Copyright
©The Author(s) 2018.
World J Hepatol. Jul 27, 2018; 10(7): 496-508
Published online Jul 27, 2018. doi: 10.4254/wjh.v10.i7.496
Published online Jul 27, 2018. doi: 10.4254/wjh.v10.i7.496
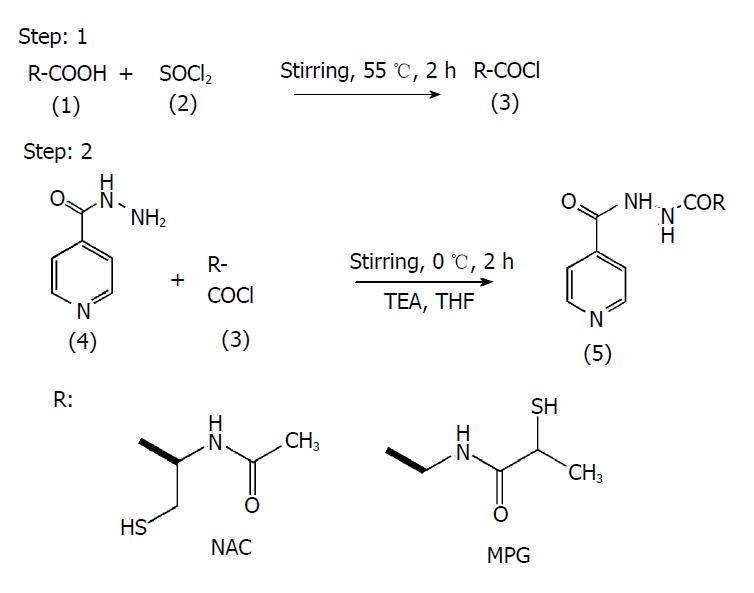
Figure 1 Scheme of synthesis for NI and MGI.
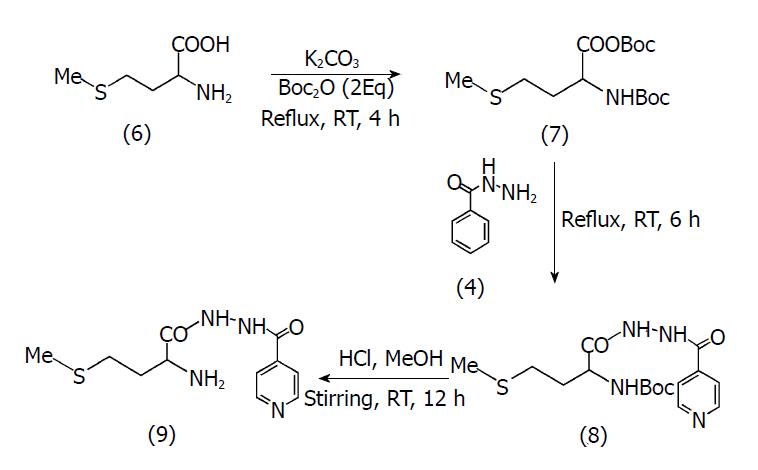
Figure 2 Scheme of synthesis for MI.
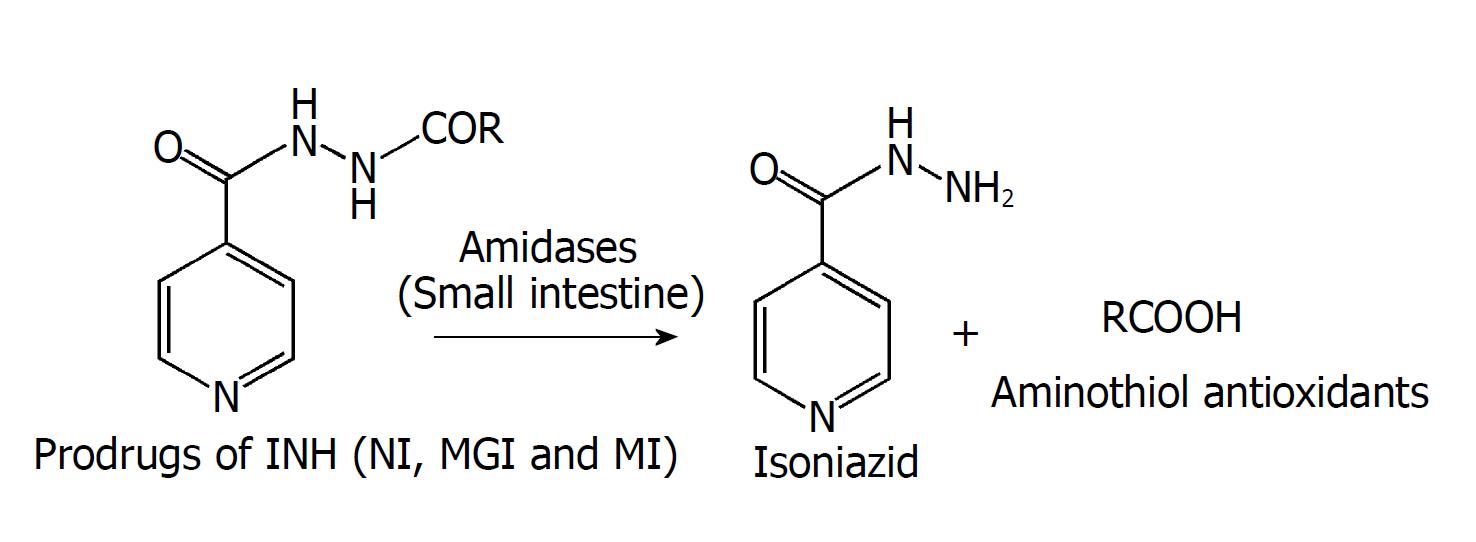
Figure 3 Proposed activation mechanism of prodrugs.
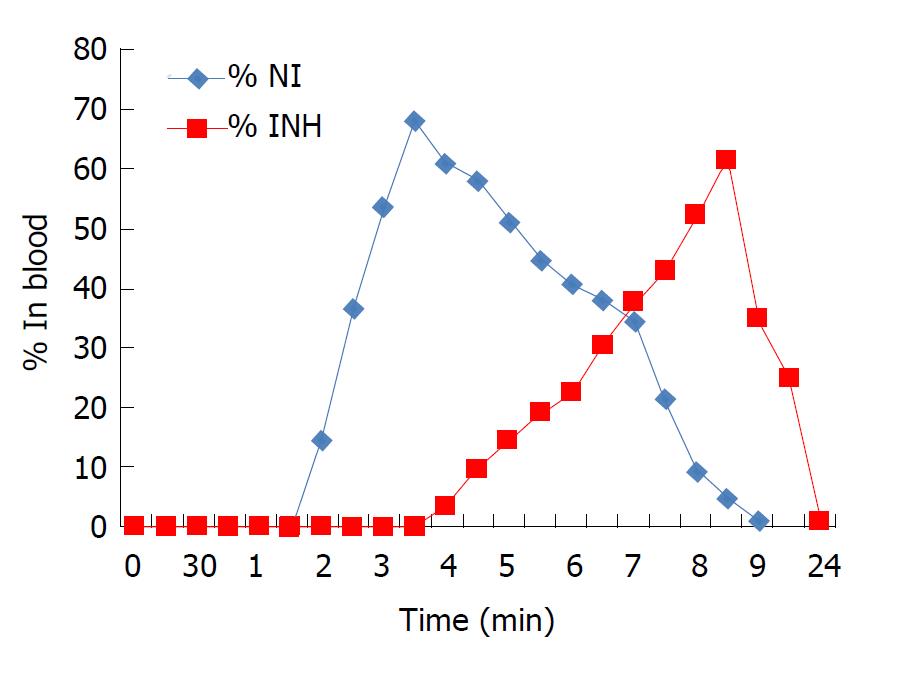
Figure 4 In vivo pharmacokinetics after oral administration of the prodrug NI (blood).
NI: Prodrug of INH and N-acetyl cysteine.
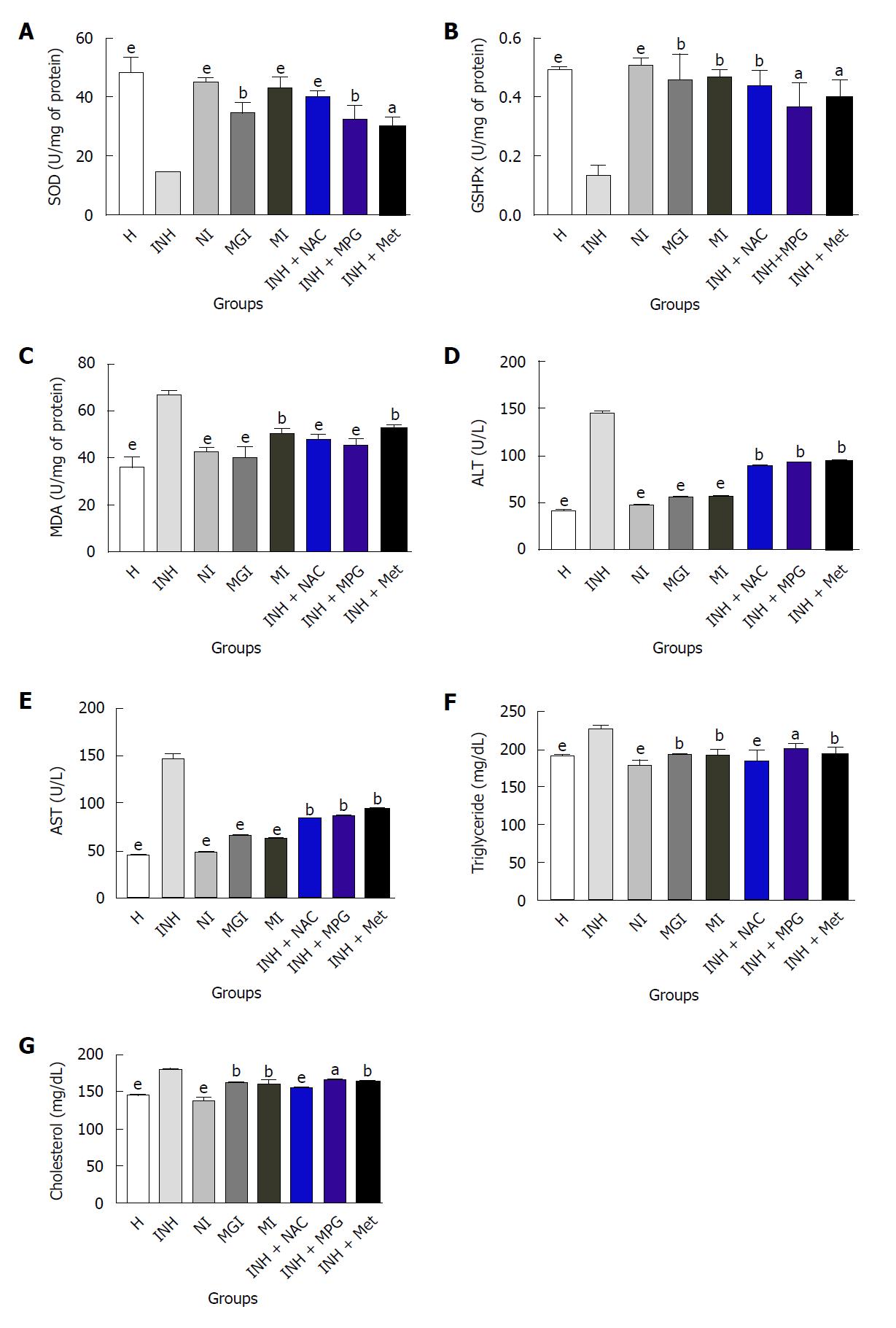
Figure 5 Effect of prodrugs on markers of liver function and oxidative stress and biochemical parameters.
Average of six readings; One-way ANOVA followed by Dunnett’s multiple comparison test, statistical significance considered at aP < 0.05, bP < 0.01, eP < 0.001 and ns: non-significant, when vs INH-treated group. H: Healthy control; INH: Isoniazid; NI: Prodrug of INH and N-acetyl cysteine; MGI: Prodrug of INH and N-(2-mercaptopropionyl) glycine; MI: Prodrug of INH and L-methionine; INH + NAC: Physical mixture of INH + N-acetyl cysteine; INH + MPG: Physical mixture of INH + N-(2-mercaptopropionyl) glycine; INH + Met: Physical mixture of INH and L-methionine.
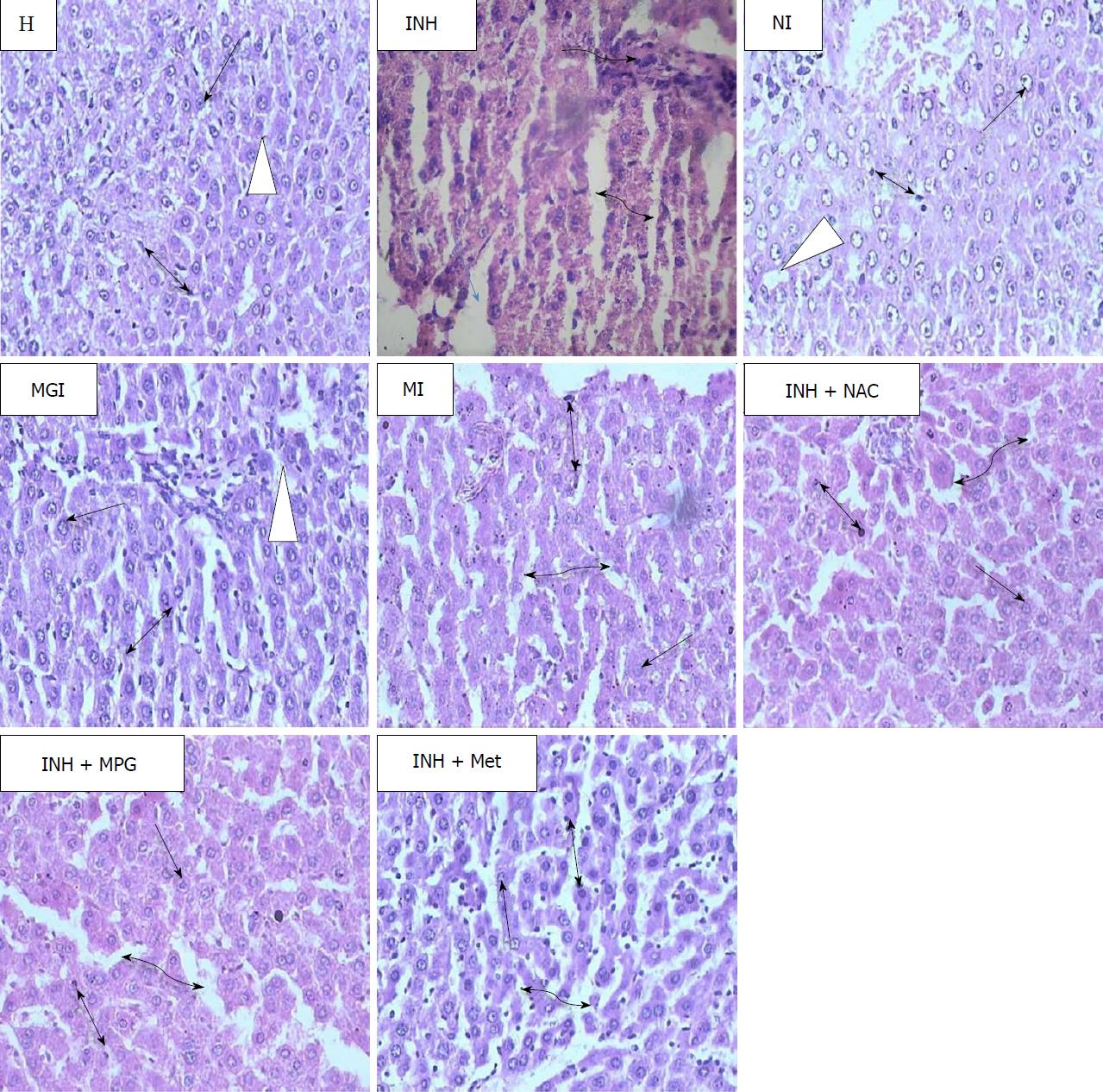
Figure 6 Photomicrographs of haematoxylin and eosin-stained histological sections of normal, isoniazid-intoxicated, prodrugs- and physical mixtures-treated rat livers.
- Citation: Bhilare NV, Dhaneshwar SS, Mahadik KR. Amelioration of hepatotoxicity by biocleavable aminothiol chimeras of isoniazid: Design, synthesis, kinetics and pharmacological evaluation. World J Hepatol 2018; 10(7): 496-508
- URL: https://www.wjgnet.com/1948-5182/full/v10/i7/496.htm
- DOI: https://dx.doi.org/10.4254/wjh.v10.i7.496