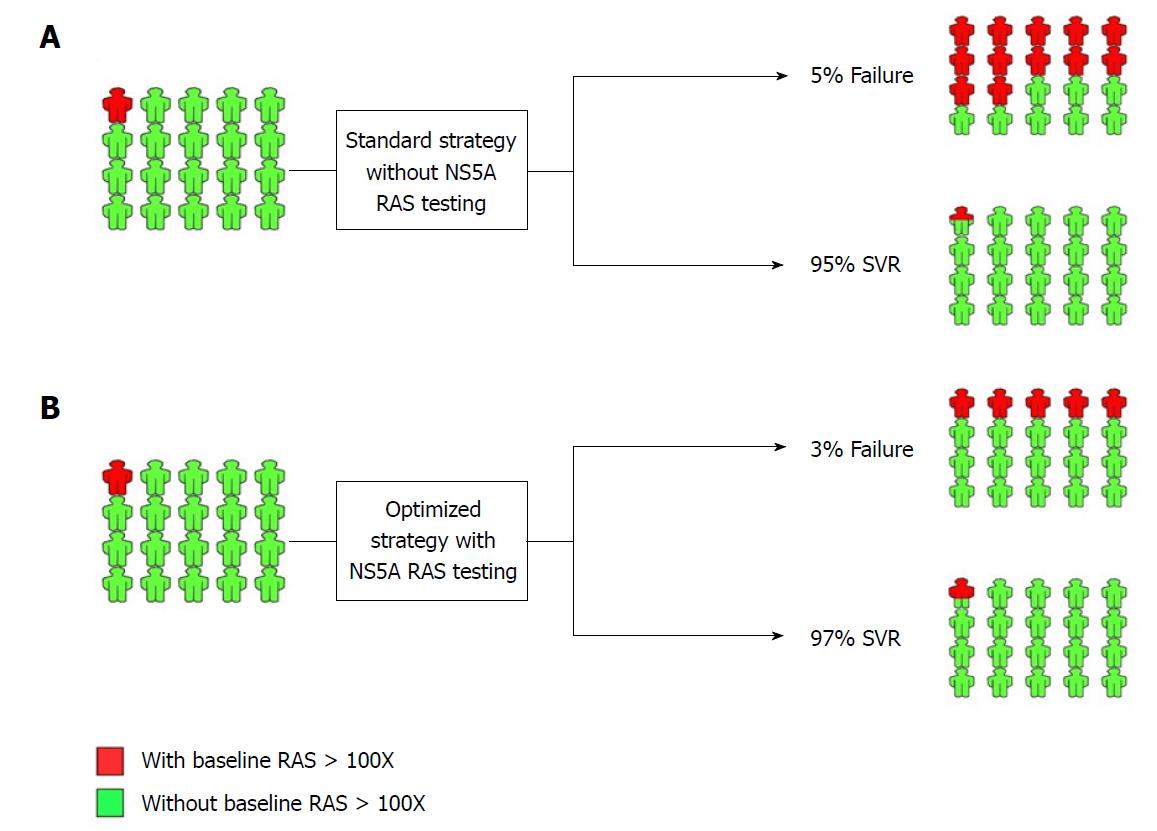
Figure 1 NS5A resistance-associated substitution testing in NS5A inhibitor-naïve patients prior to treatment with NS5A inhibitor-containing regimen.
A: Standard treatment strategy and outcome without NS5A resistance-associated substitution (RAS) testing; B: Optimized treatment strategy and outcome with NS5A RAS testing. The implementation of NS5A RAS testing is developed by decision-making based on the presence of RAS > 100X. SVR: Sustained virologic response.
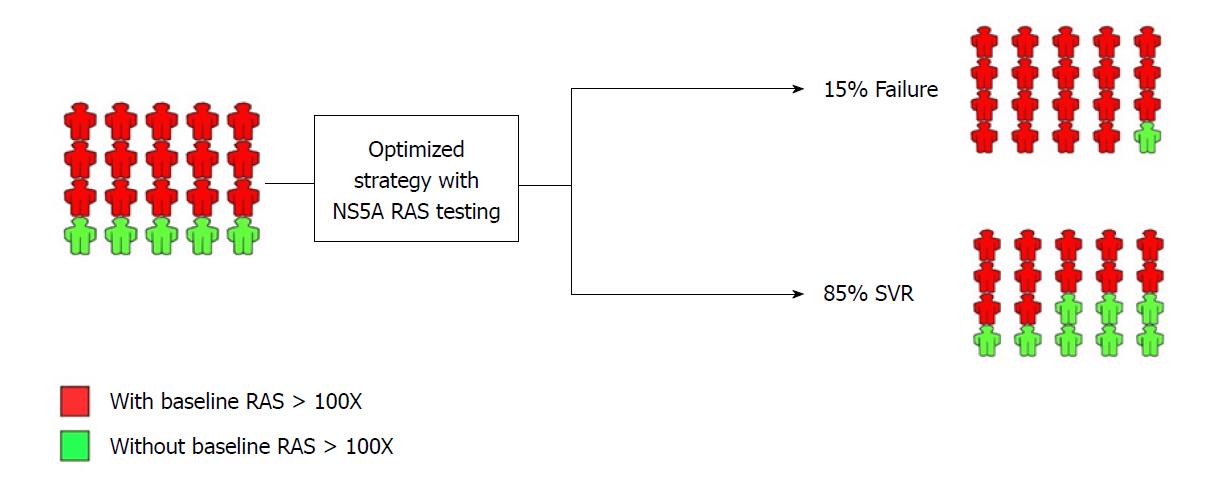
Figure 2 NS5A resistance-associated substitution testing in NS5A inhibitor-experienced patients prior to retreatment with NS5A inhibitor-containing regimen.
Optimized treatment strategy with NS5A resistance-associated substitutions (RAS) testing and the outcome is shown. The implementation of NS5A RAS testing is developed by decision-making based on the presence of RAS > 100X. SVR: Sustained virologic response.
- Citation: Sharafi H, Alavian SM. Hepatitis C resistance to NS5A inhibitors: Is it going to be a problem? World J Hepatol 2018; 10(9): 543-548
- URL: https://www.wjgnet.com/1948-5182/full/v10/i9/543.htm
- DOI: https://dx.doi.org/10.4254/wjh.v10.i9.543